Abstract
Forty-one fungal species belonging to 15 fungal genera isolated from Egyptian soil and sugar cane waste samples were tested for their capacity of producing acidity and gluconic acid. For the tests, the fungi were grown on glucose substrate and culture filtrates were examined using paper chromatography analysis. Most of the tested fungi have a relative wide potentiality for total acid production in their filtrates. Nearly 51% of them showed their ability of producing gluconic acid. Aspergillus niger was distinguishable from other species by its capacity to produce substantial amounts of gluconic acid when it was cultivated on a selective medium. The optimized cultural conditions for gluconic acid yields were using submerged culture at 30℃ at initial pH 6.0 for 7 days of incubation. Among the various concentrations of substrate used, glucose (14%, w/v) was found to be the most suitable carbon source for maximal gluconic acid during fermentation. Maximum values of fungal biomass (10.02 g/l) and gluconic acid (58.46 g/l) were obtained when the fungus was grown with 1% peptone as sole nitrogen source. Influence of the concentration of some inorganic salts as well as the rate of aeration on the gluconic acid and biomass production is also described.
Keywords: Gluconic acid production, Aspergillus niger, acidity
D-gluconic acid is an important organic acid resulting from the oxidation of D-glucose. The unique property of this acid such as low toxicity, low corrosively and complexing capability with metal ions has enabled its wide application in the food, pharmaceutical, textile, leather and other industries (Singh et al., 1999). Thus, the overall demand of this organic acid has been increased for almost 20 years and, recently it production is amounting to more than 60,000 tons per year and still growing (Singh et al., 1999; El-Enshasy, 2003). Commercially, gluconic acid is produced by three different methods, chemical oxidation of glucose with a hypochlorite solution (Kundu and Das, 1984), electrolytic oxidation of glucose solution containing a known value of bromide (Amberkar et al., 1965), and fermentation process where specific microorganisms are grown in medium containing glucose and other ingredients (Hill and Robinson, 1988; Shah and Kothari, 1983; Lee et al., 1998). The microbial fermentation process offer an attractive techniques for the gluconic acid production to alleviate the problems related to chemical production such as the inevitable side reactions and also to further economize the bioprocess (Velizarov and Beshkov, 1994; Singh et al., 1999).
A wide group of microorganisms, particularly filamentous fungi have the ability for gluconic acid production (Cochrane, 1958; Lockwood, 1975). The production of gluconic acid is mainly done in batch cultivation using several species belonging to the following fungal genera, Aspergillus, Penicillium, Fusarium, Mucor, and Gliocladium (Lockwood, 1975; Rosenberg et al., 1992; Petruccioli et al., 1994; Singh et al., 2001). Among the different fungal genera, it has been reported that the accumulation of large amounts of the gluconic acid and its salts are restricted to certain species of Aspergillus, especially A. niger which considered as the most industrially important gluconic acid producer in fermentation industry (Roukas, 2000; Sankpal et al., 2001; Sankpal and Kullkarni, 2002; El-Enshasy, 2003). Because of its industrial importance, many investigators have been worked for optimization as well as overproduction of gluconic acid by improving fungal producers (Vassilve et al., 1993; Sankpal et al., 1999; Singh et al., 2001; Sankpal and Kulkarni, 2002; El-Enshasy, 2003). Also, researchers employed conventional screening protocols to identify and develop potential indigenous fungal strains for commercial exploitation of the process (Singh et al., 1999), despite the abundant availability of commercial gluconic acid.
The main object of the present work is to isolate some local fungal strains from Egyptian soil and waste materials of sugar cane that are capable of producing large amounts of gluconic acid.
Materials and Methods
Isolation and identification of organisms
Different fungal isolates were obtained from cultivated Egyptian soil samples and waste materials of sugar cane processing from Hawamedia Distilleries Factories. The dilution plate method described by Johnson et al. (1959) and Czapek Doxs Agar medium (Oxoid, 1982) supplemented with rose bengal (1/15000, w/v) as bacteriostatic agents (Smith and Dawson, 1994) was used for isolation of fungi. For the isolation, plates were incubated at 28 ± 2℃ for 7 days, and developing fungi were purified and identified by macro-and microscopic characteristics using the following references; Gilman (1957), Barron (1968), Raper and Fennell (1977), Carmichael et al. (1980), Domsch et al. (1980) and Nelson et al. (1983). Isolated fungi were maintained on potato dextrose agar (PDA) slants and incubated at 30℃ for 7 days. The slants were stored at 4℃ and subcultured every month. The spore suspension was prepared by suspending the spores on the slant in 10 ml of sterilized distilled water.
