Abstract
Seventeen microbial species including 10 fungal taxa, two yeasts and five bacteria, were isolated from freshly prepared orange, guava and banana juices kept in open bottles at room temperature for 7 days. Eight different essential oils, from local herbs, were tested for their antimicrobial activity against these test organisms. The essential oils of Cymbopogon citratus, Ocimum basilicum and Origanum majorana were found to be highly effective against these microorganisms. Aspergillus niger, A. flavus and Saccharomyces cerevisiae, the most prevalent microorganisms in juice, showed the highest resistance against these essential oils. GC-MS analysis showed that while e-citral, a'-myrcene, and z-citral represent the major components (75.1%) of the essential oil of Cymbopogon citratus; bezynen,1-methyl-4-(2-propenyl), 1,8-cineole and trans-a'-bisabolene were the main components (90.6%) of Ocimum basilicum; whereas 3-cyclohexen-1-01,4-methyl-1(1-methylethyl)-(CAS), c-terpinene and trans-caryophyllene represent the major components (65.1%) of Origanum majorana. These three essential oils were introduced into juices by two techniques namely, fumigation and direct contact. The former technique showed more fungicidal effect than the latter one against A. flavus, A. niger, and S. cerevisiae. The essential oil of Cymbopogon citratus by comparison to other test oils showed the strongest effect against these fungi with a minimum inhibitory concentration of 1.5 µl/ml medium and a sublethal concentration of 1.0 µl/ml. The antimicrobial activity of this oil is thermostable at 121℃ for 30 min.
Keywords: Antimicrobial activity, Bacteria, Essential oils, Fungi, Yeast
Essential oils of plants and their main components show antimicrobial activity against a wide range of microorganisms including antibiotic-resistant species of bacteria and fungi (Alviano et al., 2005; Carson and Riley, 1995; El-Kabouss et al., 2002; El-Kady et al., 1993). They can also affect both yeast and filamentous fungi (Bishop and Thornton, 1997; Delaquis et al., 2002; Gowda et al., 2004; Krauze-Baranowska et al., 2002; Vagi et al., 2005) in addition to Gram-positive and Gram-negative bacteria (Ali et al., 2002; Sechi et al., 2001). Variable results have been observed depending on origin of antimicrobial substance; testing conditions and target microorganisms. The essential oil of C. citratus completely inhibited the growth of Neurospora sitophila, Penicillium digitatum, and Aspergillus parasiticus (Shadab et al., 1992), and also exhibited fungal toxicity against Aspergillus flavus (Mishra and Dubey, 1994). Its aqueous extracts completely inhibited the growth of Macrophomina phaseolina and Botryodiplodia theobramae, while it significantly reduced the growth of Gibberella fujikuroi and Fusarium solani (Bankole and Adebanjo, 1995). EL-Kamali et al. (1998) noticed that the essential oils of Nigella sativa seeds, Cymbopogon citratus leaves and Pulicaria undulata aerial parts (collected from Sudan) exhibited activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa. Recently Mejlholm and Dalgaard (2002) found that oils of Oregano and Cinnamon showed the strongest antimicrobial activity against Photobacterium phosphorium, followed by lemongrass, thyme, clove, bay, marjoram, sage and basil oils. The essential oil of Origanum majorana L., at the concentration of 1ml/ml, inhibited both A. niger and Trichoderma viride (Vagi et al., 2005).
The present investigation aimed at studying the antimicrobial activity of essential oils from some Egyptian plants against molds, yeasts and bacteria associated with the contamination of fruit juices and accordingly the possibility of using these oils as natural preservatives.
Materials and Methods
Juices
They were freshly prepared from fresh fruits of orange, guava, and banana collected from the local market of Zagazig City, Sharkia Governorate, Egypt. Fruits were washed several times with water, dried and peeled with a sterile knife. Under clean conditions berries were squeezed using handle machines or electrical blenders. The resultant juice was filtered through pasteurized double layers of cheesecloth and stored for seven days at room temperature in opened presterilized juice bottles.
Source of essential oils
The essential oils of Ocimum basilicum L. (Basil), Carium carvi L. (Caraway), Foeniculum vulgare Mill. (Fennel), Pelargonium radula L. Herit (Geranium), Cymbopogon citratus Stapf (lemongrass), Origanum majorana L. (Origanum), Mentha piperita L. (Peppermint) and Thymus capitatus (L.) Hoffmg. & Link (Thyme), were kindly supplied by Sekem Company, Hikstep, Cairo Governorate, Egypt.
Isolation
Bacteria, yeast and molds were isolated from deteriorated fruit juices using the dilution plate method (Johnson et al., 1959) on nutrient, Sabouraud's and Czapek's agar media respectively. Serial dilutions (from 10-2 to 10-5) were prepared from each juice. One ml aliquots from the appropriate dilution were transferred aseptically to each sterile Petri-dish. Agar plates of the three mentioned media were poured aseptically into the Petri-dishes. Five replicates for each dilution from each media.
The agar plates were incubated at 37℃ for 24 hr in case of bacteria and yeast and 28℃ for 5 days in case of filamentous fungi. Developing colonies of bacteria, yeasts and molds were counted, identified, and pure colonies were obtained.
Culture media
Thee media namely, nutrient agar, Sabouraud's and Czapek's were prepared according to Gams et al. (1998). Nutrient medium (g/l); bactopeptone (Difco), 5.0; beef extract (Difco), 5.0 and NaCl, 5.0. Sabouraud's medium (g/l); peptone (Difco), 10.0; glucose, 40.0; KH2PO4, 1.0; MgSO4·7H2O, 0.5. Czapek's medium (g/l); sucrose, 30.0; NaNO3, 3.0; KH2PO4, 1.0; KCl, 0.5; MgSO4·7H2O, 0.5. Agar 15 g/l was added for solidification. The media are sterilized by autoclaving at 121℃ for 15 min, unless stated otherwise. The pH of the media was adjusted to 7.0 for bacteria, 6.5 yeast growth and 5.5 for fungal growth before autoclaving. To suppress bacterial growth in case of yeast and molds isolation, 1 ml of Streptomycin solution was added to each Petri-dish before pouring media, to give as final concentration of 30~35 ppm.
Identification of microbial isolates
Bacillus subtilis, B. cereus, Staphylococcus aureus, Escherichia coli and Pseudomonas sp. were identified according to Bergey's manual of determinative bacteriology (Holt et al., 1986). Saccharomyces cerevisiae and Candida albicans were identified according to Barnett et al. (2000). Fungal isolates were purified using single spore technique and identified according to Domsch et al. (1980), Kitch and Pitt (1992), Moubasher (1993), Pitt (1979), Pitt (1986), Raper and Fennell (1977) and Raper and Thom (1968).
Antimicrobial assay of essential oils
Preparation of inocula
Inocula were prepared by growing bacterial cells in nutrient broth medium and yeast cells in Sabouraud's medium at 37℃ for 24 hr. These cell suspensions were diluted with the same broth medium to provide initial cell counts of about 105 CFU (colony forming unit)/ml. An aliquot of 1 ml is used each experiment. Test fungi were cultured on Czapek's medium, where each flask was inoculated with a mycelial disc of 5 mm diameter of five days old fungal culture.
Screening for the antimicrobial effect of essential oils
Well cut diffusion method was used in this survey. Culture plates seeded with the desired tested organisms were used in this test. Holes of "1 cm diameter" were cut using a sterilized cork borer. After which drops of water agar (15 g/l) were put in holes, then 50 µl of each essential oil were introduced into each hole. The plates were put in the refrigerator for 2 hr and incubated at 37℃ for 24 hr for yeast and bacteria and at 28℃ for 5 days for fungi. After incubation, plates were viewed and the diameters of inhibition zones were determined.
