Abstract
Effects of various preservation periods and subcultures on fruiting body formation of Cordyceps militaris were investigated using EFCC C-10995 single ascospore strains. Fruiting body formation by original strains was profuse when preserved at 4℃ for 5~6 months. Fruiting from subcultures was stable till second to sixth subcultures, after which it decreased sharply. The more the colony color of subcultures changed, the less the fruiting bodies formed. Liquid inoculum preparation of single ascospore strains in the same or separate broths did not affect fruiting body formation. Similarly, two strains C-10995-3 and C-10995-6 in different numbers during liquid inoculum preparation produced similar fruiting bodies.
Keywords: Cordyceps militaris, Fruiting body production, Original strains, Single ascospore strains, Subcultures
Cordyceps is an entomopathogenic genus which belongs to family Clavicipitaceae of Hypocreales, Ascomycota. Cordyceps militaris is the type species of Cordyceps (Seaver, 1911). It is well known for cordycepin production. At present, artificial cultivation of mycelium and fruiting bodies of C. militaris and their chemical analyses have been carried out in different parts of the world. Formation of fruiting bodies of C. militaris in artificial condition is very difficult (Sung, 1996; Sung et al., 2002).
It has been shown from cultural studies that C. militaris dominantly behaves as a bipolar heterothallic fungus (Shrestha et al., 2004). From molecular studies also, Cordyceps species, including C. militaris, have been shown to be heterothallic consisting of two mating type genes MAT1-1-1 and MAT1-2-1 (Yokoyama et al., 2003, 2004, 2005). Recently, it has been practiced to produce in vitro stromata of C. militaris, by inoculating two mating-compatible single ascospore strains in rice pupae medium (Sung et al., 2006). For regular production of stromata, freshly prepared cultures are used. It takes a long time and effort to identify mating-compatible strains among the fresh strains. There are a number of preservation techniques of fungal isolates, such as subculturing, freeze-drying and cryopreservation (Smith and Onions, 1994). Out of these, subculturing is the simplest one (Smith and Onions, 1994). Storage of subcultures at low temperatures (4~7℃) can minimize the metabolism of isolates and can prolong their life (Smith and Onions, 1994). Subculture and other long-term preservations can shorten the period for mass production of stromata. In this study, we observed the fruiting capacities of strains at regular periods of preservation at 4℃. Similarly, we also observed the fruiting body production from the subcultures of original strains at regular and successive periods.
Materials and Methods
Fungal strains
Ten single ascospores were isolated from the specimen EFCC C-10995 and grown in Sabouraud Dextrose agar plus Yeast Extract (SDAY; dextrose 40 g, peptone 10 g, yeast extract 10 g and agar 15 g per 1000 ml; pH 5.6) medium plates. The single ascospores were isolated following the method of Shrestha et al. (2004). The specimen EFCC C-10995 was collected from Sobaek Mt. of Chungcheong Province, Republic of Korea on August 30, 2003. The strains were grown on SDAY medium plates at 24±1℃ for 3 weeks, and then preserved at 4℃. The strains were referred to as original strains.
Selection of superior strains
After 3 weeks of growth on SDAY plates, all the ten original strains were inoculated in brown rice medium supplemented with silkworm pupae, commonly known as rice pupae medium, following the method of Shrestha et al. (2004). Before inoculating the strains in rice pupae medium, each strain was first grown in individual SDAY broths of 100 ml by inoculating few mycelial discs (5 mm in diameter) cut by a cork-borer. The inoculated broths were incubated in a rotary shaker (120 rpm) at 24±1℃ for 3~4 days. Liquid inocula of all the strains were inoculated in rice pupae medium in pair-wise combination following the method of Shrestha et al. (2004). After inoculation, the cultures were incubated at 20±1℃ under high humidity (70~90%) and continuous light (500~1000 lux) and observed for fruiting body production. After 40 days of incubation four strains C-10995-3, C-10995-5, C-10995-6 and C-10995-8 produced profuse fruiting bodies in the following combinations C-10995-3 × C-10995-6, C-10995-3 × C-10995-8, C-10995-5 × C-10995-6 and C-10995-5 × C-10995-8. Hence, those four strains were selected for further experiments.