Fermentation technique
Gluconic acid fermentation was carried out by submerged fermentation in 250 ml cotton wool plugged Erlenmeyer flasks with 50 ml of fermentation media: Medium I used for total acidity as well as gluconic acid production, the modified Czapek Dox Broth was of the following composition (g/l): glucose 100.0, NaNO3 3.0, yeast extract 3.0, KH2PO4 1.0, MgSO4·7H2O 0.5, KCl 0.5, and FeSO4·7H2O 0.01 having pH 6.0. Medium II, Czapek Dox Broth consisted of (g/l) sucrose 30.0, NaNO3 3.0, KH2PO4 1.0, MgSO4·7H2O 0.5, KCl 0.5 and FeSO4·7H2O 0.01 having pH 6.0. Medium III, Molliard medium (Molliard, 1992) was composed of (g/l) glucose 150.0, (NH4)2HPO4 0.388, KH2PO4 0.1 and MgSO4·7H2O 0.15 having pH 6.0. Medium IV (El-Naghy and Megalla, 1975) had the following composition: (g/l): glucose 150.0, Peptone 10.0, KH2PO4 0.5 and MgSO4·7H2O, 0.5 having pH 6.0. Medium V (El-Ktatney, 1978) contained (g/l): glucose 50.0, Peptone 2.0, KH2PO4 2.0, and MgSO4·7H2O having pH 5.5. The pH of each medium was adjusted with 1M NaOH. The sterilized media were inoculated with 2 ml of well-dispersed spore suspension of each tested fungal species (6 × 106 spores/ml). The flasks were incubated at 28℃ for up to 8 days using a rotary shaker (New Brunswick Scientific Edison N-J. USA). All the experiments were carried out in duplicate. The nitrogen sources added were yeast extract, peptone, NaNO3, NH4NO3, (NH4)2HPO4, urea, NH4Cl and KNO3.
Dry biomass estimation
The content of each flask was filtered and the mycelial residues were washed with distilled water. These mycelial residues were dried in an oven for 24 h at 90℃ till their weight are to be constant and the dry biomass was calculated in g/l of fermentation medium (Singh et al., 1999). The culture broth was pooled and the volume measured. The aliquots were also used for biochemical analysis.
Biochemical analysis
Assay of total acidity (T.A.)
The total acidity of the culture filtrates was determined by titration against standard alkaline solution (Peppler, 1967; El-Ktatney, 1978), using phenolphthalein as an indicator.
Detection of gluconic acid
The gluconic acid produced was determined qualitatively and quantitatively by chromatographic analysis (Koepsell et al., 1952; Singh et al., 1999).
Estimation of glucose
DNSA (Dinitro salicylic acid) method was used for glucose estimation (Tasun et al., 1970). The transmittance was measured at 700 nm using a spectrocolormeter (Carl-Zeiss JENA type). The amount of residual reducing sugar was determined by inference from the standard curve of different known concentrations of glucose.
Statistical analysis
The data were statistically analyzed as a complete randomized block design. The means was compared by analysis of variance (ANOVA) (Hicks, 1983).
Results and Discussion
Screening of the different fungal isolates for total acid and gluconic acid production
The amount of gluconic acid produced by isolated fungal cultures ranged from 1.00 to 58.46 g/l. The dry weight of mycelia was ranging from 2.5 to 10.02 g/l (Tables 1 and 2). A. niger showed the highest (58.46 g/l) production of gluconic acid. Most of the isolates used have been recorded as acid-producers by the aid of compendium of soil fungi (Domsch et al., 1980). In accordance with our screening, several species of Aspergillus and Penicillium in addition to some Mucorales were described by other investigators as acid producers as well as gluconic acid producers in their filtrates (El-Ktatney, 1978; Rosenberg et al., 1992; Singh et al., 1999, 2001). The screening experiments were extend to determine quantitatively the yields of gluconic acid in the twenty three selected fungi which previously showed positive results after paper chromatography analysis of the culture filtrates of the fungi grown on the modified Czapek Dox Broth.
Table 1.
The potentiality of the tested fungal species to produce total acid (ml of N NaOH/l) and gluconic acid (as shown by the paper chromatography technique) on submerged culture at 28℃ for 7 days

*(+) indicates gluconic acid production, (-) indicates no gluconic acid production.
**(ND) not detected.
Table 2.
Gluconic acid (g/l) produced by the screened fungal species on submerged culture after 7 days at 28℃

As determined by titration technique.
Other investigators developed a similar system to screen diverse fungi for their metabolic activities and in accordance with our results they considered A. niger as a very strong producer of gluconic acid in addition to other known Aspergillus spp. and Penicillium spp. (Temsah and Olama, 1999). The gluconic acid production by the proposed fungus was also reported in other studies (El-Kataney, 1978; Vassilve et al., 1993; Petruccioli et al., 1994; Singh et al., 1999). Our results revealed that a wide range of variations form low gluconic acid production as well as total acidity in its culture filtrate under the experimental conditions. These preliminary findings justified the selection of gluconic acid producing fungus for further investigation in order to enhance its productivity of gluconic acid.