Determination of minimal inhibitory concentration (MIC) of essential oils
The MIC of each oil under test was determined using two techniques namely, contact and fumigation methods.
The contact method
Broth media were prepared and sterilized in 100 ml capacity conical flasks, each containing 20 ml of the culture medium. Different amounts of the essential oils under test were added to sterilized broth medium to give the following concentration per ml: 10, 5, 4, 3, 2, 1.5, 1 and 0.5 µl. One ml Tween-80/l broth medium was added as emulsifying agent. Tween-80 (1 ml/l) did not exhibit antifungal activity. Flasks were then inoculated with one disc of 5 mm diameter for each flask of seven day old fungal culture of test fungi. While in case of yeast, flasks were inoculated with 1 ml cell suspension to give about 105 CFU/ml from 48 hr old culture. Flasks were then incubated at 28℃ for 5 days for fungal growth and at 37℃ for 24 hr for yeast cultures.
Standard curve of turbidity and cell number (per ml) was made for yeast by growing them under the same culture condition (without addition of any of the essential oils). Number of cells per ml was counted using serial dilution method as previously mentioned. At the end of the incubation period, the optical density (O.D) for yeast cultures was determined at 660 nm (using Spectronic 20 Spectrophotometer) then the cell density was calculated referring the standard curve. On the other hand, fungal growth was filtered through preweighted Whatman No. 1 filter paper. The growth of fungi was estimated gravimetrically by weighting the biomass after having dried at 80℃ until constant weights were reached. The MIC was determined from the lowest concentration at which no growth occurs.
The fumigation method
The details of this method were similar to these of the contact method except for the mode of application of the oil and the absence of Tween-80. Hence, the desired amount of the essential oil was aseptically absorbed on a piece of round, sterile filter paper suspended at the top of flasks.
 |
Determination of the main components of the most effective essential oils
The main components of three oils showing the greatest activity were determined by chemical analyses using Gas Chromatography/Mass Spectrometry (GC/MS) model Vinigan Mat SQQ 7000 at The National Research Center, Giza, Egypt.
Fungicidal-fungistatic nature of essential oils
Fungicidal-fungistatic nature of the oils was detected by the technique of Thompson (1989). According to which one ml of each of oil-inhibited yeast or mold broth medium was reinoculated onto plates of Sabouraud's or Czapek's agar medium and the revival of growth has been recorded. The appearance of new growth means this concentration of oil is fungistatic and the absence of growth means the concentration is fungicidal.
Investigation of the thermal stability of C. citratus essential oil
Discs of filter paper impregnated with sublethal concentrations SLC and MIC of C. citratus essential oil were suspended in the flasks containing media before and after autoclaving at 1.5 atmosphere for 30 min. At the end of the experiment the antifungal activity of the oil was determined as percentage of growth inhibition.
Results
Fungi and bacteria of deteriorated juice
A total of 12 genera and 17 species of microorganisms were isolated from deteriorated juices (Table 1). Orange showed a total colony count of (all organisms) 16.6 × 104, guava 7.9 × 104 and banana 6.68 × 104 CFU/ml.
Table 1.
Microbial counts and changes of pH of fresh juice exposed to air for seven days at room temperature
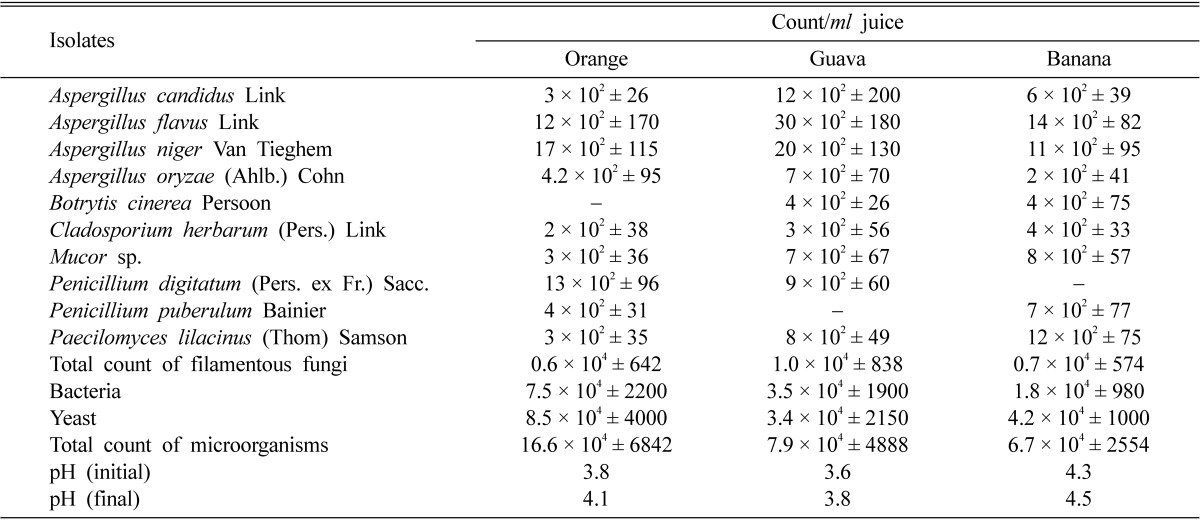
The values are mean of five replicates ± standard deviation.
Molds isolated on Czapek agar medium pH 5.5, yeasts isolated on Sabouraud's agar medium pH 6.5 and bacteria isolated on Nutrient agar medium pH 7.
Fungal counts/ml juice in descending order was 1 × 104 for guava, followed by 0.7 × 104 for banana, and 0.6 × 104 for orange. These counts belonged to 10 taxa. As for genera isolated, Aspergillus was the most predominant fungus by showing total counts of 3.6 × 103, 6.9 × 103 and 3.3 × 103 respectively from orange, guava and banana juices. It was represented by 4 species namely; A. candidus, A. flavus, A. niger and A. oryzae. Each of these species was isolated from the three juices under test. The second genus in view of total count was Penicillium. It showed total counts of 1.7 × 103, 0.9 × 103 and 0.7 × 103/ml in orange, guava and banana juices, respectively. It represented by only 2 species of which P. digitatum from orange and guava juice with total counts 1.3 × 103 and 0.9 × 103/ml and P. puberulum isolated from orange and banana juices with total counts of 4 × 102 and 7 × 102/ml, respectively. The remaining genera were represented by one species each. B. cinerea was isolated from guava and banana juices, C. herbarum, Mucor sp. and P. lilacinus were isolated from the three juices under investigation. Yeasts with total counts of 8.5 × 104, 3.4 × 104 and 4.2 × 104/ml of orange, guava and banana juices, respectively were predominant and represented by C. albicans and S. cerevisiae.
Total counts of 7.5 × 104, 3.4 × 104 and 1.8 × 104/ml bacteria were isolated from orange, guava and banana juices and were represented with 4 genera (two Gram-positive and two Gram-negative). The two Gram-positive genera namely; Bacillus, represented by B. subtilis & B. cereus and S. aureus, while the two Gram-negative genera were E. coli and Pseudomonas sp.
Antimicrobial activity of essential oils against fungi and bacteria isolated from fruit juices
Data presented in Table 2 indicated that all essential oils under test process showed antimicrobial activity against molds, yeasts and bacteria. Most of these oils delayed conidiation of fungi. The feature of antimicrobial activity varied not only from one essential oil to another but also among microorganisms. The antimicrobial activity of all tested oils was generally higher against bacteria than fungi and yeast. Botrytis cinerea and Bacillus cereus revealed the highest sensitivity to essential oils, while P. digitatum and Candida albicans revealed the lowest sensitivity. B. cinerea failed completely to grow in the presence of any of the eight essential oils. The oil of lemongrass (Cymbopogon citratus) was the most active against both molds and bacteria. In view of antimicrobial activity the oil of basil (Ocimum basilicum) comes next to lemongrass followed by thyme (Thymus capitatus) and origanum (Origanum majorana). By comparison, the remaining four essential oils showed weaker effects. In descending order activity, they came as follows: Mentha piperita, Foeniculum vulgare, Pelargonium radula, and Carium carvi.
