Abstract
Crude protein (CP) content of mechanically ground rice straw into small particles by an electric grinder and reducing value (RV) and soluble protein (SP) in the culture filtrate were lower than that of the chopped straw into 5~6 cm lengths when both ground and chopped straws were fermented with Aspergillus ochraceus, A. terreus or Trichoderma koningii, at steady conditions. The reduction rate of RV, SP and CP was 22.2, 2.4, 7.3%; 9.1, 4.9, 8.5% or 0.0, 0.0, 3.6% for the three fungi, respectively. Chemical pretreatment of straw by soaking in NH4OH for a day caused significant increase in CP of the fermented straw than the other alkali and acidic pretreatments. Gamma irradiation pretreatment of dry and wet straw with water, specially at higher doses, 100, 200 or 500 kGy, caused significant increase in RV and SP as CP in the fermented straw by any of these fungi. Chemical-physical combination pretreatment of rice straw reduced the applied dose of gamma irradiation required for increasing fermentable ability of fungi from 500 kGy to 10 kGy with approximately the same results. Significant increases in RV and SP of fermented straw generally occurred as the dose of gamma irradiation for pretreated straw, which combined with NH4OH, gradually rose. Whereas, the increase percentage in CP of fermented straw that was pretreated by NH4OH-10 kGy was 12.4%, 15.4% or 8.6% for A. ochraceus, A. terreus or T. koningii, respectively.
Keywords: Bioconversion, Gamma irradiation, Rice straw, Fermentation, Protein
Two key factors associated with the special configurations of the lingo-cellulose-hemicellulose composite slow the process of enzymatic hydrolysis. These are cellulose crystallinity and lignin protection. Thus most research efforts have been related, in some way, to one or both of them (Rolz and Humphrey, 1982). Non-biological treatments have been used to render straw and other lignocellulosic materials more digestible for cattle (Al-Masri, 1999; Al-Masri and Guenther, 1999; Banchorndhevakul, 2002) or more susceptible to microbial or enzymatic attack (Araujo and D'Souza, 1986; Babitskaya, 1986; Hoda et al., 1990; Coronel et al., 1991; Bastawde, 1992; Patel and Bhatt, 1992). Pretreatment of cellulose materials derived from various origins with different ways, such as mechanical, physical or chemical modification, before fungal attack was previously achieved (Peiris and Silva, 1987; Singh et al., 1989; Tripathi and Yadav, 1989; Chahal et al., 1991; Xin and Kumakura, 1993; Malek et al., 1994, 1998; Youssef and Aziz, 1999; Malek, 2001). Thanikachalam and Rangarajan (1992) found that crude protein content of alkali-treated rice straw and soluble protein in the culture filtrate were higher than those of the untreated, when both treated and untreated straw were fermented with Trichoderma sp, Aspergillus sp. or Fusarium sp.
The objective of this study is to determine the effect of mechanical, physical and/or chemical pretreatments on the feasibility of rice straw to microbial bioconversion into mycoprotein containing fodder.
Materials and Methods
Fungal species
The fungal species used in this study were Aspergillus ochraceus, A. terreus and Trichoderma koningii. These fungi were originally isolated and identified from the soil using Nylon net bag technique containing rice straw (Helal, 2005a). They showed the highest biodegradative ability among sixty four species tested (Helal, 2005b). The stock cultures were maintained in sterilized soil at 4℃ and subcultured in agar slants whenever required.
Pretreatment of rice straw
Mechanical and chemical pretreatments of straw were achieved according to Han and Anderson (1975), Millet et al. (1976), Rolz and Humphrey (1982) and Chahal (1982). Rice straw dried at 105℃ was ground into small particles using an electric grinder, as shown in Fig. 1. The non-ground rice stalks were chopped into 5~6 cm lengths as control. Ground or chopped rice straw was pretreated by soaking in 5% NaOH at ambient temperature for 1 h or in 3% NH4OH at room temperature for a day or 4 weeks. Acid pretreatment was made by heating with 3% H2SO4 or 5% HCl at 121℃ for 45 min or with 0.23 N HCl plus 0.15 N H3PO4 at 121℃ for 30 min. Physical pretreatment using gamma irradiation process was made in Indian cobalt-60 gamma cell at National Center for Radiation Research and Technology (NCRRT), Nasr City, Cairo, Egypt. The dose rate was 1Mg/8 hours (1.25 kGy/hr). Rice straw samples (ground, chopped, dried, or wet with water) were first packaged in plastic bags (10 g straw/bag), then in paper bags, covered with aluminum foil, and exposed to one of the following radiation doses, 5, 10, 50, 100, 200 or 500 kGy. At the same time, in combination with other treatments, plastic bags containing 10 g of ground or chopped rice straws were provided separately with 50 ml solution of each of acids or alkalies used in the chemical treatment. These bags were packaged and exposed to one of the following radiation doses: 5, 10, 50, or 100 kGy. After treatment, the straw was washed with water and added to the basal medium at pH 5.5.