Media for gluconic acid production
To choose the best substrate for acid production, the growth of the selected A. niger and its ability of producing gluconic acid was investigated by growing the fungus on some common fermentation media. As shown in Table 3, the formation of gluconic acid was found to be dependent on the chemical composition of culture media used. Both the addition and concentration of glucose and organic nitrogen in media had an effect on good gluconic acid production. A. niger gave the optimum yields of gluconic acid and mycelial growth on modified Czapek Dox medium containing sucrose as carbon source. Several workers reported the effects of glucose and organic nitrogen on the formation of gluconic acid by fungi. (El-Naghy and Megalla, 1975; El-Ktatney, 1978; Singh et al., 1999; El-Enshasy, 2003). Thus we selected medium (I) for further study in order to elucidate the optimal conditions for gluconic acid production.
Table 3.
The growth of Aspergillus niger and its production of gluconic acid in different fermentation media at 30℃ for 7 days

*Composition is described in the materials and methods.
Several conditions for gluconic acid production
Incubation period
The growth of A. niger and its production of gluconic acid were determined during the incubation period of 12 days. The results in Table 4 showed that the active growth of the fungal mycelia began after the elapse of 2 days of fermentation period and was accompanied by the increasing levels of gluconic acid production. As the growth reached in linear phase the acid yield increased exponentially and the optimum growth attained after 6 days. The maximum gluconic acid yielded up to 58.46 g/l after 7 days of fermentation.
Table 4.
Influence of fermentation period on gluconic acid production and growth of A. niger in submerged culture at 30℃ for 12 days and at pH 6.0

*Contains: 10% glucose = 150 g/l (w/v).
The kinetics of growth and gluconic acid production have been studied by several investigators (El-Naghy and Megalla, 1975; Madhavi et al., 1999; Znad et al., 2004) and their observation unequivocally supports our results. However, some workers found that an incubation period of 6 days was optimal for gluconic acid production by A. niger strains (Rao et al., 1993; Singh et al., 1999).
The pH value
The pH value is one of the most critical factors affecting on the fungal growth as well as the formation of organic acids. The profiles gluconic acid and dry mycelial mass with respect to initial pH of the fermentation medium is shown in Table 5. Over 50% yield of gluconic acid was observed in a pH range from 5 to 7. The best yield was 58.46 g/l at pH 6.0. However, the acid yield above and below this pH was poor. The suitability of pH ranges 5~6 for both the growth and gluconic acid production was also reported from other fungi (El-Naghy and Megalla, 1975; Singh et al., 1999).
Table 5.
Effect of initial pH on the gluconic acid production and growth of A. niger in submerged culture at 30℃ for 7 days
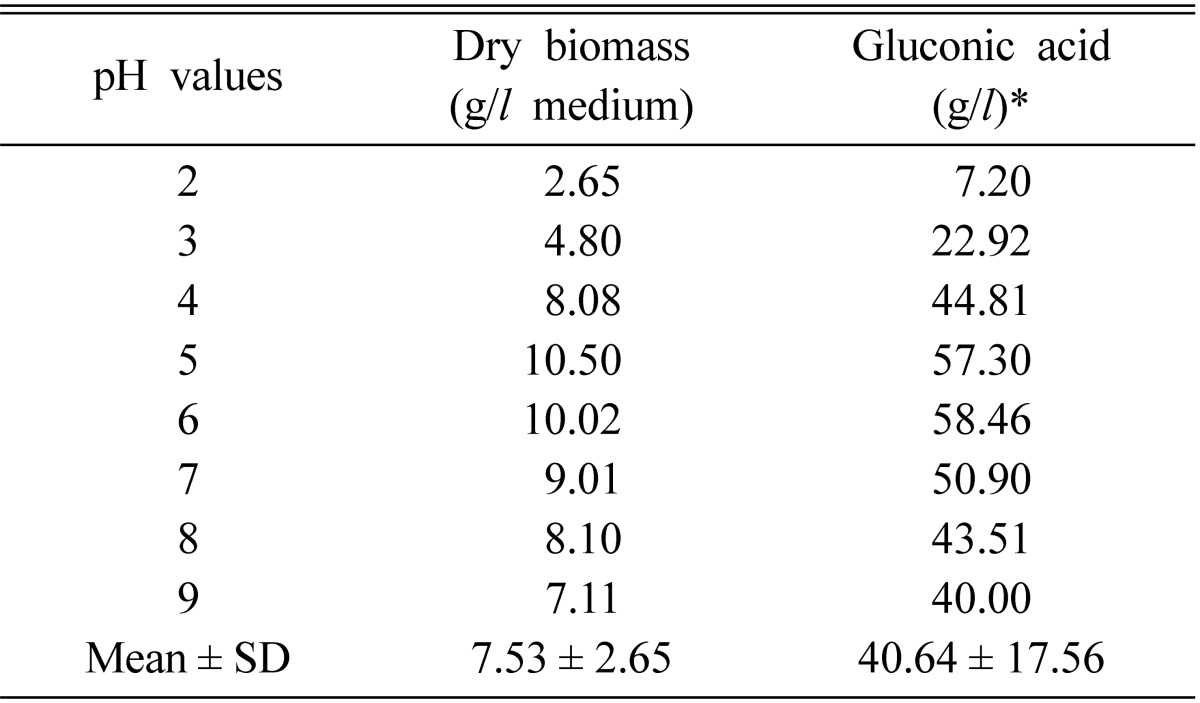
*Contains: 10% glucose = 150 g/l (w/v).