Fruiting body production of C. militaris from original strains and their subcultures at regular intervals
Original strains C-10995-3, C-10995-5, C-10995-6 and C-10995-8, being preserved at 4℃, were continuously inoculated in rice pupae medium for fruiting body production at the intervals of two weeks for next twenty-two weeks following the above method. At the time of liquid inoculum preparation, the original strains were also subcultured in fresh SDAY medium plates by transferring mycelial discs (5 mm in diameter) cut with a cork-borer and incubated at 24±1℃ for two weeks. The subcultures were referred to as first subculture. After two weeks, the original strains as well as first subcultures were again inoculated in rice pupae medium for fruiting body production, as mentioned above. At that time, the original strains were again subcultured in fresh SDAY medium plates and incubated at 24±1℃ for two weeks. After two weeks, the original strains as well as the two-weeks old first subcultures were again inoculated in rice pupae medium for fruiting body production. The process was continued for 24 weeks. The fruiting bodies were observed after 40 days of incubation.
Fruiting body production from further subcultures
The earliest subcultures of strains C-10995-3, C-10995-5, C-10995-6 and C-10995-8, after two weeks of growth, were also subcultured in fresh SDAY medium plates by transferring mycelial discs (5 mm in diameter). These subcultures were referred to as second subcultures. After two weeks of growth, the second subcultures were inoculated in SDAY broth for inoculation in rice pupae medium for fruiting body production. At the same time, they were again subcultured in fresh SDAY plates as mentioned above and were referred to as third subculture. Thus, regular subcultures were made at the intervals of two weeks till 10th subcultures. Subcultures of each generation were used for fruiting body production, as mentioned above. The fruiting bodies were observed after 40 days of incubation. Colony color of subcultures was observed following Kornerup and Wanscher (1978).
Effect of liquid inoculum type on stromata production
Two opposite mating type strains C-10995-3 and C-10995-6 were inoculated in the same broth culture as well as in separate broth cultures of SDAY before inoculating them in rice pupae medium. In the first case, mycelial discs of each strain were inoculated in the same SDAY broth and incubated in a rotary shaker before inoculating rice pupae medium, whereas in the second case, mycelial discs of the two strains were inoculated in separate SDAY broths and incubated in a rotary shaker, and ultimately inoculated in the same rice pupae medium. Fruiting bodies were observed after 40 days of incubation and compared with each other.
Effect of ratio of two strains on fruiting body production
Mycelial discs (5 mm in diameter) of both strains C-10995-3 and C-10995-6 were inoculated in the same SDAY broths of 100 ml in different numbers of 1 and 1, 1 and 2, 1 and 3, 1 and 4, 2 and 1, 3 and 1, and 4 and 1 and were incubated in a rotary shaker, as mentioned above. The broth cultures were then inoculated in rice pupae medium. Growth of in vitro fruiting bodies was observed after 40 days of incubation.
Results and Discussion
Selection of superior strains
Results showed that out of ten single ascospore strains, C-10995-1, C-10995-3, C-10995-5, C-10995-7, C-10995-9 and C-10995-10 were the same mating type in production of perithecial stromata whereas the remaining fours strains C-10995-2, C-10995-4, C-10995-6 and C-10995-8 were the opposite mating type (Table 1). Among different combinations, fruiting bodies were best produced from combinations C-10995-3 × C-10995-6, C-10995-3 × C-10995-8, C-10995-5 × C-10995-6 and C-10995-5 × C-10995-8.
Table 1.