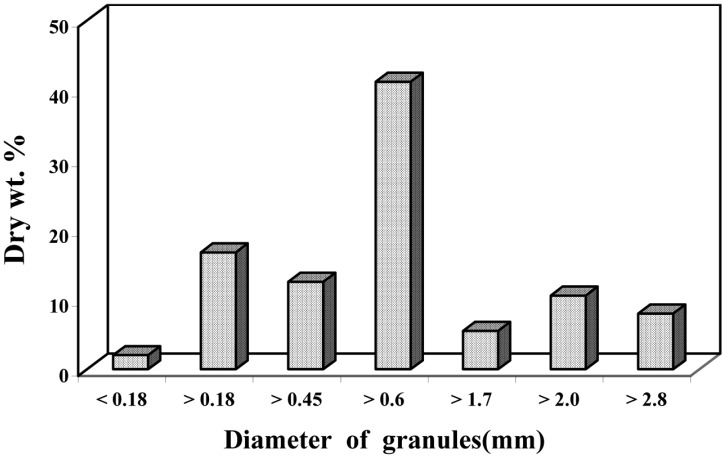
Fig. 1.
Diameter of rice straw granules (mm) ground into small particles.
Cultural conditions
Spores of each fungus were inoculated into triplicate of reagent bottles (100 ml), each containing 1.0 g ground or chopped rice straws instead of sucrose in 5 ml Czapek's broth medium containing 0.3 g NaNO3, 0.1 g KH2PO4, 0.05 g MgSO4, and 0.05 g KCl. After the pH of the medium was adjusted to 5.5, the bottles were autoclaved at 15 Lb for 20 min, and incubated in steady conditions at 27℃ for five days.
Chemical analysis of biodegraded straw
Total reducing value (mg/g dry straw) was obtained using glucose as standard by the previous method (Chaplin and Kennedy, 1987). Soluble protein (mg/g dry straw) was measured by the method of Lowery et al. (1951) with bovine albumin as standard and total nitrogen (mg/g dry straw) were determined in fermented rice straw as previously described (Allen, 1953).
Statistical analysis
The obtained data were analyzed by one-way ANOVA and multiple-way ANOVA following the way of Snedecor and Cochran (1982) and differences between means were calculated at the 5% probability level using Duncan's new multiple range tests (Duncan, 1955). Bivariate correlation matrix of the obtained data was done by using SPSS software program (ver. 8) as described by Dytham (1999).
Results
Rice straw with mechanical, chemical and/or physical pretreatments may be suitable for changing it by fungal fermentation into improved fodder with a good nutritive value as shown in the following results:
Effect of mechanical, chemical and mechanical-chemical pretreatments
Table 1 generally indicated that, non-ground rice stalks (chopped into 5~6 cm lengths) were more suitable than the fine particles (Fig. 1) for supporting fungal growth thereby increasing crude protein content and reducing sugar production. The amount of reducing sugar produced by fermentation of acidic or alkali-pretreated straw was generally higher than that of the untreated controls. This reducing sugar content significantly increased when fermented straw was pretreated by H2SO4 or HCl. The increment of reducing sugar content was usually accompanied by increase of soluble protein. The results also indicated that the crude protein content of fermented straw pretreated by NH4OH or HCl was significantly higher than those by the other pretreatments and untreated controls. In case of NH4OH pretreatment, the level of crude protein in straw pretreated for a day was increased by 7.3, 4.8 and 9.2% when fermented by A. ochraceus, A. terreus and T. koningii, respectively. Whereas, HCl pretreatment increased the crude protein content of fermented straw up to 7.3, 6.9 or 9.2% by any of these fungi as compared with the untreated controls. NaOH pretreatment caused increment in crude protein content, when straw was fermented by A. ochraceus or A. terreus (7.3 0r 5.3%), while, pretreatment with HCl plus H3PO4 increased protein content (6.6%) when straw was fermented with T. koningii. On the other hand, there was no significant difference in the crude protein content between non-treated fermenting rice straw and that pretreated by H2SO4. It is worth mentioning that there is no significant difference in reducing sugar, soluble protein and crude protein content of the rice straw, when fermented with any of the three fungi, in case of pretreatment straw by NH4OH for a day and that for 4 weeks.
Table 1.
