Abstract
This study was aimed at evaluating the antioxidant activities of methanol extract of Thamnolia vermicularis. The antioxidant activity, reducing power, superoxide anion radical scavenging and free radical scavenging activities were studied. The antioxidant activity of the extract correlated with its concentration (0.2~2 mg/ml) in the reaction mixtures containing linoleic acid. Upto 67% of lipid peroxidation was inhibited by 2 mg/ml of the lichen extract. The extract showed strong free radical scavenging activity similar to that of BHA (positive control) in a manner of concentration dependent. The lichen extract also showed moderate effects on superoxide anoin scavenging activity and reducing power, which was not so effective as that of Quercetin and BHA used as positive controls. This study suggests that T. vermicularis lichen can be used as a novel source of natural antioxidant.
Keywords: Antioxidant activity, Free radical scavenging activity, Lichen extract, Natural antioxidant, Thamnolia vermicularis
Oxygen plays very important role in human's survival, once inhaled, it undergoes a gradual reduction process and ultimately gets metabolized into water. In this process, some reactive oxygen species (ROS), such as superoxide anion radicals (O2·-), hydroxyl radicals (OH·), nonfree radical species (such as H2O2), and single oxygen (1O2) are formed (Halliwell, 1995; Sies, 1993). ROS damage membrane proteins by causing lipid peroxidation in membranes by attacking to unsaturated fatty acids (Ames et al., 1993). The damage to membrane proteins decreased the membrane permeability, activities of enzymes and receptors, and activation of cells. When free radicals attack DNA, cancer-causing mutations may occur. Therefore, antioxidant defense systems including antioxidant enzymes, food and drugs are important in the prevention of many diseases (Pietta et al., 1998; Yen and Hsieh, 1998).
Antioxidants are compounds that inhibit or delay the oxidation process by blocking the initiation or propagation of oxidizing chain reactions. Currently, synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ) are widely used in the food industry. However, restriction on the synthetic antioxidants is being imposed because of their toxicity to liver and carcinogenicity (Grice, 1986; Wichi, 1988). Therefore, the development and utilization of more effective antioxidants of natural origins are desired. In recent years, the antioxidant properties of numerous crude extracts, primary and secondary metabolites of many plants have been widely reported (Hidalgo et al., 1994; Pietta et al., 1998).
Lichens are the symbiotic organisms including a fungal partner and an algal partner and are known to have therapeutic effects on various diseases in folk medicine of many countries. Recently, much attention has been paid to lichens as resources of natural antioxidants. Scientist already investigated the antioxidant activity of some species of lichens, such as Bryoria fuscescens, Cetraria islandica, Dermatocarpon intestiniformis, Parmelia saxatilis, Peltigera rufescens, Platismatia glauca, Ramalina pollinaria, R. polymorph, Umbilicaria nylanderiana, Usnea ghattenis, and U. longissima and some of them have very good antioxidant activity (Behera et al., 2006; Gulluce et al., 2006; Halici et al., 2005; Odabasoglu et al., 2005).
Thamnolia vermicularis has commonly been used as a tea with the local name of "snow tea" in some parts of China. It has been believed to counteract inflammation and has been used in traditional Chinese medicine for hundreds or thousands of years (Wang et al., 2001). However, little study has been attempted to evaluate in vitro antioxidant activity of T. vermicularis so far. In this study, antioxidant activity of T. vermicularis was investigated to verify the potential for T. vermicularis lichen as a natural antioxidant.
Materials and Methods
Collections and identification of lichen material
Highland lichen species of T. vermicularis was collected at Mt. Da Shi (Elevation 4,500 m), Yunnan province, China in 2004. Details on locality, habit and growing conditions were previously presented (Hur et al., 2005). The lichen was identified by Mr. Wang at Kunming Botanical Institute (CAS). The lichen samples are stored in herbarium of Korean Lichen Research Institute (KoLRI) at Sunchon National University, Korea. Voucher specimen (CH040157) is also deposited in KoLRI (Fig. 1).