Table 2.
Antimicrobial activity as diameter of inhibition zone (mm) of the tested essential oils 50 µl/hole against molds, yeasts and bacteria isolated from fresh juices of orange, guava and banana
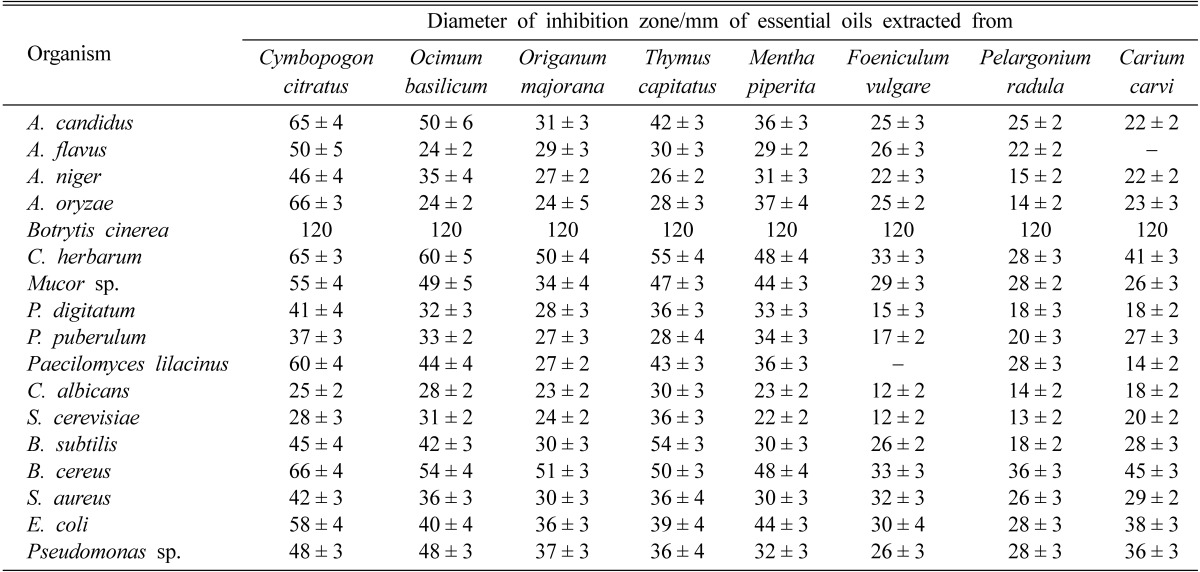
The values are mean of three replicates ± standard deviation.
The data in the present part of study revealed that the tolerance to the essential oils under test was found to be higher in filamentous fungi than that of bacteria and yeast fungi. Based on this observation the present study was extended to investigate the effect of these oils on the growth of A. flavus, A. niger and S. cerevisiae as test organisms (for being very common).
Analysis of the most effective oils
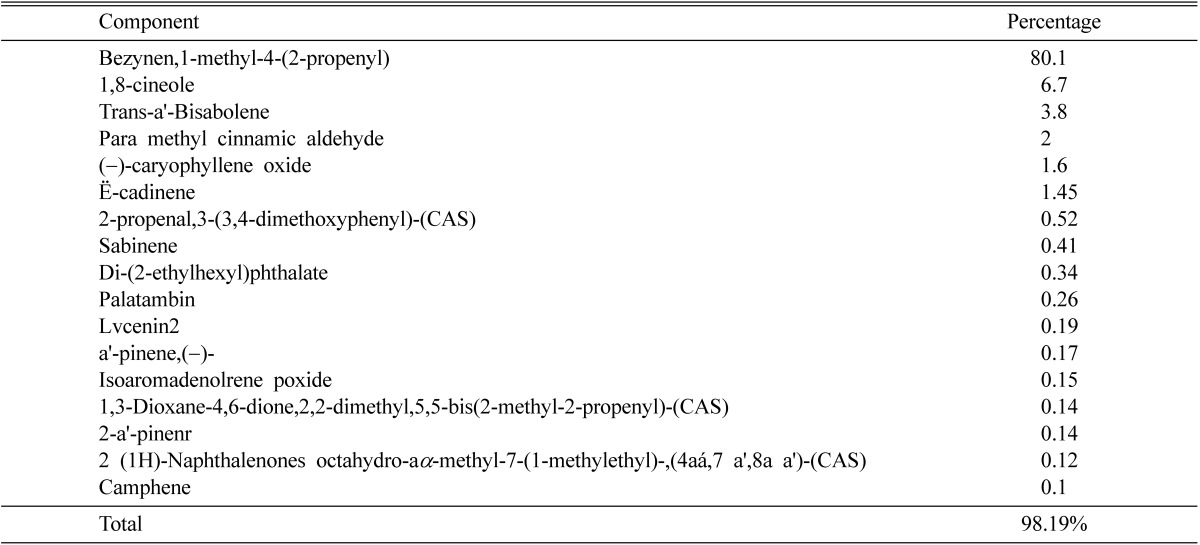
Given in Table 3~5 the main components of the essential oils of the three plants; Cymbopogon citratus, Ocimum basilicum and Origanum majorana. Where the data clearly indicated that the components are much different and the number of components in common is very low.
Table 3.
Percentages of components of essential oil extracted from Cymbopogon citratus

Table 5.