Stromata formation from single and pair-wise combinations of Cordyceps militaris EFCC C-10995 single ascospore strains (C-10995-1 to C-10995-10)
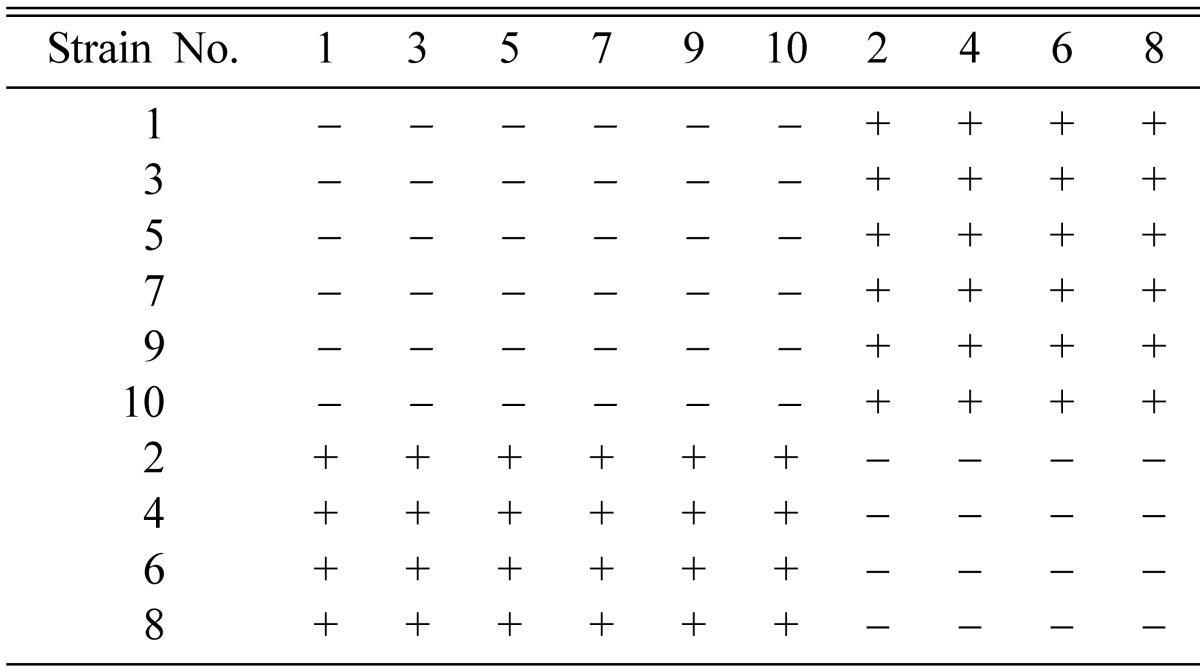
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
Production of stromata from original strains at successive periods
All the four combinations C-10995-3 × C-10995-6, C-10995-3 × C-10995-8, C-10995-5 × C-10995-6 and C-10995-5 × C-10995-8 produced profuse stromata till 18 weeks. After 20 weeks, all the combinations produced profuse stromata except C-10995-3 × C-10995-6. After 22 weeks, all the combinations produced poor fruiting bodies except C-10995-5 × C-10995-8. In general, it was observed that strains preserved at 4℃ produced fruiting bodies till 5-6 months (Fig. 1).
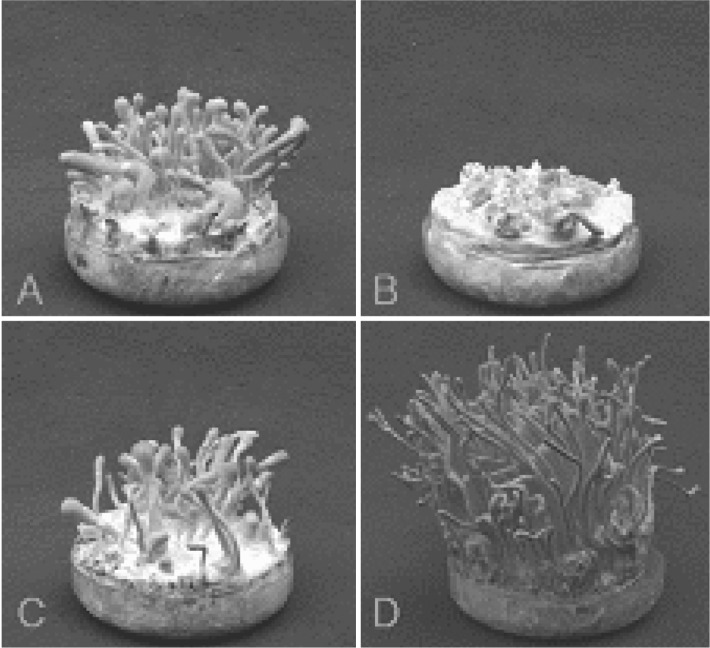
Fig. 1.