Incubation temperature
Table 6 shows the effect of incubation temperature on gluconic acid production (20~50℃). The optimum temperature for efficient fermentation for gluconic acid (58.46 g/l) and biomass yield (10.02 g/l medium) was 30℃. This temperature was also reported as optimal for maximal gluconic acid production by other authors (Moresi et al., 1991; Subba-Rao et al., 1994). At 45℃, the effect of temperature on the production of gluconic acid was negligible.
Table 6.
Effect of temperature on the gluconic acid production and growth of A. niger in submerged culture at pH 6.0 for 7 days
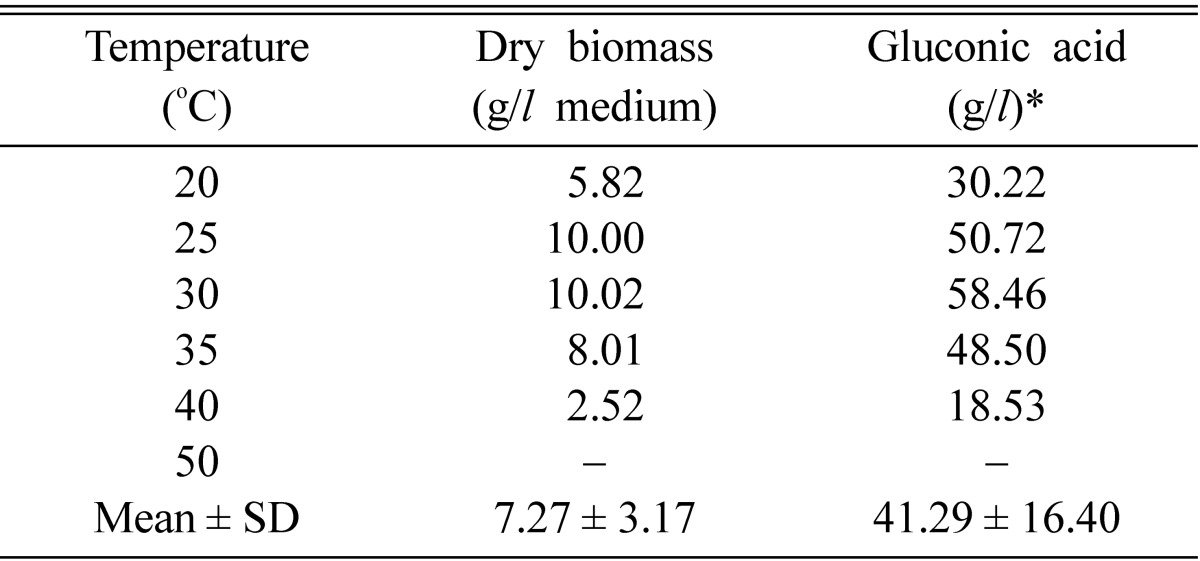
*Contains: 10% glucose = 150 g/l (w/v).
-: not detected.
Aeration
Fig. 1 shows the effect of increased aeration on the bioconversion of glucose to gluconic acid by A. niger in submerged culture. It is apparent from the data that increased gluconic acid production is affected by increasing the aeration and the size of fermentation containers. The optimal gluconic acid yield (62 g/l) was obtained in fermentation flask having 2L-capacity of containing medium.
Fig. 1.
Effect of aeration on the gluconic acid production and biomass of A. niger in submerged culture at 30℃ for 7 days.