Percentages of components of essential oil extracted from Origanum majorana
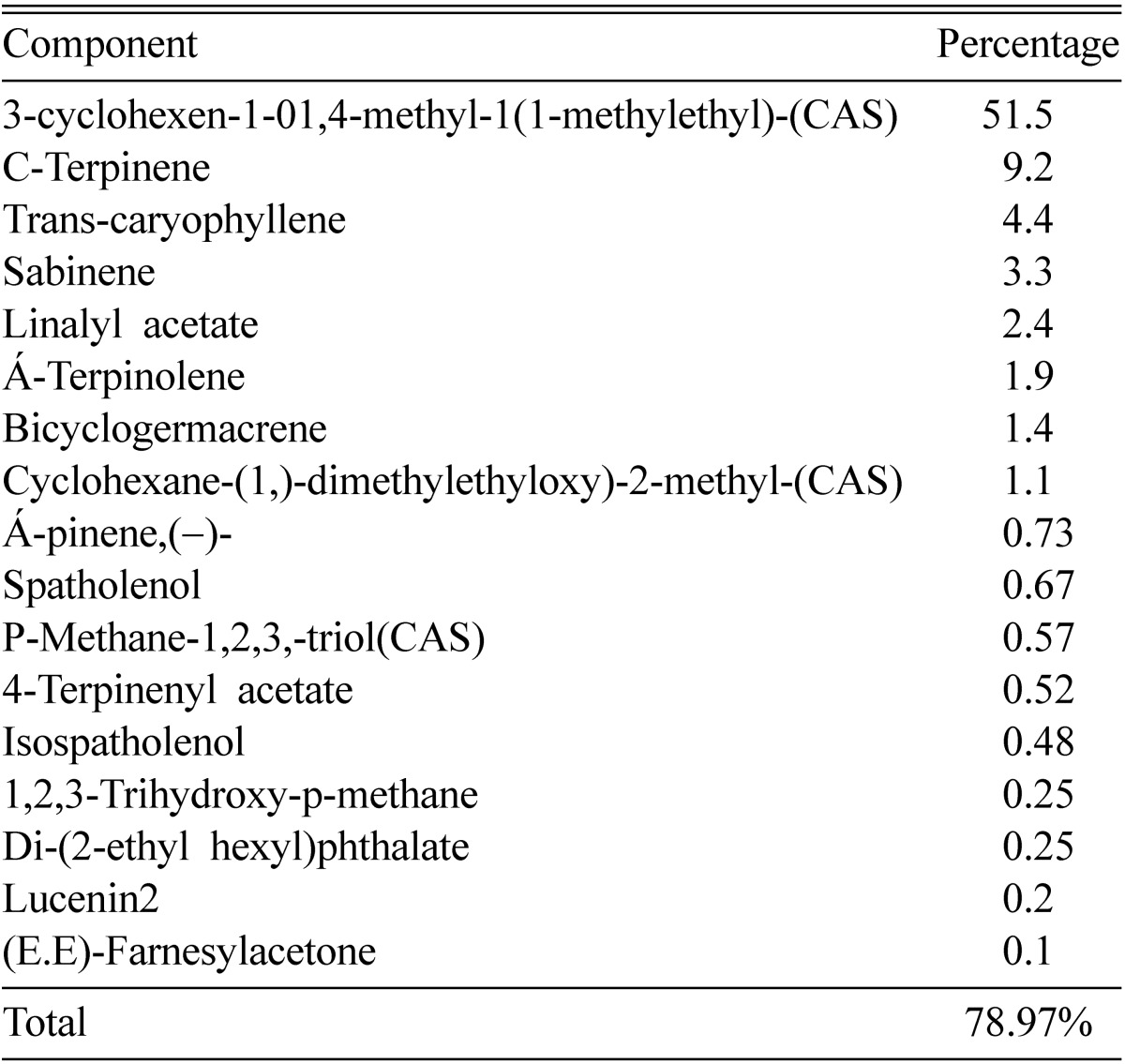
Table 3 showed that lemongrass contained 19 compounds accounting for 94.8% of the total oil components. E-citral is the major component of this oil (65.4%). Table 4 revealed that basil oil comprised 17 compounds constituting 98.19% of the total oil components. Bezynen,1-methyl-4-(2-propenyl) is the major component (80.1%). Table 5 showed that the oil of origanum contain a range of also 17 compounds accounting for 78.97% of total oil components. 3-cyclohexen-1-01,4-methyl-1(1-methylethyl)-(CAS) is the major component (51.5%).
Table 4.
Percentages of components of essential oil extracted from Ocimum basilicum
Minimum inhibitory concentration (MIC)
In Table 6 the inhibition effect of essential oils on the test fungi was studied. Oils were introduced in different concentrations as fumigants and as contact materials. The data of this Table clearly indicated that fumigation as a technique is more effective than using the contact method. Growth of A. flavus and A. niger was completely suppressed by fumigation with C. citratus at a concentration of 1.5 µl/ml. Fumigation with both of O. majorana or O. basilicum at a concentration of 5.0 µl/ml was required to inhibit growth of A. flavus. Growth of A. niger was completely inhibited by O. basilicum essential oil at a concentration of 4.0 µl and more than 5.0 µl of O. majorana essential oil/ml of growth medium. Application of these oils by the contact technique showed that higher concentrations of oils are required to induce suppression. Growth of A. flavus and A. niger required 2.0 µl of C. citratus and 10.0 µl or more of O. majorana essential oils/ml medium. Also higher concentrations of the essential oil of O. basilicum were required to inhibit growth of A. flavus and A. niger, while the former required 5.0 ml/ml the later required 4.0 µl/ml.
Table 6.
Effect of different concentrations of essential oils on the growth of test organisms
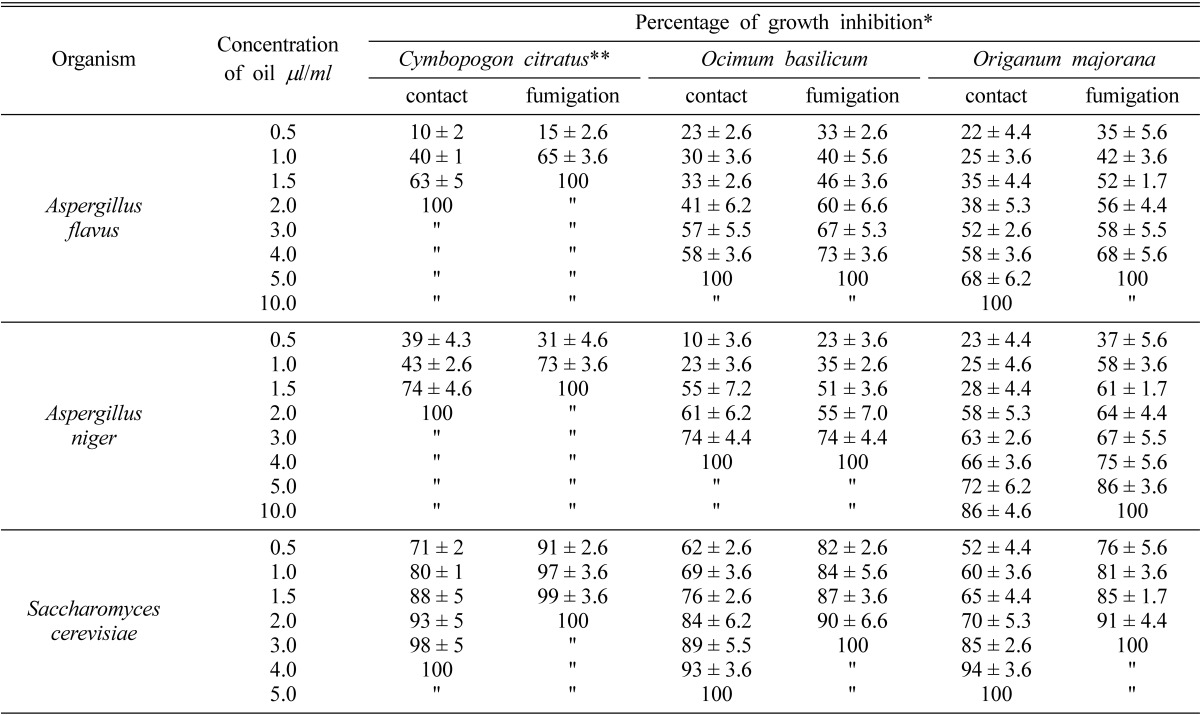
The values are mean of three replicates ± standard deviation (P < 0.01).
A. flavus & A. niger grown on Czapek's broth medium pH 5.5 at 28℃ for 5 days and S. cerevisiae grown on Sabouraud's broth medium pH 6.5 at 37℃ for 24 hr.
*For percentage of growth inhibition sees Materials and Methods.
**The results of Cymbopogon citratus were previously mentioned by Helal et al. (2006a & b; 2007).
As for S. cerevisiae data indicated that fumigation of Sabouraud's broth medium with 2.0 µl/ml of C. citratus or 3.0 µl/ml of either O. majorana or O. basilicum completely inhibited growth of S. cerevisiae. Meanwhile, 4.0 µl of C. citratus or 5.0 µl of O. basilicum or O. majorana prevented the growth of the yeast when any of these oils were applied by contact technique.
Resubculture inocula from the three test fungi inhibited by MICs of the three essential oils into nonfumigated media were negative, confirming the fungicidal effect of these concentrations. Also, C. citratus essential oil was the highest in its antimicrobial activity than the other two oils, and the sublethal (SLC) dose of this oil (1 µl/ml medium) when applied by fumigation inhibit 65%, 73% and 97% of A. flavus, A. niger and S. cerevisiae growth, respectively.
