Abstract
The germination rate and longevity of seeds of Gastrodia elata Blume have been observed for 48 weeks using Mycena osmundicola strain H-21, one of fungi stimulating seed germination. Storage condition of post-harvest seeds was observed in the different temperature ranges of -30℃, -5℃, 5℃ and 30℃ for 48 weeks. After storage period of 48 weeks, the germination rate of G. elata was 65.7% at 5℃ and 71.6% at -5℃, respectively. Although the germination rate of G. elata was 77.3% for 11 weeks at 25℃, the germination rate had been decreased gradually to 49.3% at 13 weeks, 0.3% at 23 weeks and then 0% at 25 weeks. The germination rate was reached to the level of 10% for 2 weeks at -30℃ and then decreased to 0%.
Keywords: Gastrodia elata Blume, Germination rate, Mycena osmundicola, Storage condition
Gastrodia elata Blume belongs to the Orchidaceae, and has been known to be distributed widely in Korea, China and Japan. The dried tubers of G. elata have been used as a traditional Chinese herb for curing human diseases such as vertigo, blackout, headache, gemiplegia and convulsions epilepsy under the name of "Cheonma" for several centuries in Asian countries (Huang, 1985; Chang and But, 1986).
G. elata is aphyllous and achlorophyllous orchid plant, and has been known to need a symbiont necessary to the growth of G. elata under the natural conditions. Armillaria mellea, one of these symbionts has been engaged in the growth of G. elata in the form of energy metabolism (Kusano, 1911; Zhang and Li, 1980; Choi and Lee, 1983; Hong et al., 1990).
Zhang and Li (1980) observed the biological relationship between G. elata and A. mellea. They pointed out ontogenesis of G. elata has four stages: for example, four stages such as seedling formation, tuber formation, flowering and fruiting. They found that there were two modes of infection of A. mellea on G. elata. Under normal conditions, A. mellea has been known to infect the cortical layer of G. elata. On the contrary, the digestive cells posses both the functions of defense and infecting hyphae. Some researchers suggested a pathological infection often was caused under unfavorable conditions (Zhang and Li, 1980; Sung et al., 1996).
To develop tubers of G. elata from its seedlings, the renewal of its vegetative organ has been known to depend on A. mellea (Zhang and Li, 1980; Lee, 1983; Sung et al., 1995).
Although G. elata has been cultured widely in Korea and China, Korean farmers are faced with some troubles in tuber production of G. elata. The yields of G. elata have been recently decreased owing to the degeneration of spawn tuber arisen from a successive asexual reproduction and technical problem of managing fungal organisms caused by the mistake of farmers themselves (Guo and Xu, 1991; Hong et al., 2002). One possible way to solve these problems had been suggested elaborately to use seeds instead of vegetative propagation (Clements et al., 1986).
Since the seeds of G. elata are not only very small but do not possess an endosperm, the germination rate of its seed is poor or not at all in nature (Nakamura, 1982: Xu and Guo, 1990). Therefore, many researchers have done to elucidate some mechanism by applying the viewpoint of histology and enzymology to seed germination of G. elata.
Though A. mellea has an outstanding effect on tuber formation of G. elata, some strains of A. mellea have been known to inhibit the seed germination of G. elata in nature (Xu and Mu, 1990).
Guo and Xu (1991) overcame an obstacle of degeneration of G. elata by using seed propagation method. Also, they pointed out some enzyme promoted seed germination in orchidaceae plant. Especially, they demonstrated that estrase isozyme of six fungal strains could promote the seed germination of G. elata by comparing the enzyme patterns of five fungal strains with those of Mycena osmundicola (Guo and Xu, 1991; Li et al., 1999). To develop protocorm from seeds, Xu and Mu (1990) observed the symbiotic relationship of both M. osmundicola and G. elata. They reported the cytological observation that hyphae of M. osmundicola invaded seed coat in the process of seed germination of G. elata. To obtain the nutrient sources necessary to sprout seeds of G. elata, the seeds should be cultured with some fungi helpful to formation of protocorm, because the seed is small and does not contain an endosperm in itself (Xu and Guo, 1990; Li et al., 2000).
Hong et al. (2002; 2004) have observed optimal factors of seed germination such as an optimal medium in the storage of seeds, protocorm formation, optimum range of temperatures and substrates suitable for fungal species promoting seed germination (Hong et al., 2002, 2004).