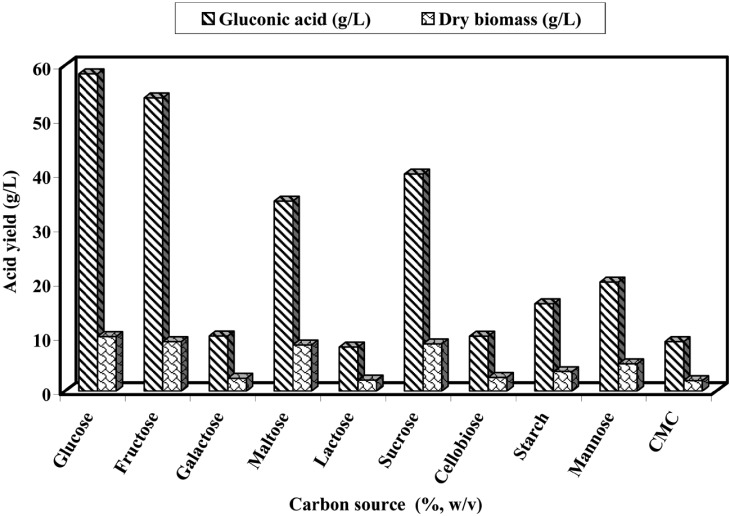
Carbon sources
The results of fermentation using different carbon compounds are summarized in Fig. 2. The data indicated that glucose and fructose were found to be the best carbon followed by sucrose and maltose for gluconic acid production as well as for the growth of the experimental fungus. On the other hand, CMC (carboxy methyl cellulose), lactose, galactose and cellobiose seemed unsuitable for gluconic acid production and mycelial biomass yield. A similar situation has been described for gluconic acid production by other investigators (Rosenberg et al., 1992; Madhavi et al., 1999; Singh et al., 1999). Their observation indicated that D-glucose was the best carbon source not only for gluconic acid production but also for biomass production by different fungal strains.
Fig. 2.
Effect of different carbon sources (10% w/v), CMC and starch (5% w/v) on the gluconic acid production and biomass of A. niger at pH 6.0 and 30℃ for 7 days in submerged culture.
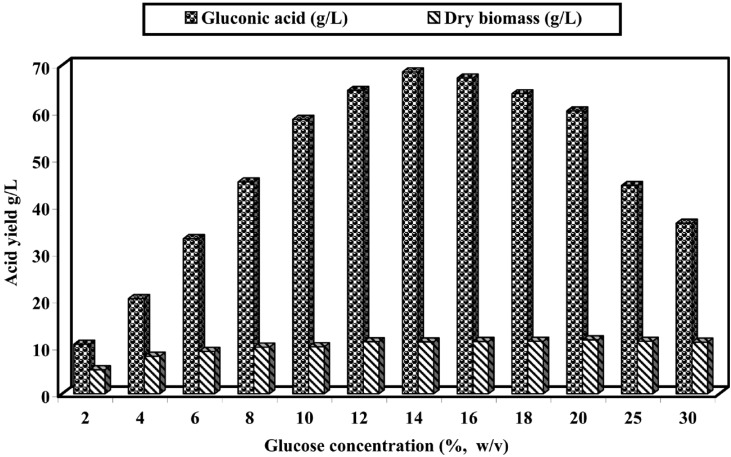
Glucose concentration
The concentration of carbon sources plays an important role on the conversion of glucose into gluconic acid. Thus, different glucose concentrations (2~30%, w/v) were used to investigate the effect of carbon level on this fermentation reaction (Fig. 3). It was observed that the better conversion in relatively dilute medium containing 10~20% glucose. Beyond this concentration, gluconic acid production decreased while the biomass yield remained relatively constant. These results coincided with those previously reported for several fungal species and indicated that the gluconic acid production was dependent on substrate concentration (El-Naghy and Megalla, 1975; Moresi et al., 1992; Singh et al., 1999; Sankpal and Kulkarni, 2002). Thus, the enhancement in the gluconic acid production can be attributed to the induction of glucose oxidase at increased glucose level in fermented medium (Ptruccioli and Federica, 1993; El-Enshasy, 2003).
Fig. 3.
Effect of different glucose concentrations (%w/v) on gluconic acid production and biomass of A. niger in submerged culture at pH 6.0 and 30℃ for 7 days.
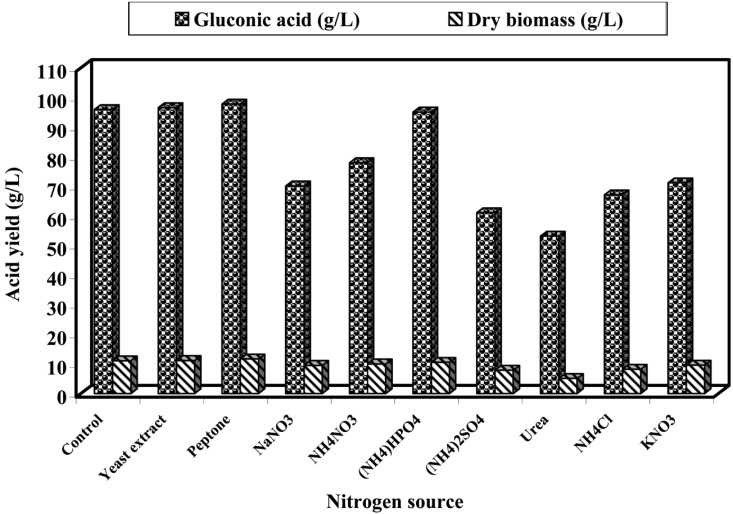
Nitrogen sources
On an equivalent nitrogen basis, the mixture of NaNO3 and yeast extract was replaced by NaNO3, peptone, yeast extract, NH4NO3, (NH4)2HPO4, (NH4)2SO4, KNO3, NH4C, and urea (Fig. 4). The results revealed that peptone was the best source for gluconic acid and biomass production (97.8 and 11.7 g/l, respectively) followed by yeast extract, control (mixture of NaNO3 and yeast extract), (NH4)2HPO4, and NH4NO3. Urea was the least favorable nitrogen source. Other investigators have confirmed the selective induction of nitrogenous compounds on gluconic acid production and growth of several fungi (El-Neghy and Megalla, 1975; Singh et al., 1999).