Effect of mechanical, chemical and mechanical-chemical pretreatments on total reducing value (RV), soluble protein (SP) and crude protein (CP) of fermented rice straw by A. ochraceus, A. terreus and T. koningii at 27℃ for 5 days
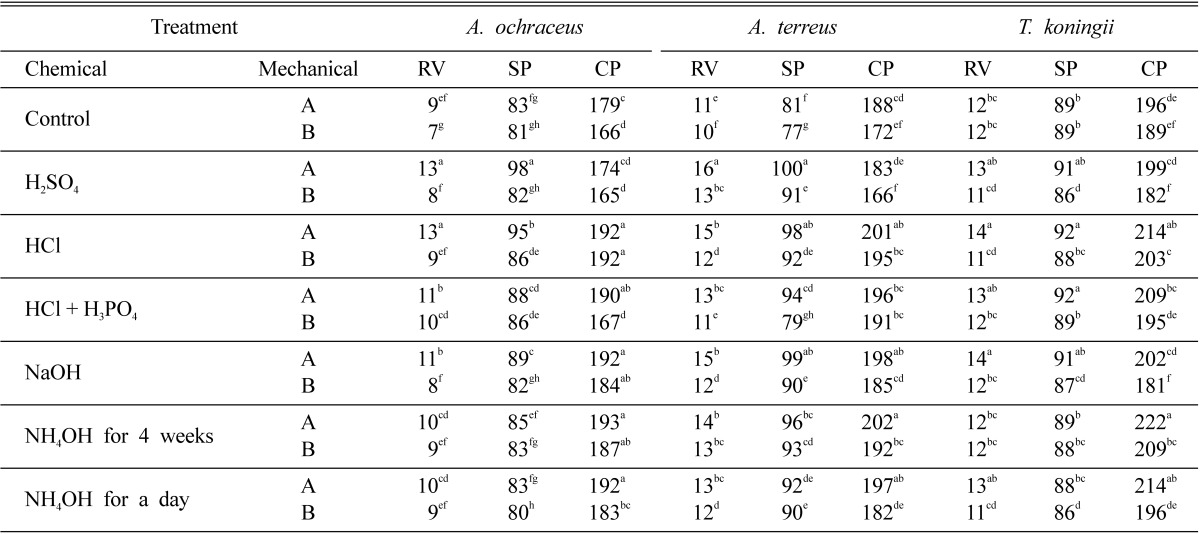
Means in the same column having the same superscript letter are not significantly different at P < 0.05.
A = chopped rice stalks 5~6 cm lengths. B = ground rice straw as in Fig. 1.
Combined effect of mechanical and/or physical pretreatment using gamma irradiation
Table 2 generally implied that in most cases, irradiation pretreatment of straw did not make significantly differ in the contents of reducing sugar, soluble protein and crude protein between chopped and ground fermented straws. In contrast, reducing sugar and soluble protein were gradually increased in the culture filtrate of the tested fungi grown on straw preirradiated with different doses from 5 to 500 kGy gamma radiation. Also pre-irradiated wet straw gave better result than pre-irradiated dried straw. On the other hand, crude protein contents of fermented straw of pre-irradiated by 5, 10 or 50 kGy were not significantly changed, while significant increase gradually occurred in that of pre-irradiated by 100, 200 or 500 kGy. Pretreatment of wet straw with 500 kGy significantly increased the contents of reducing sugar, soluble protein and crude protein after fermentation with A. ochraceus, A. terreus or T. koningii. The percentage of increase in soluble protein of ground wet straw after fermentation with any of these fungi was 61.7, 72.7 and 46.1%, and that in crude protein was 23.5, 22.1 or 12.7%, respectively. Reducing sugar was amplified more than four fold when irradiation dose increased up to 500 kGy.