Fruiting body formation from different combinations of single ascospore strains of Cordyceps militaris EFCC C-10995 after 10th subculture. A, C-10995-3 × C-10995-6; B, C-10995-3 × C-10995-8; C, C-10995-5 × C-10995-6 and D, C-10995-5 × C-10995-8.
Production of fruiting bodies from subcultures
Not only the original strains, but their first subcultures also produced fruiting bodies profusely. The combination C-10995-3 × C-10995-6 produced fruiting bodies from their first subcultures till ninth time. Another combination C-10995-3 × C-10995-8 produced fruiting bodies till 8th time. The remaining combinations C-10995-5 × C-10995-6 and C-10995-5 × C-10995-8 produced fruiting bodies till 10th time. However, it was observed that first subcultures at later ages produced less fruiting bodies. Thus, strains older than 18~20 weeks as well as their subcultures are not recommended for mass fruiting body production.
Fruiting body production from regular subcultures of isolates
Combinations C-10995-3 × C-10995-6 and C-10995-3 × C-10995-8 produced fruiting bodies till sixth subcultures. After sixth subculture, amount of fruiting bodies started to decrease. Similarly, another combination C-10995-5 × C-10995-8 produced fruiting bodies till only third subculture, after which the amount of fruiting bodies decreased sharply. The last combination C-10995-5 × C-10995-6 produced fruiting bodies till only second subculture, after which no fruiting bodies were produced. Among the four strains, colony color of strains C-10995-3 and C-10995-6 almost remained orange to light orange, while that of C-10995-8 changed from light orange to light yellow (Table 2). C-10995-5 showed the highest change from orange to white (Table 2). It was observed that the decrease in fruiting body production followed the changes in colony color of the subcultures of the strains. For example, the combination C-10995-3 × C-10995-6 produced profuse fruiting bodies till sixth subculture. Both the strains showed the least change in colony color of their subcultures. Other combination C-10995-3 × C-10995-8 also produced constant fruiting bodies till sixth subcultures. In this combination, only C-10995-8 showed slightly more change in colony color of its subcultures. In case of other two combinations C-10995-5 × C-10995-8 and C-10995-5 × C-10995-6, they produced fruiting bodies till only third and second subcultures, respectively. In those combinations, although the strains C-10995-6 and C-10995-8 showed little or no change in colony color of their subcultures, the other strain C-10995-5 showed the highest change in colony color of its subcultures. Thus, it was observed that the more the color of the subcultures changed, the less the fruiting bodies were formed. In this study, the effect of change in colony color of subcultures was found linked to the fruiting body production.
Table 2.
Variation in colony color of single ascospore strains of Cordyceps militaris after regular subcultures
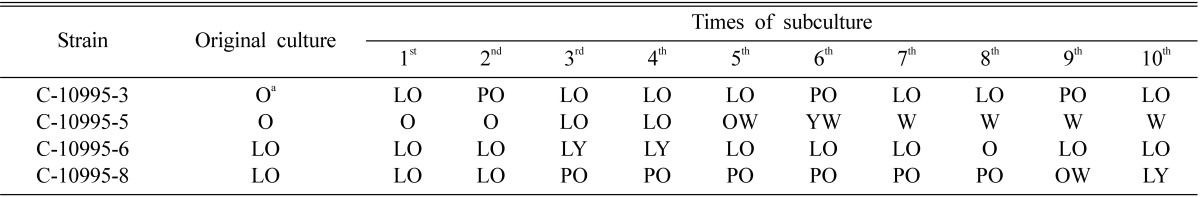
aPigmentation descriptions according to Kornerup and Wanscher (1978): O, orange (5A6, 5A7); LO, light orange (5A4, 5A5); LY, light yellow (4A4, 4A5); PO, pale orange (5A3); OW, orange white (5A2); YW, yellowish white (4A2) and W, white (5A1).