Fig. 4.
Effect of different nitrogenous compounds on gluconic acid production and biomass of A. niger in submerged culture at pH 6.0 and 30℃ for 7 days.
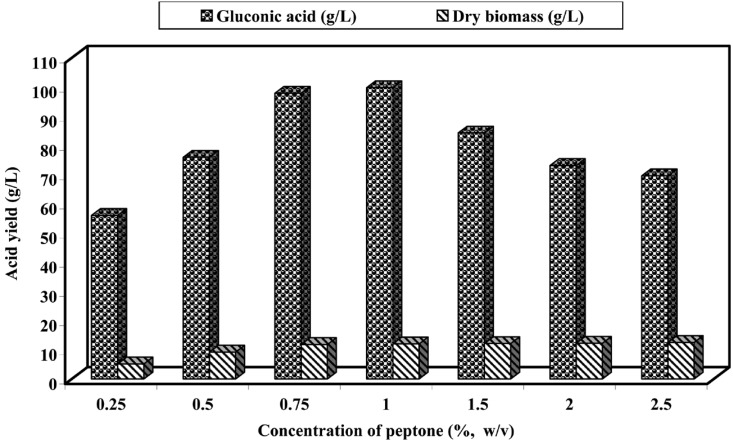
The results of the quantitative effect of peptone on both parameters are presented in Fig. 5. It could be seen that maximum gluconic acid and biomass yields (i.e. 99.80 and 12.01 g/l, respectively) was obtained at 1.0% peptone concentration in the basal medium containing 14.0% (w/v) glucose. Generally, gluconic acid production decreased above or below this peptone level. However, mycelial biomass depended on peptone level and increased with the increase of peptone concentration in bioprocess. El-Naghy and Magalla (1975) and El-Ktatney (1978) have been found that 1.0% peptone gave the optimum gluconic acid production by P. puberulum and A. carneus.
Fig. 5.
Effect of different concentrations of peptone on gluconic acid production and biomass of A. niger in submerged culture (14% glucose) at pH 4.0 and 30℃ for 7 days.
Inorganic salts
In order to enhance the rate of conversion of glucose to gluconic acid, the influence of several inorganic salts constituting the fermented medium were investigated (Fig. 6). Addition of MgSO4·7H2O up to concentration of 0.025% showed stimulatory effect on gluconic acid production i.e. 100.24 g/l. High concentrations decreased the formation of gluconic acid, while the highest mycelial dry weight was obtained at 0.05% MgSO4·7H2O i.e. 12.01 g/l. On the other hand, the addition of KH2PO4 up to 0.05% induced gluconic acid yield from 57.5 to 71.78% and then decreased, with high concentrations of this salt (Fig. 7). But, the dry biomass remained constant at high concentration of KH2PO4. These observations coincided with those previously mentioned by other investigators (El-Naghy and Megalla, 1975; Singh et al., 1999) who found that the addition of 0.05% KH2PO4 increased gluconic acid production by A. niger ORS-4. Also, El-Ktatney (1978) reported that the mycelial dry weight of A. carneus increased with more additional concentration of KH2PO4 but gluconic acid decreased at the higher concentrations. The potassium chloride free medium did not have significant influences on the gluconic acid production as well as fungal dry biomass (Fig. 8). The highest gluconic acid production was obtained with 0.025% and decreased thereafter. In general, Singh et al. (1999) reported that the concentrations of inorganic salts greater than 0.1% with an initial pH 6.5 was not beneficial for gluconic acid production by A. niger ORS-4.
Fig. 6.
Effect of different concentrations of MgSO4·7H2O on gluconic acid production and mycelial dry biomass of A. niger in submerged culture (14% glucose and 1% peptone) at pH 6.0 and 30℃ for 7 days.
Fig. 7.
Effect of different concentrations of KH2PO4 on gluconic acid production and mycelial dry biomass of A. niger in submerged culture (14% glucose and 1% peptone) at pH 6.0 and 30℃ for 7 days.