Table 2.
Combined effect of mechanical-physical pretreatments using gamma irradiation on total reducing value (RV), soluble protein (SP) and crude protein (CP) of fermented rice straw by A. ochraceus, A. terreus and T. koningii at 27℃ for 5 days
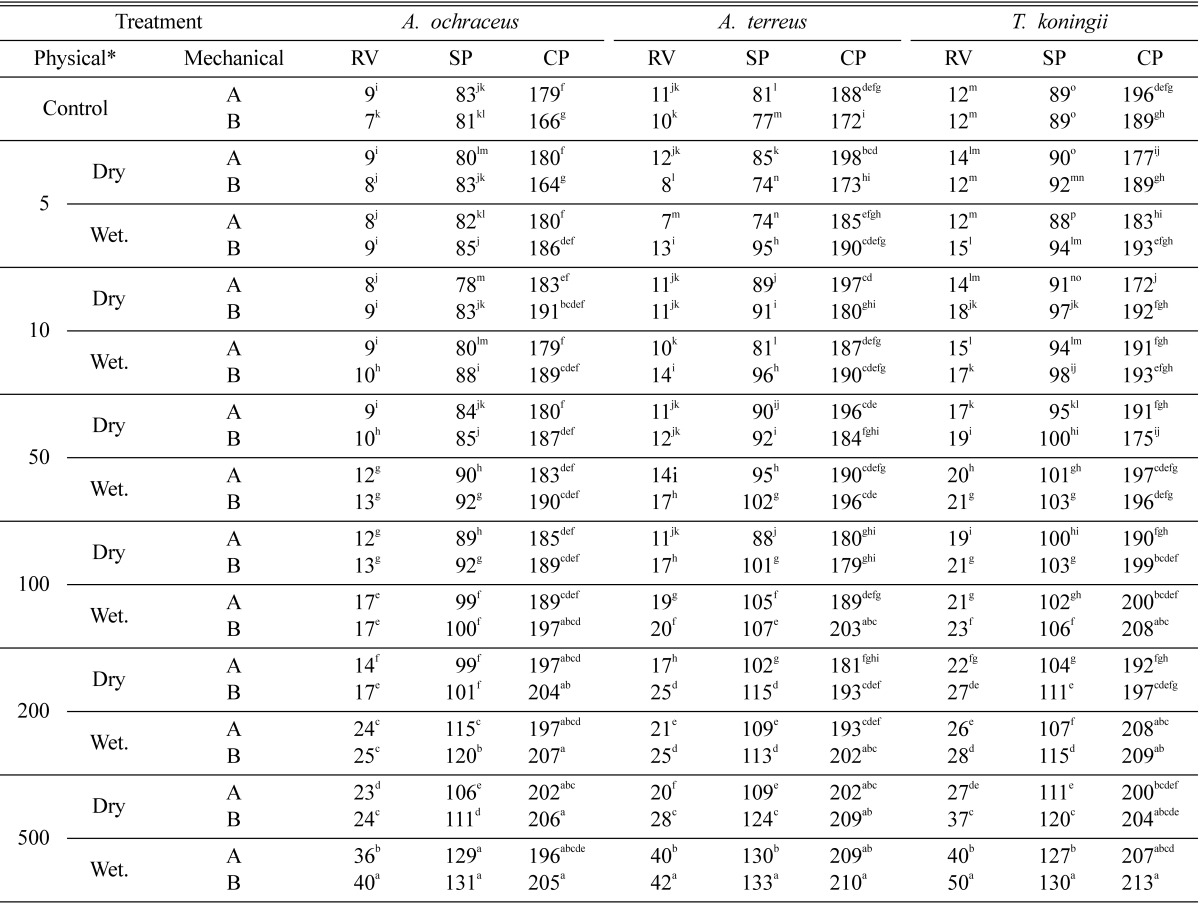
Means in the same column having the same superscript letter are not significantly different at P < 0.05.
A = chopped rice stalks 5~6 cm lengths. B = ground rice straw as in Fig. 1.
*Physical treatment = exposure to doses of gamma radiation/kGy.
Combined effect of mechanical-chemical-physical pretreatment
As indicated in the previous results (Table 1), the data in Table 3 emphasized that the fermentation which was obtained using chopped straw (5~6 cm lengths) was better than that of ground straw. The results also indicated that chemical-physical pretreatment using low doses of gamma-radiation (5 or 10 kGy) enhanced the fermentation process. Each of the three fungi significantly increased the protein content in the fermented straw with chemical-physical pretreatment rather than in the fermented straw with either chemical or physical pretreatment. The type of chemical pretreatment combined with the physical pretreatment was also effective. High level of reducing sugar and soluble protein were obtained when straw was pretreated with H2SO4-or HCl-gamma radiation while, high protein content was obtained when straw pretreated with NH4OH- or HCl-gamma radiation. The highest protein content 217, 233 or 241 mg/g in fermented straws by A. ochraceus, A. terreus and T. koningii was obtained when the chopped straw was pretreated with NH4OH-10 kGy.
Table 3.