Thermal stability of C. citratus essential oil as antifungal agent
For being the highest in antimicrobial activity with a sublethal dose of 1 µl/ml when applied by fumigation technique C. citratus was selected to study its thermal stability. The data presented in Table 7 demonstrate that the percentage of growth inhibition is nearly the same and there is no significant difference between the antifungal activity of autoclaved and nonautoclaved C. citratus oil at SLC and MIC against the three test fungi.
Table 7.
Effect of autoclaving on the antimicrobial activity of Cymbopogon citratus oil fumigated against test organisms
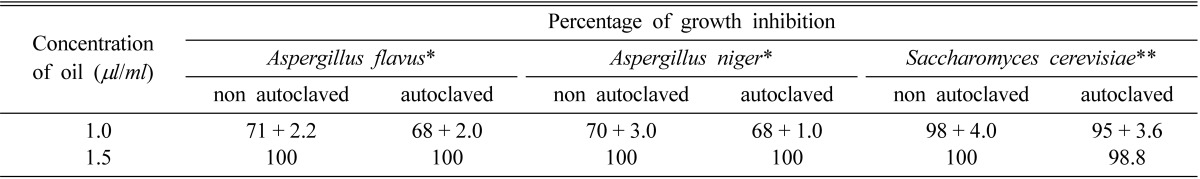
The values are mean of three replicates ± standard deviation.
*A. flavus and A. niger grown on Czapek's broth medium pH 5.5 at 28℃ for 5 days.
**S. cerevisiae grown on Sabouraud's broth medium pH 6.5 at 37℃ for 24 hr.
Discussion
In the present study, microbial count (CFU/ml) of deteriorated juice showed that orange hold higher counts by comparison to guava and banana. Counts of total fungi (Molds and yeasts) were higher than that of bacteria. The increasing in fungal count should be attributed to the high acidic pH values of these fruit juices "3.6~4.3" (Braddock, 1999; Chen et al., 1993). The most prevalent fungi were species belonging to the genera of Aspergillus (4 species), Penicillium (2 species) and yeasts (2 species). In a previous study of Hemida (2004) found that many of the preceding species especially A. niger, A. flavus, P. digitatum and S. cerevisiae were also predominant among the mycoflora associated with several juices and foodstuffs. Fermentative yeasts, particularly S. cerevisiae, are known to be common spoilage agents in refrigerated citrus juices, because of their capacity to produce copious amounts of CO2 and ethanol (Braddock, 1999; Chen et al., 1993). Essential oils and their constituents are contemporary applied in food preservation and in the manufacture of medicinal antimicrobial agents and disinfectants (Voda et al., 2003). Botrytis cinerea is the only organism that failed completely to grow in the presence of any of the eight oils tested (Table 2). Except caraway oil (against A. flavus) and fennel oil (against Paecilomyces lilacinus), all essential oils tested in the present study showed inhibitory activity against all microorganisms isolated from orange, guava and banana juices. Inhibition of microbial growth by essential oils has been previously recorded. While Romagnoli et al. (2005) noticed that P. digitatum was inhibited with 1.25 µl/ml and B. cinerea with 10 µl/ml of Tagetes patula essential oil, Daferea et al. (2000) also noticed that 400 µl/ml Origanum majorana essential oil totally inhibited the mycelial growth of P. digitatum. The same oil inhibited the growth of the common spoilage fungus A. niger at the concentration of 10 µl/ml broth with 91.5% inhibition effect (Baratta et al., 1998). In the present study, lemongrass oil revealed the greatest potential of antimicrobial activity against all test organisms (10 molds, 2 yeasts and 5 bacteria). This oil was followed by basil, thyme and origanum oils. Dube et al. (1989) showed that the essential oil of Ocimum basilicum at a concentration of 1.5 ml/l completely suppressed the mycelial growth of 22 species of fungi including the mycotoxin producing strains of A. flavus and A. parasiticus. In a comprehensive study by Pattnaik et al. (1996) the antimicrobial activity of some essential oils against 22 bacterial strains, including Gram-positive cocci and rods, Gram-negative rods and 12 fungi were studied using disc diffusion method. Lemongrass, eucalyptus and peppermint essential oils were found to be effective against all tested bacterial strains. They noticed also that aegle and palmarosa oils inhibited 21 bacteria, patchouli and ageratum oils inhibited 20, and citronella and geranium oils were inhibitory to 15 and 12 strains, respectively. All the test fungal species were inhibited by 7 oils namely aegle, citronella, geranium, lemongrass, orange, palmarosa and patchouli. Eucalyptus and peppermint oils were effective against 11 fungal species. Ageratum oil was inhibitory to only 4 of the test fungi. The minimum inhibitory concentration of eucalyptus, lemongrass, palmarosa and peppermints oils ranged from 0.16 µl/ml to 720 µl/ml for 18 bacteria and from 0.25 µl/ml to 10 µl/ml for 12 fungi. A screening of the level of inhibitory activity among 51 essential oils tested by Hili et al. (1997) using drop diffusion method, showed that the value varied from 0.3 to 90% of total growth of Escherichia coli, Staphylococcus aureus, Schizosaccharomyces pombe, Saccharomyces cerevisiae, Candida albicans and Torulopsis utilis. In a comparison study, Mejlholm and Dalgaard (2002) observed that Oregano, cinnamon and lemongrass oils possess strong antimicrobial activity by comparison with thyme, clove, bay marjoram, sage and basil oils. They reduced the growth rate of the seafood spoilage microorganism Photobacterium phosphoreum. In a study by Guynot et al. (2003) showed that out of 17 essential oils tested only seven namely; cinnamon leaf, lemongrass, thyme, bay, clove, peppermint and basil inhibited growth of Eurotium amstelodami, E. herbariorum, E. repens, E. rubrum, A. flavus, A. niger and P. corylophilum commonly causing deterioration of bakery products. Nielsen and Rios (2000) have proved that volatile substances from mustard, cinnamon, garlic and clove essential oils were efficient in the control of common bread spoilage fungi. However comparison of the data obtained by different studies is difficult; because of differences in plants extract compositions, in methodologies followed to assess antimicrobial activity and in test microorganisms chosen (Hammer et al., 1999).