Fig. 8.
Effect of different concentrations of KCl on gluconic acid production and mycelial dry biomass of A. niger in submerged culture (14% glucose and 1% peptone) at pH 6.0 and 30℃ for 7 days.
In summary, the data obtained in this study indicated that a good yield of the desired products depended on the presence of appropriate physical conditions and nutritional requirements during fermentation process. Thus, it can be concluded from this report that experimental strain of A. niger could be employed for producing gluconic acid on a large scale as grown on the optimization bioprocess. Further work is in progress for improving producing strain as well as economizing the process of production.
References
- 1.Ambekar GR, Thadani SB, Doctor VM. Production of calcium gluconate by Penicillum chrysogenum in submerged culture. Appl Microbiol. 1965;13:713–719. doi: 10.1128/am.13.5.713-719.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron GL. The general of Hyphomycetes from soil. Baltimore, USA: The Williams and Wilkins; 1998. [Google Scholar]
- 3.Carmichael JW, Brycekendrick W, Conners IL, Sigler L. Genera of Hyphomycetes. Canada: The Univ. of Alberta Press; 1980. [Google Scholar]
- 4.Cochrane VW. Physiology of fungi. London: John Wiley and Sons, Inc; 1958. [Google Scholar]
- 5.Domsch KH, Gams W, Anderson T. Compendium of soil fungi. Academic Press; 1980. [Google Scholar]
- 6.El-Enshasy HA. Production of gluconic acid by free and immobilized cells of recombinant Aspergillus niger in batch cultures. Egypt J Biotechnol. 2003;13:187–201. [Google Scholar]
- 7.El-Ktatney MS. Studies on the production of organic acid by some locally isolate fungi. Egypt: Bot. Dept. Fac. Sci. Assiut Univ.; 1978. M.Sc. Thesis. [Google Scholar]
- 8.El-Naghy MA, Megalla SE. Gluconic acid production by Penicillium puberulum. Folia Microbiol. 1975;20:504–508. doi: 10.1007/BF02891710. [DOI] [PubMed] [Google Scholar]
- 9.Giluman JC. A manual of soil fungi. Ames. Iowa USA: Iowa State Univ. Pres; 1957. [Google Scholar]
- 10.Hicks CR. Fundamental concepts in the design of experimental CBS. New York: College Publishing; 1983. [Google Scholar]
- 11.Hill GA, Robinson CW. Morphological behavior of S. cerevisiae during continuous fermentation of calcium gluconate. Biotechnol Lett. 1988;11:805–810. [Google Scholar]
- 12.Johnson LE, Curl EA, Bond JH, Fribourgy HA. Methods for studying soil microflora-plant disease relationship. Minn. USA: Burgess Pub. Co.; 1959. [Google Scholar]
- 13.Koepsell HJ, Stodola FH, Sharpe ES. Production of α-ketoglutarate in glucose oxidation by Pseudomonas fluorescens. J Am Chem Soc. 1952;74:5142–5144. [Google Scholar]
- 14.Kundu PN, Das A. Utilization of cheap carbohydrate sources for production of calcium gluconate by Penicillim funiculosum mutant MN-238. Ind J Exp Biol. 1984;22:279–281. [PubMed] [Google Scholar]
- 15.Lockwood LB. Orgainc acid production in the filamentous fungi. In: Smith HE, Berry DE, editors. Industrial Mycology. Vol. 1. London: Edward Arnold (Pub.) Itd; 1975. p. 140. [Google Scholar]
- 16.Lee HW, Pan JG, Lebeault JM. Calcium gluconate form glucose substrate. Appl Microbial Biotechol. 1998;49:9–15. [Google Scholar]
- 17.Madhavi CH, Subramani S, Datta MD. Fermentative production of gluconic acid using cheese whey. J Food sci Technol. 1999;36:361–364. [Google Scholar]
- 18.Molliard M. Sure une nouvelle fermentation acide production parle Sterigmatocyti niger. Comp Rend. 1992;147:981–903. [Google Scholar]
- 19.Moresi M, Parente E, Mazzatura A. Effect of dissolved oxygen concentration on repeated production of gluconic acid by immobilized mycelia of Aspergillus niger. Appl Microbiol Biotechnol. 1991;36:320–323. [Google Scholar]
- 20.Moresi M, Parente E, Mazzatura A. Gluconic acid production from grape must surplus by immobilized mycelia of Aspergillus niger. Ann Microbiol Enzimol. 1992;42:173–184. [Google Scholar]
- 21.Nelson EP, Taussow TA, Marasas WFO. Fusarium species, an illustrated manual for identification. Univ. Park and London: The Pennsylvania state Univ. Press; 1983. [Google Scholar]