Combined effect of mechanical-chemical-physical pretreatments on total reducing value (RV), soluble protein (SP) and crude protein (CP) of fermented rice straw by A. ochraceus, A. terreus and T. koningii at 27° for 5 days
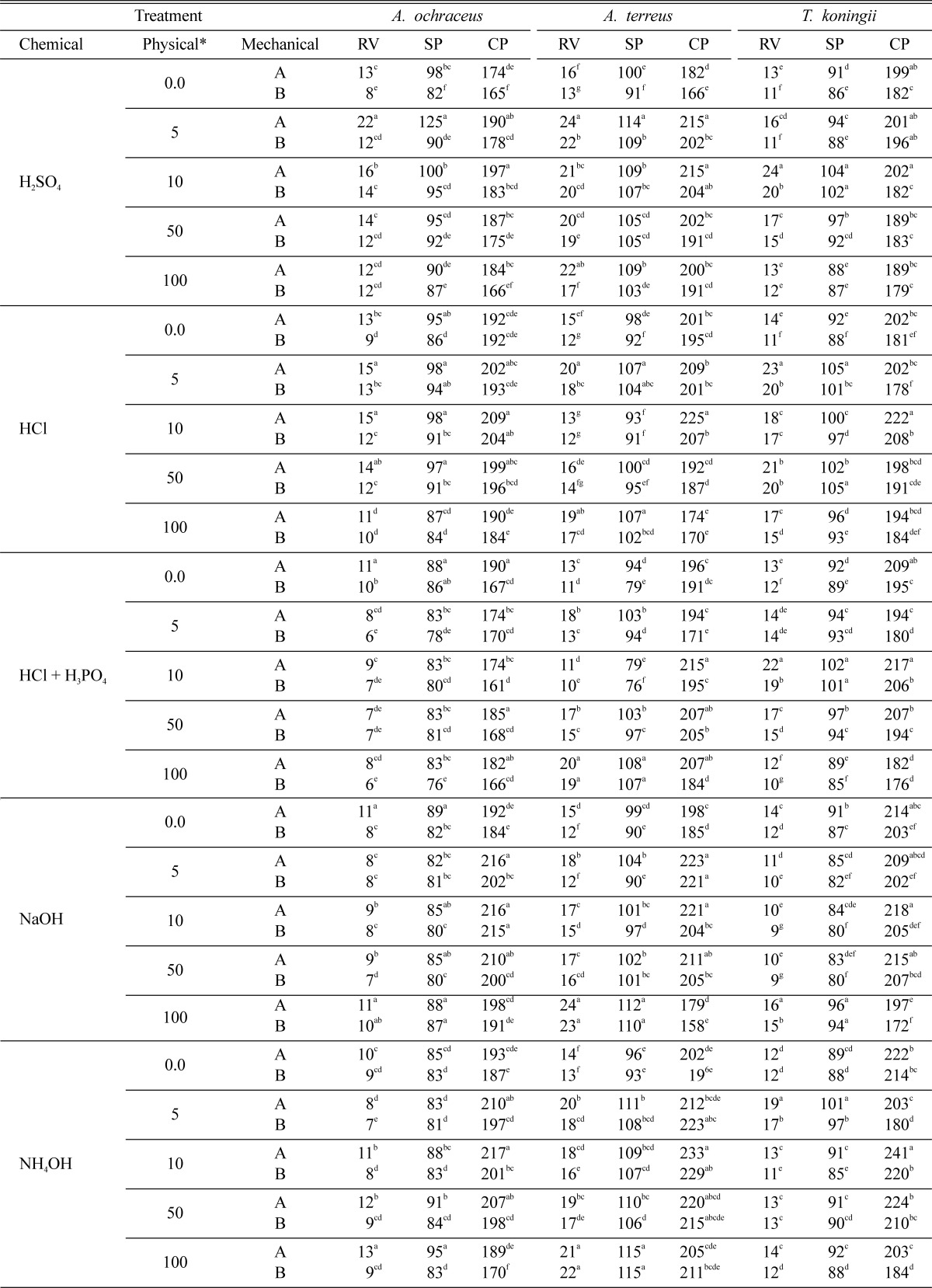
Means in the same column for the same chemical treatment having the same superscript letter are not significantly different at P < 0.05.
A = chopped rice stalks 5~6 cm lengths. B = ground rice straw as in Fig. 1.
*Physical treatment = exposure to doses of gamma radiation/kGy.
Discussion
Cellulose of rice straw which constitutes 39.7% does not occur in pure form and always associated with lignin 13.4%, hemicellulose 25.8% and silica 13.0% (Helal, 2005b). Thus naturally occurring cellulose is not readily susceptible to microbial attack. Pretreatments by either mechanical or chemical and/or physical method could change the structure of lignocellulosic materials or even simply affecting the lignin-hemicellulose cellulose (LHC) complex and thus facilitate the growth of fungi with high cellulolytic activity. Aspergillus ochraceus, A. terreus and Trichoderma koningii were chosen through screening of several rice straw degrading fungi. These fungi produced fermentable products containing 24.3, 25.0 and 23.2% crude protein, respectively (Helal, 2005b). In the present study, reducing sugar, soluble protein and crude protein content of chopped fermentable straw by the three fungi were generally higher than those in the ground straw under static conditions. The increase in crude protein content of straw reached 7.8, 9.3 or 3.6% by these fungi, respectively. This may be attributed to the good aeration between the stalks (5~6 cm lengths) of chopped straw which absorbed basal medium by capillary state during fermentation, than that between the fine particles (0.18~2.8 mm in Fig. 1) of ground straw saturated with the basal medium. The problem which can be resolved in the following studies by turning ground straw. During pretreatment the cellulose materials of various origins by different ways, the average particle size and the polymerization degree of the substrates practically did not affect the enzymatic hydrolysis catalyzed by cellulase complex of T. reesei and A. niger (Kolyosov and Sinitsyn, 1981; Rivers and Emert, 1988). Also, the particle size reduction and pressure cooking of the substrate had a little effect on cellulose digestibility by cellulolytic microorganisms (Han and Callihan, 1974). Milling had a little effect on the extent of cellulose, hemicellulose and lignin utilization by co-culture of Sporotrichum pulverulentum and Chaetomium cellulolyticum (Prendergast et al., 1983). Fungi varied in their ability to decompose milled straw, and milling increased the amount of straw decomposed but did not alter the relative success of the isolates (Harper and Lynch, 1985). Harper and Lynch (1985) added that, the effect of milling (0.5~2.0 mm) may break open parts in the cells of the straw in which the fungi are unable to enter and increase decay following milling. They suggested that parts of the straw may be non-lignified yet and protected by lignified areas which surrounded them. Pretreatment before enzymatic hydrolysis increases the surface area of the cellulose by reducing particle size. Coverse et al. (1990) explained that opening up pores by hydrolysis of the xylan and melting the lignin at high temperature cause agglomeration and lignin acts as an inhibitor during hydrolysis since cellulases adsorbed on lignin cannot hydrolyze cellulose. During hydrolysis, these pores expand and collapse, causing the exposed surface area of cellulose to vary in a complex manner, thus affecting cellulase's adsorption and the rate of hydrolysis.