Fig. 1.
Habit of Thamnolia vermicularis (CH040157) growing with other lichens (Steroucaulon sp.) and mosses on the soil.
Preparation of the methanol extract
Air-dried and fractioned lichen thalli (5 g) were extracted 2 times with 200 ml methanol for 48 hours at room temperature with a shaking attachment. The combined methanol extract was filtered and then concentrated in vacuo at 40℃ using a Rotary Evaporator. The residuals obtained were stored in a freezer at -20℃ until further study.
Antioxidant activity determination
The antioxidant activity of T. vermicularis was determined according to the thiocyanate method (Mitsuda et al., 1996). Lichen extracts of 0.2, 0.5, 1 and 2 mg/ml in methanol solution were added to 50 µl linoleic acid. The mixed solution was incubated at 37℃ for 1 hour. Then 0.1 ml of ammonium thiocyanate (100 g/l) and 0.1 ml FeCl2 (10 g/l) was added into 0.1 ml mixed solution. The antioxidant activity was determined by reading the absorbance at 500 nm. The solution without the extract was used as a negative control and one with same concentration of ascorbic acid was used as a positive control.
Reducing power determination
The reducing power of T. vermicularis was determined following Oyaizu's method (Oyaizu, 1986). Lichen extracts of 0.2, 0.5, 1 and 2mg/ml in methanol solution were mixed with 2.5 ml phosphate buffer (0.2M, pH 6.6) and 2.5 ml of potassium ferricyanide (K3Fe(CN)6; 10g/l), then the mixture was incubated at 50℃ for 20 min. Afterwards, 1.5 ml of trichloroacetic acid (100 g/l) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The supernatant solution (0.5 ml) was mixed with 0.5 ml of FeCl3 (1 g/l) and the absorbance was measured at 700 nm. A reaction mixture without extract was used as a negative control and one with the same concentration of BHA was used as a positive control.
Superoxide anion scavenging activity determination
Measurement of superoxide anion scavenging activity of T. vermicularis was performed according to the protocol of Nishimiki method, (Nishimik et al., 1972) with slight modifications. One ml of nitroblue tetrazolium solution (100 µM NBT in 100 mM phosphate buffer, pH 7.4), 1 ml NADH solution (468 µM in 100 mM phosphate buffer, pH 7.4) and 1ml of lichen extracts (0.2, 0.5, 1 and 2 mg/ml in methanol solution) were mixed. The reaction started by adding 100 µl of phenazine methosulphate solution (60 µM PMS in 100 mM phosphate buffer, pH 7.4) to the mixture, which was then incubated at 30℃ for 15 min. The absorbance was measured at 560 nm. A reaction mixture without the extract was used as a negative control and one with the same concentration of Quercetin was used as positive control.
Free radical scavenging activity determination
The free radical scavenging activity of T. vermicularis was determined by Blois' method (Blois, 1958). One ml of the extract solution in methanol (containing 0.2, 0.5, 1, 2 mg of dried extract) was added into 0.1 mM of 1,1-diphenyl-2-picryl-hydrazil (DPPH) methanol solution. After 30 min of incubation, the absorbance was measured at 517 nm. A reaction mixture without the extract was used as a negative control and one with the same concentration of BHA was used as a positive control.