They concluded that favorable seed germination of G. elata depended on optimal temperatures during the storage of its seeds.
In this experiment, we tried to check the optimal storage period and temperature for the storage of post-harvest seeds.
Materials and Methods
Microbial cultures and inocula
Mycena osmundicola strain H-21, one of fungal strains inducing seed germination of G. elata was received from Guo (Institute of Medicinal Plant Development, China, Xu and Guo, 1989) and then maintained on PDA for the culture (Hong et al., 2002). For a germination assay of G. elata, the culture media which M. osmundicola H-21 was cultured were prepared by mixing fallen leaves of Quercus acutissima with rice bran at the ratio of 8 : 2 (V/V). The culture media were inoculated with M. osmundicola, and then cultured for 4 weeks at 25℃ (Hong et al., 2004). After 4 weeks of incubation, the culture media were fully colonized with M. osmundicola. Several pieces of leaves which were infested with M. osmundicola were placed on water agar. Several hundred seeds were spread on the surface of leaves infested with M. osmundicola, and then cultured for 48 weeks at different temperatures. The optimal conditions of seeds were checked in the range of 5℃, 25℃, -5℃ and -30℃, respectively.
Observation of seed germination
After seeds were spread on leaves, the germination rate was evaluated periodically Germination rate was observed every week for 2 months, and then two or four weeks. The degree and evaluation of seed germination were performed by using light microscope (40 ×). The seed germination was evaluated by swollen form of seeds and then converted into the percentage. The experiment was done with three replications.
Results and Discussions
The storage of post-harvest seeds has been carried out in 4 different temperatures for 48 weeks. After 48 weeks of the storage, the germination rate of G. elata was 65.7% at 5℃ and 71.6% at -5℃, respectively. Although the germination rate was 77.3% for 11 weeks at 25℃. The germination rate was decreased rapidly to 49.3% at 13 weeks and stopped nearly at 23 weeks. The germination rate was reached to the level of 10% for 2 weeks at -30℃ and decreased to 0% (Table 1 and Fig. 1).
Table 1.
Germination rate of G. elata on the leaves of Quercus acutissima inoculated with Mycena osmundicola
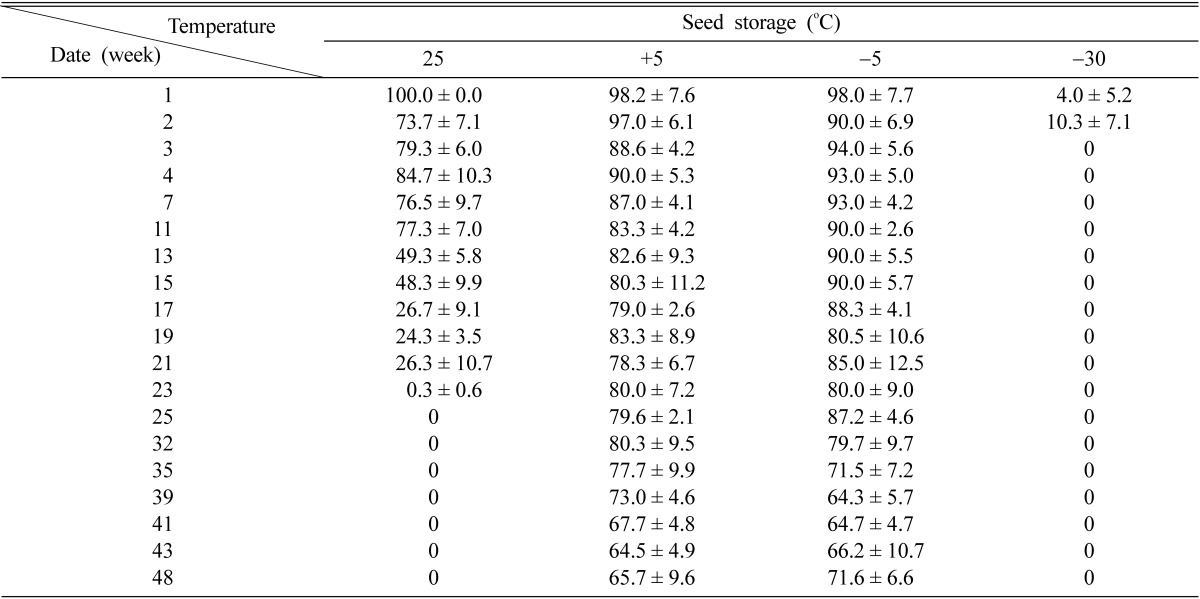
*Germination rate has been observed for 12 months (48 weeks).