The chemically pretreated straw acted as better substrates during fermentation than the physically pretreated straw (Tripathi and Yadav, 1989). The degree of cellulose degradation by a chemical microbial process was dependent upon the nature of cellulose and the kind of pretreatment of the substrate (Han et al., 1971). In this study soaking rice straw in NH4OH for a day at room temperature gave better results than other chemicals pretreatments combined with high temperature above 100℃ (Table 1). The crude protein content of fermented chopped straw pretreated with NH4OH raised 7.3, 4.8 or 9.2% using A. ochraceus, A. terreus or T. koningii, respectively. The same trend was found by the works of Jackson (1978) and Weichert (1991) that treatment of straw with ammonia (gas or solution) had a great appeal because ammonia did not leave residual alkali as NaOH and it increased the nitrogen content of the material by 0.8 to 1.0% units. The drastic hydrolysis conditions of complex substrates which contain cellulose produced not only simpler carbohydrates but also possible growth inhibitors derived from the degradation of lignin or other polyphenols. Chemical delignification has the advantage that it is a rapid process, but expensive and poses a potential pollution problem. Therefore, microbial delignification is gaining attention as a possible alternative to the chemical methods currently in use (Kirk et al., 1978, 1979).
Results in this investigation also showed that gamma irradiation pretreatment of rice straw generally increased reducing sugar in the culture filtrate of the fermented ground straw by any of these fungi from 7, 10 or 12 to 40, 42 or 50 mg/g dry straw as the doses increased up to 500 kGy. Whereas, the crude protein content of fermented straw decreased at low doses 5, 10 and 50 kGy but increased gradually at 100, 200 or 500 kGy. On the other hand, there were significant increases in reducing sugar, soluble protein and the crude protein contents of the fermented preirradiated-wet straw than the fermented preirradiated-dry straw (Table 2). Ionizing radiation as gamma lowers the degree of the polymerization of cellulose and lignin, and partially disrupts the lignocellulosic complex (Millet et al., 1978; Malek et al., 1998; Malek, 2001).
The combined effect of gamma irradiation with chemical pretreatment of rice straw (Table 3) clearly showed that NH4OH or HCl combined with 10 kGy gave better results in the crude protein content of fermented straw by the three tested fungi. This combined effect of NH4OH-10 kGy increases the crude protein content to 13.0, 18.3 or 12.6% in the chopped fermented straw by A.ochraceus, A. terreus or T. koningii, respectively, as compared with NH4OH pretreatment alone (21.2, 24.6 or 26.2%) as compared with 10 kGy pretreatment alone, and 10.7, 11.5 or 16.4% as compared with 500 kGy pretreatment alone, under the same conditions, respectively. Conversion of rice straw to chemical stock feed is usually based on acid or enzymatic hydrolysis of cellulose to glucose but ionizing radiation 100 MRAD could be an effective alternative (Brenner, et al., 1977). They added that other methods of hydrolysis combined with irradiation (10 MRAD) promises well economically. The combined effect of gamma radiation doses 100, 150 or 200 kGy and 2, 4 or 6 g NaOH/25 ml water/100 g digestible matter (Al-Masri, 1999) and also, 5% urea-200 kGy (Al-Masri and Guenther, 1999) resulted in a larger increase in the digestible energy and a better effect by reducing the concentration of cell-wall constituents: neutral-detergent fiber, acid-detergent fiber and acid-detergent lignin of wheat straw, cotton seed shell, peanut shell, soybean shell, extracted olive cake and extracted unpeeled sunflower seeds, than in the individual treatments. Also, the combinational effect of 20% urea-200 kGy gamma on Thai rice straw and corn stalk gave a higher percentage decrease in neutral detergent fiber, acid detergent fiber, acid detergent lignin and cellulose, hemicellulose and lignin and cutin in comparison with urea pretreatment alone (Banchorndhevakul, 2002).