- 22.Nour-Eldein OO. Production of gluconic acid by fermentation. Cairo UAR: Fac. Agric. Ain Shams Univ.; 1972. M.SC. Thesis. [Google Scholar]
- 23.Oxide Limited. The Oxoid manual of culture media, ingredients, and other laboratory service. Fifth edition. England: Turner graphic, Ltd; 1982. [Google Scholar]
- 24.Peppler HG. Microbial Technology. New York: Reinhold Pub. Corp.; 1967. [Google Scholar]
- 25.Petruccioli M, Federica F. Glucose oxidase production by Penicillium variabile P16: effect of medium composition. J Appl Bacteriol. 1993;75:369–372. [Google Scholar]
- 26.Petruccioli M, Piccioni P, Fenice M, Federica F. Glucose oxidase, catalase and gluconic acid production by immobilized mycelium of Penicillium variabile. P16. Biotechnol Lett. 1994;16:939–942. [Google Scholar]
- 27.Rao DS, Panda T, Subba RD. Comparative analysis of calcium gluconate techniques for the production of gluconic acid by Aspergillus niger. Bioprocess Eng Berlin. 1993;8:203–207. [Google Scholar]
- 28.Raper KB, Fennell DI. The genus Aspergillus. Baltimore, USA: Williams and Wilkins; 1977. [Google Scholar]
- 29.Rosenberg M, Svitel J, Rosenbergova I, Sturdik E. Gluconic acid production by Aspergillus niger with oxygen supply by hydrogen peroxide. Bioprecess Eng. 1992;7:309–313. [Google Scholar]
- 30.Roukas T. Citric and gluconic acid production from fig by Aspergillus niger using solid-state fermentation. J Induse Microbiol Biotechnol. 2000;25:298–304. doi: 10.1038/sj.jim.7000101. [DOI] [PubMed] [Google Scholar]
- 31.Shah DN, Kothri RM. Glucose oxidase rich Aspergillus niger strain an economical substrate for the preparation of table grade calcium gluconate. Biotechnol Lett. 1993;15:35–40. [Google Scholar]
- 32.Sankpal NV, Joshi AP, Sutar II, Kulkarni BD. Continuous production of gluconic acid by Aspergillus niger immobilized on a cellulosic support: study of low pH fermentative behavior of Aspergillus niger. Processes Biochem. 1999;35:317–325. [Google Scholar]
- 33.Spankpal NV, Cheema JJS, Jambe SS, Julkarni BD. An artificial intelligence tool for bioprocesses monitoring: Application to continuous production of gluconic acid by immobilized Aspergillus niger. Biotechnol Lett. 2001;23:911–916. [Google Scholar]
- 34.Spankpal NV, Kulkarni BD. Optimization of fermentation conditions for gluconic acid production using Aspergillus niger immobilized on cellulose micro-fabrics. Process Biochem. 2002;37:1345–1350. [Google Scholar]
- 35.Singh OV, Pereira BMJ, Singh RP. Isolation and characterization of a potent fungal strain Aspergillus niger ORS-4 for gluconic acid production. J Sci Industrial Res. 1999;58:594–600. [Google Scholar]
- 36.Singh OV, Sharma A, Singh RP. Gluconic acid production by Aspergillus niger mutant ORS-4.410 in submerged and solid state surface fermentation. Indian J Exp Boil. 2001;39:691–696. [PubMed] [Google Scholar]
- 37.Smith NR, Dawson VT. The bacteriostatic action of rose bengal in media used for plate counts of soil fungi. Soil Sci. 1944;58:467–471. [Google Scholar]
- 38.Subba-Rao D, Panda T, Roa DS. Comparative analysis of different whole cell immobilized Aspergillus niger catalysts for gluconic acid fermentation using pretreated can molasses. Bioproc Eng. 1994;11:209–212. [Google Scholar]
- 39.Tasun K, Chose, Ghen Sugar determination by DNS method. Biotechol Bioeng. 1970;12:921. [Google Scholar]
- 40.Temash S, Olama ZA. Optimization of glucose oxides and gluconic acid production by Penicillim purpurescens using Banana waste under solid-state fermentation. J Medical Research Institute. 1999;20:54–62. [Google Scholar]
- 41.Teskova K, Vicheva A. Kinetics of the gluconic acid biosynthesis by strain Aspergillus niger 13, 37. Acta Microbial Bulg. 1993;30:51–55. [PubMed] [Google Scholar]
- 42.Vassilev NB, Vassilve MCH, Spessova DI. Production of gluconic acid by Aspergillus niger immobilized on polyurethane foam. Appl Microbiol Biotechnol. 1993;39:285–288. doi: 10.1007/BF00192079. [DOI] [PubMed] [Google Scholar]
- 43.Velizarov S, Bechkov V. Production of free gluconic acid by cells of Gluconobacter oxidans. Biotechnol Lett. 1994;16:715–720. [Google Scholar]