Results
Antioxidant activity of T. vermicularis on lipid peroxidation
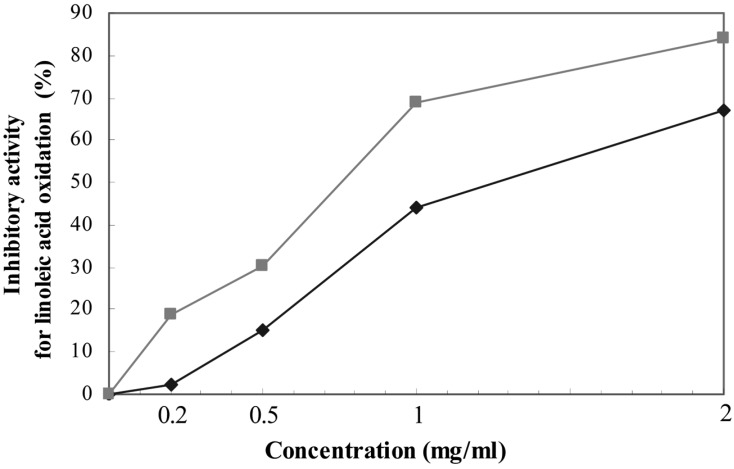
Methanol extracts of T. vermicularis exhibited notable antioxidative activity against lipid peroxidation (Fig. 2). Increasing the concentrations of the extract from 0.2 to 2 mg/ml caused increased antioxidative capacity of the reaction mixtures. The extract exhibited significant inhibition of linoleic acid oxidation (67%) at the concentration of 2.0 mg/ml, compared to the negative control. However, the antioxidant activity was slightly lower than that of ascorbic acid (+ control) at the tested concentrations.
Fig. 2.
Inhibitory activity for lipid peroxidation of methanol extract of T. vermicularis (♦) and ascorbic acid (■). Amounts of lichen extract were present in 0.05 ml of linoleic acid mixture. The control was the linoleic acid mixture without extract.
Free radical scavenging activity of T. vermicularis
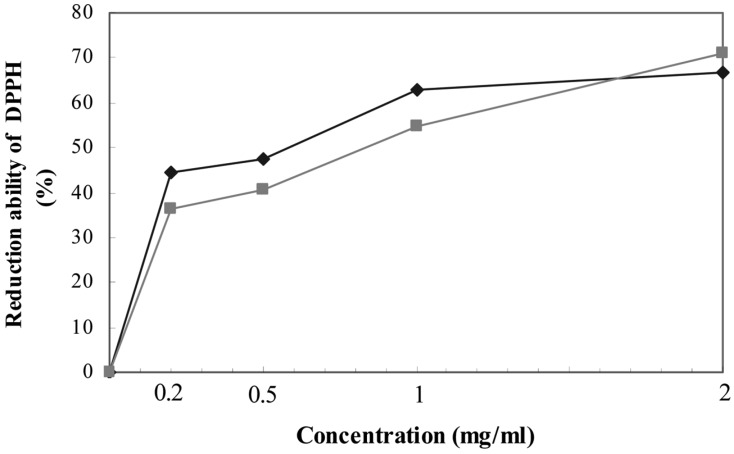
DPPH is present as a stable free radical in aqueous or methanol solution and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. It is usually used as a substrate to evaluate the antioxidative activity of antioxidants. Free radical scavenging ability of the extract and BHA, a positive control, is presented in Fig. 3. Removal of free radical increased by 36 to 72% in accordance with the increase of the concentrations of the extract from 0.2 to 2 mg/ml, compared to the negative control and moreover, the scavenging ability of the extract was as strong as that of BHA at all the concentrations tested. Significant correlation was found between the free radical scavenging activity and the concentration of lichen extract or the compounds used as positive controls.
Fig. 3.
Free radical scavenging activity at various concentrations of methanol extract from T. vermicularis (♦) and BHA (■) by 1,1-diphenyl-2-picrylhydrazyl (DPPH). The control was the DPPH methanol solution (0.1 mM) without the extract.