Essential oils are natural mixtures of hydrocarbons (terpenes), oxygen-containing (alcohols, aldehydes, ketones, carboxylic acids, ethers, lactones) and sulfur-containing (sulfides, disulfides and trisulfides) organic substances of plant and animal origin (Voda et al., 2003). El-Kady et al. (1993) stated that there is a relationship between the chemical structure of the most abundant compounds in the essential oil and the antimicrobial activity. It would also be worthy to be cited here that the composition of any plant essential oil is influenced by the presence of several factors such as; local, climate, plant species, methodology and experimental conditions (Daferea et al., 2000; Mishra and Dubey, 1994; Prudent et al., 1995). These factors may alter the biological and antimicrobial activities of the oils produced (Shu and Lawrence, 1997; Vardar-Ünlü et al., 2003). Distillation time and temperature can also significantly affect the oil constituents (Janssen et al., 1987). The oil chemistry profile obtained from GC-MS analysis of these oils showed that the major components of three tested oils were terpenoids. Cymbopogon citratus oil contain e-citral (65.4%), z-citral (3.0%) and a-myrcene (6.7%) Ocimum basilicum oil contain bezynen,1-methyl1-4-(2-propenyl) 80%, cineole 6.7% and bisabolene 3.8% in, Origanum majorana oil contain 51.5% 3-cyclohexen-1-01,4-methyl-1(1-methylethyl)-(CAS), 9.2% C-terpinene and 4.4% Trans-caryophyllene.. It is well known that terpenoids possess strong antimicrobial activity (Singh et al., 2002; Vagi et al., 2005). Among these terpenoids, Citral, geraniol and citronellol showed the highest antifungal activities (Viollon and Chaumont, 1994). According to Suhr and Nielsen (2003) the main components of Cymbopogon citratus oil are; D-limonene, 3.14%; geranial 4.2%, geranial (citral a), 31.93% and neral (citral b) 45.99%. Chemically, citral is an isomeric mix of geranial and neral, both are well known antimicrobial agents of prominent activity against bacteria and fungi (Guynot et al., 2003; Inouye et al., 2001; Kim et al., 1995). Citral is thought to be responsible for the resistance toward post-harvest fungal infections of lemons (Rodov et al., 1995) and preventing spoilage induced by food borne organisms (Kim et al., 1995). Citral is the main constituent of Cymbopogon citratus essential oil in the present study and also in other studies by Hammer et al. (1999), Inouye et al. (2001) and Friedman et al. (2004). The antimicrobial action of Backhousia citroidora oil was believed to be directly related to its high citral content (Wilkinson et al., 2003). A great number of components such as; terpinene, cineole, pinene, sabinene recorded in the oils of Ocimum basilicum and Origanum majorana were also noticed by Christoph et al. (2000) and Cox et al. (2001) in Melaleuca alternifolia (tea tree oil), in Thymus revolutus oil by Karaman et al. (2001) in T. x-porlock and T. eriocalyx oils by Rasooli and Abyaneh (2004) and also in Origanum vulgare oil by Sahin et al. (2004). In the present study linalool (as a component of lemongrass oil) and linalyl acetate (as a component of origanum oil) are present also in Salvia sclarea essential oil, both exhibited antifungal activity against Sclerotinia sclerotiorum, Sclerotium cepivorum and Fusarium oxysporum (Pitarokili et al., 2002). It has been concluded that the antimicrobial activity of essential oils can differ from that of their major constituents when tested separately probably due to the presence of synergistic or antagonistic effects resulting from the minor components (Lis-Balchin et al., 1998a, b; Pitarokili et al., 2002).
Aspergillus flavus and A. niger are saprophytic molds capable of growing upon a wide range of organic substrates and often cause deterioration of stored food materials (Samson et al., 1995). These species are known to produce mycotoxins notably A. flavus (aflatoxin) and A. niger (nigragillin, malformins, naphthoquinones and oxalic acids) (Frisvad, 1988; Northolt and Soentoro, 1988; Pitt and Hocking, 1997). They are potentially able to cause mycotic diseases to human and other vertebrates (de Hoog et al., 2000). S. cerevisiae is the most common spoilage agent in refrigerated citrus juices (Braddock, 1999; Chen et al., 1993). In the present study growth of these fungi is inhibited by each of the eight essential oils tested (Table 2), especially C. citratus, O. basilicum and O. majorana essential oils. Although the majority of these essential oils are classified as Generally Recognized As Safe (GRAS) (Kabara, 1991), their use in foods as preservatives is often limited due to flavor concentrations, since effective antimicrobial doses may exceed organoleptically acceptable levels. Therefore, there is an increasing demand for accurate knowledge of the minimum inhibitory (effective) concentrations (MIC) of essential oils to enable a balance between the sensory acceptability and antimicrobial efficacy. This could be achieved in vitro using dilution method which provides more quantitative results as recommended by Manou et al. (1998). The present data showed that C. citratus essential oil appear to be more toxic than that of O. basilicum and O. majorana essential oils against A. flavus, A. niger and S. cerevisiae. It caused complete growth inhibition of the three fungi at 1.5 or 2.0 µl/ml medium when applied by fumigation or contact methods, respectively. i.e., the MIC in case of fumigation method is less than that when the oils applied by contact method in the medium. Mishra and Dubey (1994) recorded that lemongrass oil exhibited a broad spectrum of fungitoxicity by inhibiting the growth of 35, 45 and 47 fungal species, which cause deterioration of stored food commodities including A. flavus and A. niger. This oil was also found to be the second highest active as anti Trichophyton among seven oils namely; cinnamon bark, thyme, perilla, lavender, tea tree and citron essential oils. The antifungal activity of these oils against Trichophyton mentagrophytes and T. rubrum was more enhanced by vapor action than solution contact (Inouye et al., 2000, 2001). These authors suggested that this might be caused by the combined effect of vapor action on mycelia or spores and action after absorption on agar. The different MIC values obtained for each of the three essential oils applied by fumigation or contact methods show that the level of antimicrobial activity of essential oils is closely dependent on the screening method used (Delespaul et al., 2000). It has been reported that the antifungal effect of essential oils is dependent on the application method, for example, larger phenolic compounds such as thymol and eugenol (Thyme, cinnamon and clove) had the best effect when applied directly to the medium, whereas smaller compounds such as allyl isothiocyanate and citral (mustard and lemongrass) were most efficient when added as volatiles (Suhr and Nielsen, 2003).
Data obtained in the present study, showed that C. citratus essential oil was not affected when preautoclaved at 121℃ for 30 min. This thermostable nature of the oil fungitoxicity was previously reported by Mishra and Dubey (1994) at concentrations of 1000 and 1500 ppm after 5~100℃ treatments. They added that this oil was also non phytotoxic, exhibited no animal toxicity, more efficacious than 10 synthetic fungicides and its potency is not affected by increasing the density of the inoculums of the tested A. flavus. These advantages and others such as antitoxic property (Helal et al., 2007) and antioxidant activity (Helal et al. unpublished data) increase the possibility of using C. citratus essential oil for juice preservation in future studies.