Fig. 1.
The seed germination of Gastrodia elata.
1. The seed of G. elata. 2. The seeds of G. elata spread on the mycelial culture of Mycena osmundicola, one of fungi inducing seed germination. 3. The seeds of G. elata undergoing seed germination. The swollen portion (SP) of seed was considered as seed germination. 4. The small protocorm of G. elata formed on the surface of oak leaf infested with M. osmundicola.
Xu and Guo (1989) reported that some fungus was associated symbiotically with seed germination of G. elata. They isolated Mycena osmundicola as a symbiotic fungus inducing seed germination of G. elata, and also demonstrated the symbiotic relationship of both G. elata and M. osmundicola.
Guo and Xu (1990) reported that the seed germination of G. elata depended entirely on M. osmundicola in the embryonic cells of the plant. However, it is reasonable that further development of the protocorm needs an invasion of A. mellea.
Xu and Mu (1990) reported the cytological observation on an invading hyphae of M. osmundicola in the process of seed germination of Gastrodia elata. It was proved from M. osmundicola that G. elata obtained the nutrient sources necessary to sprout seeds of G. elata. Therefore, it is reasonable that the seeds of G. elata should be cultured with some fungi such as M. osmundicola capable of inducing seed germination of G. elata (Xu and Mu, 1990).
The hyphal mass of M. osmundicola inducing seed germination is enclosed and digested by embryonic cytoplasm after hyphae of M. osmundicola penetrates the embryo through suspensor cells, and then meristem cells begin to be divided. The digested hyphae penetrates into large cells to be further digested. Consequentially, the embryonic part enlarges to form tissue, and continues to grow under the system of meristem cells using the nutrition thus obtained (Xu and Mu, 1990). On nutrient sources necessary to seed germination of G. elata, Xu and Mu (1990) proved that seeds of Gastrodia elata lack an endosperm and other stored nutrition, and thus do not easily germinate. The nutrition necessary for seed germination is derived from M. osmundicola invading cells of embryo. It has been known that the leaves of Q. acutissima, one of coniferous trees are not only considered as a culture medium for the favorable growth of M. osmundicola but also offer an indirect nutrient source for seed germination of G. elata (Xu et al., 1990).
Hong et al. (2004) pointed out that optimum temperature for seed germination was 25℃ for the storage of 1 month, and the substrate suitable for a favorable growth of M. osmundicola was Q. acutissima. Also, Xu and Mu (1990) clarified seed germination of G. elata was optimal within the range of 3~4 weeks after seed harvest. In this experiments, the mycelial growth of M. osmundicola was favorable on the leaves of Quercus acutissima (Hong et al., 2004). After seed harvest, seed germination of G. elata was continued at 5℃ and -5℃ during storage period of 48 weeks. Although the germination rate of G. elata was kept to the level of about 70% for 11 weeks at 25℃, the germination rate was decreased rapidly after 11 weeks. On the other hand, the seed germination of G. elata was stopped nearly within 2 weeks at -30℃ (Table 1).
Our result was different excessively with that of Guo and Xu (1991). They have tested seed germination of G. elata for storage period of 1 month. With that result they recommended that storage period of 1 month was optimal for seed germination of G. elata, because the germination rate was decreased rapidly below 50% after 1 month (Unpublished data, personal communication). However, it was observed from our results that germination rate of G. elata was more than 60% at 5℃ or -5℃ during storage period of 48 weeks. The reason that germination rate of G. elata was more than 60% at 5℃ or -5℃ seems to be attributable to the influence of an optimal temperature and M. osmundicola. Hong et al. (2004) tested storage conditions and optimum temperature of G. elata. They concluded that the culture condition of G. elata was optimal at 25℃. Also, they pointed out that storage of seed pod was good in the range of -5℃ or 5℃ for storage period of 1 month. This was the same as our result. Therefore, it could be proved in the case of G. elata that the desirable storage of seeds could be kept for 1 year (or 48 weeks) at -5℃ or 5℃ and the propagation of seeds could be probable for 1 year after their harvest.