In conclusion, the crude protein content of rice straw was improved with fermentation by any of the three fungi specially when the straw was pretreated with chemical-physical combination (3% NH4OH-10 kGy). This pretreatment will be considered during the conductive studies for increasing the feasibility of rice straw to change into a good nutritive fodder.
References
- 1.Allen MB. Exp. in soil bacteriology. 1st ed. Burgess Publ. Co; 1953. [Google Scholar]
- 2.Al-Masri MR. In vitro digestible energy of some agricultural residues, as influenced by gamma irradiation and sodium hydroxide. Appl Radiat Isot. 1999;50:295–301. [Google Scholar]
- 3.Al-Masri MR, Guenther KD. Changes in digestibility and cell-wall constituents of some agricultural by-products due to gamma irradiation and urea treatments. Radiat Phys Chem. 1999;55:323–329. [Google Scholar]
- 4.Araujo A, D'Souza J. Enzymatic saccharification of pretreated rice straw and biomass production. Biotechnol Bioeng. 1986;28:1503–1509. doi: 10.1002/bit.260281008. [DOI] [PubMed] [Google Scholar]
- 5.Babitskaya VG. Mycelial fungi as producers of protein biomass of lignocellulosic substrates. Mikologiya I Fitopathologia. 1986;20:377–385. [Google Scholar]
- 6.Banchorndhevakul S. Effect of urea and urea-gamma treatments on cellulose degradation of Thai rice straw and corn stalk. Radiat Phys Chem. 2002;64:417–422. [Google Scholar]
- 7.Bastawde KB. Cellulolytic enzymes of a thermotolerant Aspergillus terreus strain and their action on cellulosic substrates. World J Microbiol Biotechnol. 1992;8:353–368. doi: 10.1007/BF01200683. [DOI] [PubMed] [Google Scholar]
- 8.Brenner W, Rugg B, Rogers C. Radiation processing. New York, USA: New York Univ.; 1977. Radiation treatment of solid wastes; pp. 389–401. (See FSTA (1979) 11 5G 346) [Google Scholar]
- 9.Chahal DS. Bioconversion of lignocelluloses into food and feed rich in protein. In: Subba RAO NS, editor. Advances in agricultural microbiology. New Delhi: Oxford and IBH Pub. Co; 1982. pp. 551–584. [Google Scholar]
- 10.Chahal DS, Khan SM, Maher MJ. Production of mycelial biomass of oyster mushrooms on rice straw; Proceedings of the 13th International Congress on the Science and Cultivation of Edible Fungi; Dublin, Irish Republic. 1991. pp. 709–716. [Google Scholar]
- 11.Chaplin MF, Kennedy JF. Carbohydrate analysis. Oxford and Washington: IRL Press; 1987. [Google Scholar]
- 12.Coronel LM, Mesina OG, Joson LM, Sobrejuanite EE. Cellulase and xylanase production of Aspergillus fumigatus, a thermophilic fungus. Philippine J Sci. 1991;120:283–303. [Google Scholar]
- 13.Coverse AO, Ooshima H, Burns DS, Greenboum E, Wyman CE. Kinetics of enzymatic hydrolysis of lignocellulosic materials based on surface area of cellulose accessible to enzyme and enzyme adsorption on lignin and cellulose. Appl Biochem Biotechnol. 1990;24-25:67–73. [Google Scholar]
- 14.Duncan DB. Multiple range and multiple (F) test. Biometrics. 1955;11:1–42. [Google Scholar]
- 15.Dytham C. Choosing and using statistics: A biologist's guide. London, UK: Blackwell Science Ltd.; 1999. p. 147. [Google Scholar]
- 16.Han YW, Anderson AW. Semi-solid fermentation of rye grass straw. Appl Microbiol. 1975;27:159–165. [Google Scholar]
- 17.Han YW, Callihan CD. Cellulose fermentation: Effect substrate pretreatment on microbial growth. Appl Microbiol. 1974;27:159–165. doi: 10.1128/am.27.1.159-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han YW, Dunlap CE, Callihan CD. Single cell protein from cellulosic wastes. Food Technol. 1971;25:130–133. [Google Scholar]
- 19.Harper SHT, Lynch JM. Colonization and decomposition of straw by fungi. Trans Br Mycol Soc. 1985;85:655–661. [Google Scholar]