References
- 1.Ali NM, Hohtar M, Shaari K, Rahmanii M, Ali AM, Jantan I. Chemical composition and antimicrobial activities of the essential oils of Cinnamomum aureofulvum Gamb. J Essent Oil Res. 2002;14:135–138. [Google Scholar]
- 2.Alviano WS, Mendonca-Filho RR, Alviano DS, Bizzo HR, Souto-Padron T, Rodrigues ML, Bolognese AM, Alviano CS, Souza MMG. Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol Immunol. 2005;20:101–105. doi: 10.1111/j.1399-302X.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 3.Bankole SA, Adebanjo A. Inhibition of growth of some plant pathogenic fungi using some Nigerian plants. Int J Tropical Plant Diseases. 1995;13:91–95. [Google Scholar]
- 4.Baratta MT, Dorman HJD, Deans SG, Figueiredo AC, Barroso JG, Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr J. 1998;13:235–244. [Google Scholar]
- 5.Barnett JA, Payne RW, Yarrow D. Yeasts: characteristics and identification. 3rd ed. Cambridge, U.K: Cambridge Uni. Press; 2000. [Google Scholar]
- 6.Bishop CD, Thornton IB. Evaluation of the antifungal activity of the essential oils of Monarda citriodora var. citriodora and Melaleuca alternifolia on post-harvest pathogens. J Essent Oil Res. 1997;9:77–82. [Google Scholar]
- 7.Braddock RJ. Handbook of citrus by products and processing technology. New York: John Wiley & Sons, Inc.; 1999. Single strength orange juices and concentrate; pp. 53–83. [Google Scholar]
- 8.Carson CF, Riley TV. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol. 1995;78:264–269. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen CS, Shaw PE, Parish ME. Orange and tangerine juices. In: Nagy S, Chen CS, Shaw PE, editors. Fruit juice processing technology. Auburndale, Fla: Agscience; 1993. pp. 110–165. [Google Scholar]
- 10.Christoph F, Kaulfers PM, Stahl-Biskup E. A comparative study of the in vitro antimicrobial activity of tea tree oils with special reference to the activity of B-Triketones. Planta Med. 2000;66:556–560. doi: 10.1055/s-2000-8604. [DOI] [PubMed] [Google Scholar]
- 11.Cox SD, Mann CM, Markham JL. Interactions between components of the essential oil of Melaleuca alternifolia. J Appl Microbiol. 2001;91:492–497. doi: 10.1046/j.1365-2672.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- 12.Daferera DJ, Ziogas BN, Polissiou MG. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J Agric Food Chem. 2000;48:2576–2581. doi: 10.1021/jf990835x. [DOI] [PubMed] [Google Scholar]
- 13.de Hoog GS, Guarro J, Gene J, Figueras MJ. Atlas of clinical fungi. 2nd ed. The Netherlands: CBSU; 2000. [Google Scholar]
- 14.Delaquis PJ, Stanich K, Girard B, Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol. 2002;74:101–109. doi: 10.1016/s0168-1605(01)00734-6. [DOI] [PubMed] [Google Scholar]
- 15.Delespaul Q, Debillerbeck VG, Roques CG, Michel G, Marquier-Vinuales C, Bessiere JM. The antifungal activity of essential oils as determined by different screening methods. J Essent Oil Res. 2000;12:256–266. [Google Scholar]
- 16.Domsch KH, Gams W, Anderson T. Compendium of soil fungi. London and New York: Academic Press; 1980. [Google Scholar]
- 17.Dube S, Upadhyay PD, Tripathi SC. Antifungal, physicochemical and insect repelling activity of the essential oil of Ocimum basilicum. Can J Bot. 1989;67:2085–2087. [Google Scholar]
- 18.El-Kabouss A, Charrouf Z, Faid M, Garneau F, Collin G. Chemical composition and antimicrobial activity of the leaf essential oil Argania spinosa L. Skeels. J Essent Oil Res. 2002;14:147–149. [Google Scholar]
- 19.El-Kady IA, El-Maraghy SSM, Mostafa EM. Antibacterial and antidermatophyte activities of some essential oils from spices. Qatar Univ Sci J. 1993;13:63–69. [Google Scholar]
- 20.El-Kamali HH, Ahmed AH, Mohamed AS, Yehia AA, El-Tayeb I, Ali AA. Antibacterial properties of essential oils from Nigella sativa seeds, Cymbopogon citratus leaves and Pulicaria undulata aerial parts. Fitoterapia. 1998;69:77–78. [Google Scholar]
- 21.Friedman M, Henika PR, Levin CE, Mandrell RE. Antibacterial activities of plant essential oils and their components against Escherichia coli 0157:H7 and Salmonella enterica in apple juice. J Agric Food Chem. 2004;52:6042–6048. doi: 10.1021/jf0495340. [DOI] [PubMed] [Google Scholar]
- 22.Frisvad JC. Fungal species and their specific production of mycotoxins. In: Sanson RA, Van Reenen-Hoekstra ES, editors. Introduction to Foodborne Fungi. Baarn, The Netherlands: Centraalbureau Voor Schimmelcultures; 1988. pp. 239–249. [Google Scholar]
- 23.Gams W, Hoekstora ES, Aptroot A. CBS course of mycology. 4th ed. Baarn, The Netherlands: Centraalbureau Voor Schimmelcultures; 1998. [Google Scholar]
- 24.Gowda NKS, Malathi RU, Suganthi RU. Effect of some chemical and herbal compounds on growth of Aspergillus parasiticus and aflatoxin production. Animal Feed Sci Tech. 2004;116:281–291. [Google Scholar]
- 25.Guynot ME, Ramos AJ, Seto L, Purroy P, Sanchis V, Marin S. Antifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products. J Appl Microbiol. 2003;94:893–899. doi: 10.1046/j.1365-2672.2003.01927.x. [DOI] [PubMed] [Google Scholar]
- 26.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 27.Hemida SK. Influence of some commercial oils on growth of fungi and their degrading enzymes. Egypt J Biomed Sci. 2004;16:365–380. [Google Scholar]
- 28.Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-Khair EM. Effect of Cymbopogon citratus L. essential oil on the growth and morphogenesis of Saccharomyces cerevisiae ML2 strain. J Basic Microbiol. 2006a;46:375–386. doi: 10.1002/jobm.200510084. [DOI] [PubMed] [Google Scholar]
- 29.Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-Khair EM. Effect of Cymbopogon citratus L. essential oil on the growth and morphogenesis of Aspergillus niger ML2 strain. J Basic Microbiol. 2006b;46:456–469. doi: 10.1002/jobm.200510106. [DOI] [PubMed] [Google Scholar]
- 30.Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-Khair EM. Effect of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2 strain. J Basic Microbiol. 2007;47 doi: 10.1002/jobm.200610137. (in press) [DOI] [PubMed] [Google Scholar]
- 31.Hili P, Evans CS, Veness RG. Antimicrobial action of essential oils: the effect of dimethylsulphoxide on the activity of cinnamon oil. Lett Appl Microbiol. 1997;24:269–275. doi: 10.1046/j.1472-765x.1997.00073.x. [DOI] [PubMed] [Google Scholar]
- 32.Holt JG, Sneath PHA, Mair MS, Sharpee ME. Bergey's Manual of systematic bacteriology, vol. 2. 428 east Preston Street, Baltemore, MD 211202, U.S.A: Williams & Wilkins; 1986. [Google Scholar]
- 33.Inouye S, Tsuruoka M, Watanabe M, Takeo K, Akao M, Nishiyama Y, Yamaguchi H. Inhibitory effect of essential oils on apical growth of Aspergillus fumigatus by vapour contact. Mycoses. 2000;43:17–23. doi: 10.1046/j.1439-0507.2000.00538.x. [DOI] [PubMed] [Google Scholar]
- 34.Inouye S, Tsuruoka T, Uchida K, Yamaguchi H. Effect of sealing and Tween 80 on the antifungal susceptibility testing of essential oils. Microbiol Immunol. 2001;45:201–208. doi: 10.1111/j.1348-0421.2001.tb02608.x. [DOI] [PubMed] [Google Scholar]
- 35.Janssen AM, Scheffer JJC, Baerheim Svendeaen A. Antimicrobial activity of essential oils: a 1976-86 literature review. Aspects of the test methods. Planta Med. 1987;53:395–398. doi: 10.1055/s-2006-962755. [DOI] [PubMed] [Google Scholar]
- 36.Johnson LF, Curl EA, Bond JH, Fribourg HA. Methods for studying soil microflora-plant diseases relationships. Minneqpolis: Burgess; 1959. [Google Scholar]