Acknowledgement
This work was supported by the research program of Dongguk University in 2004.
References
- 1.Chang HM, But PH. Pharmacology and Application of Chinese Materia Medica. Vol. I. Singapore: World Scientific; 1986. p. 185. [Google Scholar]
- 2.Choi MJ, Lee JY. Physiological and ecological studies on mycelia of Armillaria mellea. Korean J Mycol. 1983;11:79–84. [Google Scholar]
- 3.Clements MA, Muir H, Cribb PJ. A preliminary report on the symbiotic germination of European terrestrial orchids. Kew Bull. 1986;41:437–445. [Google Scholar]
- 4.Guo SX, Xu JT. Studies on the cell ultrastructure in the course of Gastrodia elata digesting Mycena osmunidicola Lange and Armillaria mellea Fr. Acta Mycologica Sinica. 1990;9:218–225. [Google Scholar]
- 5.Guo SX, Xu JT. Esterase isozyme of fungi promoting seed germination of Gastrodia elata etc. Med Plants Orchidaceae. 1991;26:524–526. [Google Scholar]
- 6.Hong IP, Kim HK, Park JS, Kim KP, Lee MW, Guo SX. Physiological characteristics of symbiotic fungus associated with the seed germination of Gastrodia elata. Mycobiology. 2002;30:22–26. [Google Scholar]
- 7.Hong IP, Nam SH, Jung IY, Sung GB, Nam HW, Cheong JC, Park JS, Hur H, Lee MW. Studies on the conditions of seed germination of Gastrodia elata. Korean J Mycol. 2004;32:39–44. [Google Scholar]
- 8.Hong JS, Kim MK, So GJ, Kim TH. Studies on the mycelial cultivation and the rhizomorph production of Armillaria mellea. Korean J Mycol. 1990;18:149–157. [Google Scholar]
- 9.Huang ZL. Pharmacologic studies and clinical applications of Gastrodia elata Bl. J Modern Develop Trad Media. 1985;5:251–254. [PubMed] [Google Scholar]
- 10.Kusano S. Gastrodia elata and its symbiotic association with Armillaria mellea. Imperial University of Tokyo. J College Agr. 1911;4:1–65. [Google Scholar]
- 11.Lee JY. Artificial culture methods of Gastrodia elata. No.15661. Korea Patent. 1983
- 12.Li F, Guo SX, Xiao PG. A study on the mycorrhizal microstructure of six orchids. Chinese Bull Bot. 2000;17:73–79. [Google Scholar]
- 13.Li F, Guo SX, Xiao PG. Ultrastructure changes during the symbiotic development of Gastrodia elata associated with Mycena orchidicola. Mycosystema. 1999;8:431–435. [Google Scholar]
- 14.Li F, Guo SX, Xiao PG. Study on structure and localization of acid phosphatase of mycorrhiza root of Cymbidium sinense (Orchidaceae) Acta Botanica Yunnanica. 1999;21:197–201. [Google Scholar]
- 15.Nakamura SJ. Nutritional conditions required for the nonsymbiotic culture of an achlorophyllus orchid Galeola septentrionalis. New Phytol. 1982;90:701–715. [Google Scholar]
- 16.Sung JM, Jung BS, Yang KJ, Lee HK. Production of Gastrodia elata tuber using Armillaria spp. Korean J Mycol. 1995;23:61–70. [Google Scholar]
- 17.Sung JM, Jung BS, Moon HW, Kim SH. Studies on collection and spawn manufacture of Armillaria spp. for production of Gastrodia elata. Korean J Mycol. 1996;24:127–134. [Google Scholar]
- 18.Xu JT, Mu C. The relation between growth of Gastrodia elata protocorms and fungi. Acta Bot Sinica. 1990;32:26–31. [Google Scholar]
- 19.Xu JT, Guo SX. Studies on nutrition source of seed germination of Gastrodia elata. Bl. Mellea Acta Bot Sinica. 1990;22:57–62. [Google Scholar]
- 20.Xu JT, Guo SX. Fungus associated with nutrition of seed germination of Gastrodia elata-Mycena osmundicola Lange. Acta Mycol Sinica. 1989;8:221–226. [Google Scholar]
- 21.Zhang WJ, Li BF. Biological relationship of Gastrodia elata and Armillaria mellea. Acta Bot Sinica. 1980;22:57–62. [Google Scholar]