Superoxide anion radical scavenging activity of T. vermicularis
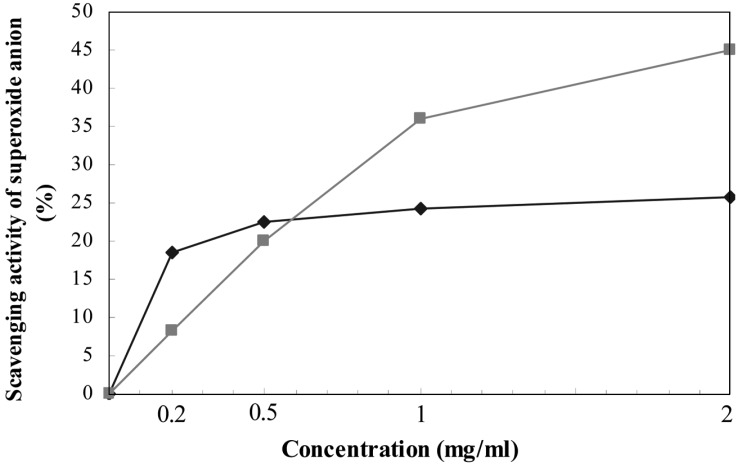
Superoxide anion radical was also removed by the lichen extract with more effectively than by the control compound by 20% at the concentrations tested (Fig. 4). Unlike lipid peroxidation inhibition and free radical scavenging activity, the activity was not correlated with concentration. The lichen extract of lower concentrations (0.2 and 0.5 mg/ml) showed higher scavenging activity than Quercetin (a positive control) of same concentrations, but positive control shows higher activity than the lichen extract at higher concentrations (1.0 and 2.0 mg/ml). Quercetin also showed superoxide anion scavenging activity in a concentration-dependent manner.
Fig. 4.
Superoxide anion scavenging activity at various concentrations of methanol extract from T. vermicularis (♦) and Quercetin (■). Each reaction was performed in 2 ml of reaction mixture. The control reaction was performed in a reaction mixture without the extract.
Antioxidant activity of T. vermicularis on reducing power
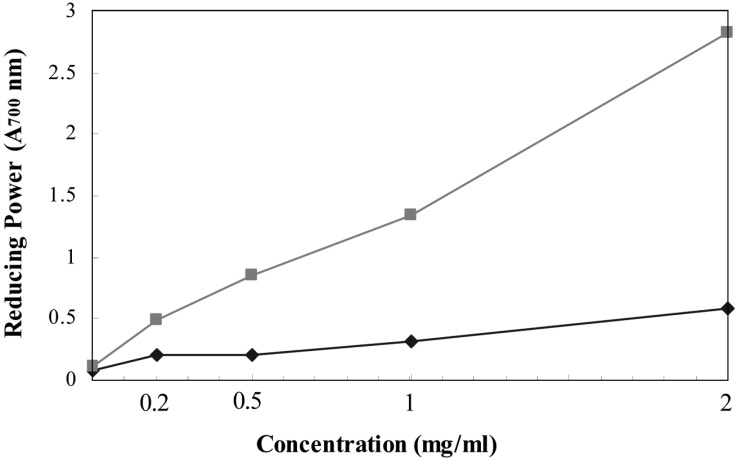
The lichen extract exhibited much weaker reducing power at the tested concentrations, compared to BHA. Both the lichen extract and BHA showed reducing power in a concentration-dependent manner (Fig. 5).
Fig. 5.
Reducing power at various concentrations of methanol extract from T. vermicularis (♦) and BHA (■). Each reaction was performed in 3 ml of reaction mixture. The control reaction was performed in a reaction mixture without the extract.
Discussion
All these results indicated that the methanol extract of T. vermicularis showed apparent antioxidant activity and DPPH radical scavenging activity, however moderate reducing power and superoxide anion scavenging activity compared to the compounds used as positive controls. The concentrations of lichen extracts tested in this study were much lower than those employed in other studies. For example, Behera et al. (2006) demonstrated that methanol extract of Usnea ghattensis exhibited strong antioxidant activity in the range of 2 to 20 mg/ml. Although T. vermicularis showed 67% inhibition of lipid peroxidation at the concentration of 2 mg/ml, U. ghattensis exhibited only 3.8% inhibition at the same concentration. This suggests that T. vermiculairs can produce stronger antioxidant substances or larger amount of antioxidants in the thalli than other lichen species. It is thus concluded that T. vermicularis can be used as a potential natural resource of antioxidants.