Effect of inoculum type on fruiting body production
Not much difference was observed between two types of liquid inoculum preparation. For fruiting body production, it is necessary that the opposite mating type strains be inoculated in the same rice pupae medium. It should be made certain that both strains are viable and can grow in rice pupae medium. When the two strains are grown in separate broths, it can be observed whether both strains are growing vigorously or not by visual observation or by their dry weights. But, during their growth in the same broth, it is not possible to observe the growth of each individual strain. From this study, it was shown that the two opposite mating type strains could be grown either in the same broth or separate broths before inoculating rice pupae medium. Due to the reason of simplicity, the first type of liquid inoculum preparation is much easier than the second one since a single broth culture is enough to prepare the inoculum in the first type, whereas the second type of inoculum preparation demands twice the amount of work than the first one.
Effect of different ratios of two strains on fruiting body production
Similar fruiting bodies were produced when the ratio of two strains C-10995-3 and C-10995-6 differed in a range of 1 : 1, 1 : 2, 1 : 3, 1 : 4, 2 : 1, 3 : 1 and 4 : 1 in broth culture. It could be observed that the two strains grew together without any interference between them during the broth culture. It is assumed that the individual strains can attain the same level of growth in broth culture despite the difference in their initial concentration. The other assumption is that for stromata production, equal amount of mycelial growth is not necessary.
Acknowledgement
The authors wish to acknowledge the financial support from Korea Science and Engineering Foundation (KOSEF) to Entomopathogenic Fungal Culture Collection (EFCC), Kangwon National University, and Biogreen 21 Rural Development Administration. Also, acknowledgement goes to Cordyceps Research Institute for providing facilities to carry out this study.
References
- 1.Kornerup A, Wanscher JH. Methuen handbook of colour. 3rd ed. London: Eyre Methuen; 1978. [Google Scholar]
- 2.Seaver FJ. The Hypocreales of North America-IV. Mycologia. 1911;3:207–230. [Google Scholar]
- 3.Shrestha B, Kim HK, Sung GH, Spatafora JW, Sung JM. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotech Bioprocess Engin. 2004;9:440–446. [Google Scholar]
- 4.Smith D, Onions AHS. The preservation and maintenance of living fungi. Wallingford: CAB International; 1994. [Google Scholar]
- 5.Sung JM. Insect-Borne Fungi of Korea. Seoul: Kyo-Hak Publishing Co. Ltd; 1996. [Google Scholar]
- 6.Sung JM, Choi YS, Shrestha B, Park YJ. Investigation on artificial fruiting of Cordyceps militaris. Korean J Mycol. 2002;30:6–10. [Google Scholar]
- 7.Sung JM, Park YJ, Lee JO, Han SK, Lee WH, Choi SK, Shrestha B. Selection of superior strains of Cordyceps militaris with enhanced fruiting body productivity. Korean J Mycol. 2006;34:131–137. doi: 10.4489/MYCO.2006.34.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama E, Yamagishi K, Hara A. Structures of the mating-type loci of Cordyceps takaomontana. Appl Environ Microbiol. 2003;69:5019–5022. doi: 10.1128/AEM.69.8.5019-5022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama E, Yamagishi K, Hara A. Development of a PCR-based mating type assay for Clavicipitaceae. FEMS Microbiol Lett. 2004;237:205–212. doi: 10.1016/j.femsle.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama E, Yamagishi K, Hara A. Heterothallism in Cordyceps takaomontana. FEMS Microbiol Lett. 2005;250:145–150. doi: 10.1016/j.femsle.2005.07.004. [DOI] [PubMed] [Google Scholar]