- 37.Kabara JJ. Phenols and chelators. In: Russell NJ, Gould GW, editors. Food Preservatives. Blackie: London; 1991. pp. 200–214. [Google Scholar]
- 38.Karaman S, Digrak M, Ravid V, Ilcim A. Antibacterial and antifungal activity of the essential oils of Thymus revolutus Celak from Turkey. J Ethnopharmacol. 2001;76:183–186. doi: 10.1016/s0378-8741(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Marshall MR, Wei C. Antibacterial activity of some essential oil components against five food borne pathogens. J Agric Food Chem. 1995;43:2839–2845. [Google Scholar]
- 40.Kitch MA, Pitt JI. A laboratory guide to the common Aspergillus species and their Teleomorphs published by common wealth scientific and Industrial Research Organization. Sydney: Division of Food Processing (CSIRO); 1992. [Google Scholar]
- 41.Krauze-Baranowska M, Mardarowicz M, Wiwart M, Poblocka L, Dynowska M. Antifungal activity of the essential oils from some species of the genus Pinus. Z Naturforsch. 2002;57:478–482. doi: 10.1515/znc-2002-5-613. [DOI] [PubMed] [Google Scholar]
- 42.Lis-Balchin M, Buchbauer G, Hirtenlehner T, Resch M. Antimicrobial activity of Pelargonium essential oils added to a quiche filling a model food system. Lett Appl Microbiol. 1998a;27:207–210. doi: 10.1046/j.1472-765x.1998.t01-1-00423.x. [DOI] [PubMed] [Google Scholar]
- 43.Lis-Balchin M, Deans SG, Eaglesham E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr J. 1998b;13:98–104. [Google Scholar]
- 44.Manou L, Bouillard L, Devleeschouwer MJ, Barel AO. Evaluation of the preservative properties of Thymus vulgaris essential oil in topically applied formulations under a challenge test. J Appl Microbiol. 1998;84:368–376. doi: 10.1046/j.1365-2672.1998.00353.x. [DOI] [PubMed] [Google Scholar]
- 45.Mejlholm O, Dalgaard P. Antimicrobial effect of essential oils on the seafood spoilage microorganism Photobacterium phosphoreum in liquid media and fish products. Lett Appl Microbiol. 2002;34:27–31. doi: 10.1046/j.1472-765x.2002.01033.x. [DOI] [PubMed] [Google Scholar]
- 46.Mishra AK, Dubey NK. Evaluation of some essential oils for their toxicity against fungi causing deterioration of stored food commodities. Appl Environ Microbiol. 1994;60:1101–1105. doi: 10.1128/aem.60.4.1101-1105.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moubasher AH. Soil fungi in Qatar and other Arab countries. centre for scientific and applied research. University of Qatar. The Doha Modern Printing Press; 1993. [Google Scholar]
- 48.Nielsen PV, Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol. 2000;60:219–229. doi: 10.1016/s0168-1605(00)00343-3. [DOI] [PubMed] [Google Scholar]
- 49.Northolt MD, Soentoro PSS. In: Introduction to Foodborne Fungi. Samson RA, Van Reenen-Hoekstra ES, editors. Barrn, The Netherlands: Centraalbureau voor Schimmelculures; 1988. [Google Scholar]
- 50.Pattnaik S, Subramanyam VR, Kole C. Antibacterial and Antifungal activity of ten essential oils in vitro. Microbios. 1996;86:237–246. [PubMed] [Google Scholar]
- 51.Pitarokili D, Couladis M, Petsikos-Panayotarou N, Tzakou O. Composition and antifungal activity on soil-borne pathogens of the essential oil of Salvia sclarea from Greece. J Agric Food Chem. 2002;50:6688–6691. doi: 10.1021/jf020422n. [DOI] [PubMed] [Google Scholar]
- 52.Pitt JI. The genus Penicillium and its teleomorphic states Eupenicilium and Talaromyces. London and New York: Academic Press; 1979. [Google Scholar]
- 53.Pitt JI. A laboratory guide to common Penicillium species. Sydney: Commonwealth scientific and Industrial Research Organization (CSIRO). Division of Food Processing; 1986. [Google Scholar]
- 54.Pitt JI, Hocking AD. Fungi and food spoilage. London: Blackie Academic and Professional; 1997. [Google Scholar]
- 55.Prudent D, Perineau F, Bessiere JM, Michel GM, Baccou JC. Analysis of the essential oil of wild oregano form Martinique (Coleus aromaticus Benth.). Evaluation of its bacteriostatic and fungistatic properties. J Essent Oil Res. 1995;7:165–173. [Google Scholar]
- 56.Raper KB, Fennell DI. The genus Aspergillus Robert E. Huntington, New York: Krieger Publishing Company; 1977. [Google Scholar]
- 57.Raper KB, Thom C. A manual of the Penicillium. New York and London: Hafner Publishing Company; 1968. [Google Scholar]
- 58.Rasooli I, Abyaneh MR. Inhibitory effects of Thyme oils on growth and aflatoxin production by Aspergillus parasiticus. Food Control. 2004;15:479–183. [Google Scholar]
- 59.Rodov V, Ben-Yehoshua S, Fang DQ. Ashkenazi preformed antifungal compounds of lemon fruit: citral and its relation to disease resistance. J Agric Food Chem. 1995;43:1057–1061. [Google Scholar]
- 60.Romagnoli C, Bruni R, Andreotti E, Rai MK, Vicentini CB, Mares D. Chemical characterization and antifungal activity of essential oil of capitula from wild Indian Tagetes patula L. Protoplasma. 2005;225:57–65. doi: 10.1007/s00709-005-0084-8. [DOI] [PubMed] [Google Scholar]
- 61.Sahin F, Gulluce MM, Daferera D, Sokmen A, Sokmen M, Polissiou M, Agar C, Ozer H. Biological activities of the essential oils and methanol extract of origanum vulgare ssp. Vulgare in the eastern Anatolia region of Turkey. Food Control. 2004;15:549–557. [Google Scholar]
- 62.Samson AR, Hoekstra ES, Frisvad JC, Filtenborg O. Introduction to food-borne fungi. The Netherlands: CBS; 1995. p. 64. [Google Scholar]
- 63.Sechi LA, Lezcano I, Nunez N, Espim M, Dupre I, Pinna A, Molicotti P, Fadda G, Zanetti S. Antibacterial activity of ozouized sunflower oil (Oleozon) J Appl Microbiol. 2001;90:279–284. doi: 10.1046/j.1365-2672.2001.01235.x. [DOI] [PubMed] [Google Scholar]
- 64.Shadab O, Hanif M, Chaudhary FM. Antifungal activity by lemongrass essential oils. Pakistan J Sci Int Res. 1992;35:246–249. [Google Scholar]
- 65.Shu CK, Lawrence BM. Reasons for the variation in composition of some commercial essential oils. In: Risch SJ, Ho CT, editors. ACS symposium series: Col.660. Spices, Flavor chemistry and antioxidant properties. 1997. pp. 138–159. [Google Scholar]
- 66.Singh G, Singh OP, Maurya S. Chemical and biocidal investigations on essential oils of some Indian Curcuma species. Progress in Crystal Growth and Characterization of Materials. 2002;45:75–81. [Google Scholar]
- 67.Suhr KI, Nielsen PV. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J Appl Microbiol. 2003;94:665–674. doi: 10.1046/j.1365-2672.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- 68.Thompson DP. Fungitoxic activity of essential oil components on food storage fungi. Mycologia. 1989;81:151–153. [Google Scholar]
- 69.Vagi E, Simandi B, Suhajda A, Hethelyi E. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res Int. 2005;38:51–57. [Google Scholar]
- 70.Vardar-Ünlü G, Candan F, Sokmen A, Daferera D, Polissiou M, Sokmen M, Donmez E, Tepe B. Antibacterial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey var. Pectinatus (Lamiaceae) J Agric Food Chem. 2003;51:63–67. doi: 10.1021/jf025753e. [DOI] [PubMed] [Google Scholar]
- 71.Viollon C, Chaumont JP. Antifungal properties of essential oils and their main components upon Cryptococcus neoformans. Mycopathologia. 1994;128:151–153. doi: 10.1007/BF01138476. [DOI] [PubMed] [Google Scholar]
- 72.Voda K, Boh B, Vrtacnik M, Pohleven F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int Biodeterior Biodegrad. 2003;51:51–59. [Google Scholar]
- 73.Wilkinson JM, Hipwell M, Ryan T, Cavanagh HMA. Bioactivity of Backhousia citriodora: Antibacterial and Antifungal activity. J Agric Food Chem. 2003;51:76–81. doi: 10.1021/jf0258003. [DOI] [PubMed] [Google Scholar]