- 20.Helal GA. Bioconversion of straw into improved fodder: Fungal flora decomposing rice straw. Mycobiology. 2005a;33:150–157. doi: 10.4489/MYCO.2005.33.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helal GA. Bioconversion of straw into improved fodder: Mycoprotein bioconversion and cellulolytic activity of rice straw decomposing fungi. Mycobiology. 2005b;33:90–96. doi: 10.4489/MYCO.2005.33.2.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoda GEM, Alian AM, Nagwa MES, Fadel MA. Enzymatic hydrolysis of some cellulosic wastes for fodder yeast production 1. Transformation of wastes to fermentable sugars. Annals Agric Sci Cairo. 1990;35:143–155. [Google Scholar]
- 23.Jackson MG. Treating straw for animal feeding, animal production and health. Paper No. 10. Rome: FAO; 1978. [Google Scholar]
- 24.Kirk TK, Higuchi T, Chang HM, editors. Lignin biodegradation: microbiology, chemistry, and potential applications. Boca Raton, Florida: Vols. 1 and 2 CRC Press; 1979. [Google Scholar]
- 25.Kirk TK, Yang HH, Keyser P. The chemistry and physiology of the fungal degradation of lignin. Developm Industr Microbiol. 1978;19:51–61. [Google Scholar]
- 26.Klyosov AA, Sinitsyn AP. Enzymatic hydrolysis of cellulose. IV. Effect of major physico-chemical and structural features of the substrate. Bioorg Khim. 1981;7:1801–1812. [Google Scholar]
- 27.Lowery OH, Resebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Malek MA. Ligno-cellulose degradation in solid-state fermentation of irradiated rice straw by Pleurotus sajor-caju fungi. Bangladesh Veterinarian. 2001;18:148–152. [Google Scholar]
- 29.Malek MA, Chowdhury NA, Matsuhashi S, Hashimoto S, Kume T. Radiation and fermentation treatment of cellulosic wastes. Mycoscience. 1994;35:95–98. [Google Scholar]
- 30.Malek MA, Matsuhashi S, Kume T. Chemical composition and digestibility of rice straw fermented by selected fungi. Int J Mushroom Sci. 1998;2:27–32. [Google Scholar]
- 31.Millet MA, Baker AJ, Sattar LD. Physical and chemical pretreatments for enhancing cellulose saccharification. Biotechnol Bioeng Symposim. 1976;6:125–153. [PubMed] [Google Scholar]
- 32.Malek MA, Baker AJ, Sattar LD. The use of organic residues in rural communities. Biotechnol Bioeng. 1978;20:107–113. [Google Scholar]
- 33.Patel MM, Bhatt RM. Optimization of the alkaline peroxide pretreatment for the delignification of rice straw and its applications. J Chem Technol Biotechnol. 1992;53:253–263. [Google Scholar]
- 34.Peiris PS, Silva I. Hydrolysis of rice straw to fermentable sugars by Trichoderma enzymes. MIRCEN J. 1987;3:57–65. [Google Scholar]
- 35.Prendergast P, Booth A, Colleran E. Protein enrichment of pretreated lignocellulosic materials by fungal fermentation. In: Ferranti MP, Fiechter A, editors. Production and feeding of single cell protein. Barking, ESSEX (UK): Appl. Sci.; 1983. pp. 96–100. [Google Scholar]
- 36.Rivers DB, Emert CH. Factors affecting the enzymatic hydrolysis of bagasse and rice straw. Biol wastes. 1988;26:90–95. [Google Scholar]
- 37.Rolz C, Humphrey A. Microbial biomass from renewables: Review of alternatives. Adv Biochem Eng. 1982;198:1–53. [Google Scholar]
- 38.Singh K, Sondhi HS, Neelakantan S. Bioconversion of wheat straw with cellulolytic moulds in submerged culture fermentation. Indian J Ani Nutrit. 1989;6:140–144. [Google Scholar]
- 39.Snedecor GW, Cochran WG. Statistical methods. 6th ed. London, UK: Blackwell Science Ltd.; 1982. p. 147. [Google Scholar]
- 40.Thanikachalam A, Rangarajan M. Bioconversion of rice straw into protein rich feed. Madras Agric J. 1992;79:138–141. [Google Scholar]
- 41.Tripathi JP, Yadav JS. Selection of pre-treatment for an alkalophilic Coprinus fermentation of wheat straw in a two - stage process. Int J Ani Sci. 1989;4:128–133. [Google Scholar]
- 42.Weichert D. Nitrogen as a guide element in bioconversion of lignocellulosics. Mikrobiologicheskii Zhurnal. 1991;53:103–111. [Google Scholar]
- 43.Xin LZ, Kumakura M. Effect of radiation pretreatment of enzymatic hydrolysis of rice straw with low concentrations of alkali solution. Bioresour Technol. 1993;43:13–17. [Google Scholar]
- 44.Youssef BM, Aziz NH. Influence of gamma-irradiation on the bioconversion of rice straw by Trichoderma viride into single cell protein. Cytobios. 1999;97:171–183. [Google Scholar]