Thamnolic acid, didepside, has been known to be the main secondary metabolite of T. vermicularis which has been used in traditional Chinese medicine to counteract inflammation for hundreds years (Wang et al., 2001). The compound may be responsible for the higher antioxidant activity of the extract of T. vermicularis. Further study might be focus on the isolation and identification of the antioxidative components from T. vermicularis. As the growth of lichen in nature is very slow, isolation and artificial cultivation of the lichen-forming fungi may be required for further progress in mass production of the compound.
Acknowledgement
This work was supported by the 21C Frontier Microbial Genomics and Application Center Program, Ministry of Science & Technology (Grant MG05-0101-5-0), Republic of Korea. Miss Luo is thankful for the finical support from the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-C00049).
References
- 1.Ames BN, Shigennaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behera BC, Verma N, Sonone A, Makhija U. Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT - Food Sci Technol. 2006;39:80–85. [Google Scholar]
- 3.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. [Google Scholar]
- 4.Grice HC. Safety evaluation of butylated hydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food Chem Toxicol. 1986;24:1127–1130. doi: 10.1016/0278-6915(86)90298-x. [DOI] [PubMed] [Google Scholar]
- 5.Gulluce M, Aslan A, Sokmen M, Sahin F, Adiguzel A, Agar G, Sokmen A. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorph and Umbilicaria nylanderiana. Phytomedicine. 2006;13:515–521. doi: 10.1016/j.phymed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Halici M, Odabasoglu F, Suleyman H, Cakir A, Asland A, Bayir Y. Effects of water extract of Usnea logissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine. 2005;12:656–662. doi: 10.1016/j.phymed.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell B. How to characterize an antioxidant: an update? Biochem Soc Symp. 1995;61:73–101. doi: 10.1042/bss0610073. [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo ME, Quilhot FW, Lissi E. Antioxidant activity of depsides and depsidones. Phytochemistry. 1994;37:1585–1587. doi: 10.1016/s0031-9422(00)89571-0. [DOI] [PubMed] [Google Scholar]
- 9.Hur JS, Wang LS, Oh SO, Kim GH, Lim KM, Jung JS, Koh YJ. Highland marcolichen flora of north-western Yunnan, China. J Microbiol. 2005;43:228–236. [PubMed] [Google Scholar]
- 10.Mitsuda H, Yuasumoto K, Iwami K. Antioxidation action of indole compounds during the autoixdation of linoleic acid. Eiyo to Shokuryo. 1996;19:210–214. [Google Scholar]
- 11.Nishimiki M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–853. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 12.Odabasoglu F, Asland A, Cakir A, Suleyman H, Karagoz Y, Bayir Y, Halici M. Antioxidant activity, reducing power and total phenolic contents of some lichen species. Fitoterapia. 2005;76:216–219. doi: 10.1016/j.fitote.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nutri. 1986;44:307–315. [Google Scholar]
- 14.Pietta P, Simonetti P, Mauri P. Antioxidant activity of selected medicinal plants. J Agric Food Chem. 1998;46:4487–4490. [Google Scholar]
- 15.Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang LS, Narui T, Harada H, Culberson CF, Culberson WL. Ethnic uses of lichens in Yunnan, China. Bryologist. 2001;104:345–349. [Google Scholar]
- 17.Wichi HP. Enhanced tumor development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium. Food Chem Toxicol. 1988;26:717–723. doi: 10.1016/0278-6915(88)90072-5. [DOI] [PubMed] [Google Scholar]
- 18.Yen GC, Hsieh CL. Antioxidant activity of extracts from Du-Zhong (Eucommia ulmoides) toward various lipid peroxidation in vitro. J Agric Food Chem. 1998;46:3952–3957. [Google Scholar]