Abstract
Purpose
The purpose of the current study was to examine the effect of dexamethasone (Dex) at various concentrations on the apoptosis and mineralization of human periodontal ligament (hPDL) cells.
Methods
hPDL cells were obtained from the mid-third of premolars extracted for orthodontic reasons, and a primary culture of hPDL cells was prepared using an explant technique. Groups of cells were divided according to the concentration of Dex (0, 1, 10, 100, and 1,000 nM). A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed for evaluation of cellular viability, and alkaline phosphatase activity was examined for osteogenic differentiation of hPDL cells. Alizarin Red S staining was performed for observation of mineralization, and real-time polymerase chain reaction was performed for the evaluation of related genes.
Results
Increasing the Dex concentration was found to reduce cellular viability, with an increase in alkaline phosphatase activity and mineralization. Within the range of Dex concentrations tested in this study, 100 nM of Dex was found to promote the most vigorous differentiation and mineralization of hPDL cells. Dex-induced osteogenic differentiation and mineralization was accompanied by an increase in the level of osteogenic and apoptosis-related genes and a reduction in the level of antiapoptotic genes. The decrease in hPDL cellular viability by glucocorticoid may be explained in part by the increased prevalence of cell apoptosis, as demonstrated by BAX expression and decreased expression of the antiapoptotic gene, Bcl-2.
Conclusions
An increase in hPDL cell differentiation rather than cellular viability at an early stage is likely to be a key factor in glucocorticoid induced mineralization. In addition, apoptosis might play an important role in Dex-induced tissue regeneration; however, further study is needed for investigation of the precise mechanism.
Keywords: Apoptosis, Cell differentiation, Cell survival, Dexamethasone, Periodontal ligament
INTRODUCTION
Isolation of adult stem cells from the human periodontal ligament (PDL) has presented new opportunities for tissue engineering [1,2]. Following periodontal treatment, periodontal regeneration entails the reformation of all components of the periodontium: the gingival connective tissue, periodontal ligament, cementum, and alveolar bone. Some studies have confirmed that undifferentiated mesenchymal cells in the PDL are essential for osteogenesis and cementogenesis in periodontal tissues and can differentiate into osteoblasts or cementoblasts [3,4].
Various factors affect differentiation of PDL cells into osteoblastic cells, including β-glycerophosphate (β-GP), ascorbic acid (AA), and dexamethasone (Dex). β-GP is known to act as a source of phosphate ion for mineralization, reducing the activity of ALP and protein formation [5] and increasing the formation of matrix vesicles [6]. AA is known to enhance the accumulation of collagen-stimulating osteoblastic phenotypes [7]. Cells from the PDL are heterogeneous, containing several cell types, including fibroblasts and mineralized tissue-forming cells, which require the presence of Dex, a member of the glucocorticoid class of hormones, for osteoblastic differentiation [8-11]. Dex potentiates the effects of other osteogenic cells, including rat calvarial cells [12], on osteoblastic differentiation, and has been used to enhance osteogenic, chondrogenic, and adipogenic differentiation of mesenchymal stromal cells [13]. An increase in apoptosis of osteocytes, hypertrophic chondrocytes, and bone marrow cells was also observed in mice receiving high doses of glucocorticoids [14].
Once differentiated osteoblasts have completed their bone-forming function, they are entrapped in the matrix and become either osteocytes or bone lining cells. However, analysis of available data from histomorphometric examinations of human bone has revealed that 50-70% of osteoblasts at the remodeling site cannot be accounted for [15]. The "missing" osteoblasts appear to have died, possibly by apoptosis, and if apoptosis does occur in osteoblasts or their progenitors, osteoblastogenesis might be diminished [16]. On the other hand, one study reported that apoptotic bodies derived from vascular smooth muscle cells act as nucleating structures for calcium crystal formation [17]. Another study demonstrated that the expression patterns of apoptosis and antiapoptosis-related genes were regulated in a temporal manner by Dex during mineralization of hPDL cells [18] and apoptosis-related factors were associated with the osteoblastic differentiation of hPDL cells [19]. Taken together, an association of differentiation of hPDL cells into osteoblastic cells with apoptosis may be considered. However, the precise mechanism of mineralization and apoptosis of hPDL cells according to the concentration of Dex has not been established and remains to be examined.
The purpose of the current study was to examine the effect of Dex on the apoptosis and osteogenic differentiation of hPDL cells by evaluating cellular viability, alkaline phosphatase (ALP) activity, mineralization, and related genes of hPDL cells induced by various concentrations of Dex.
MATERIALS AND METHODS
Isolation and culture of hPDL cells
PDL tissues were obtained from the mid-third of premolars extracted for orthodontic reasons. Patients provided written consent for harvesting the PDL before extraction, and the primary culture of PDL cells was prepared using an explant technique, described previously [19]. Briefly, the PDL tissues were cut into small pieces and incubated in a biopsy medium at 37℃, 5% CO2, and 95% humidity. The cells were cultured to confluence in 100-mm culture dishes with Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/µL penicillin, and 100 µg/µL streptomycin. After reaching confluence, the cells were passaged with 0.25% trypsin/0.1% ethylenediaminetetraacetic acid. Cells between the third and fifth passage were used in the current experiments. To determine the effects of Dex, the cells were divided into five groups according to the concentration of Dex (0, 1, 10, 100, and 1,000 nM), based on previous reports [20-23]. The medium was changed every three days throughout the entire experiment.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for cellular viability
MTT assay was performed for the evaluation of cellular viability. Four samples per Dex group were evaluated, and the hPDL cells were seeded at a density of 30 cells/mm2; 50 µg/µL AA, 10 mM β-GP, and Dex (0, 1, 10, 100, and 1,000 nM) were added to the medium at a time designated as day 0.
On days 1, 4, and 7, 50 µL of prewarmed (37℃) MTT solution was added to each well and cultured for 3 hours. The reaction was then stopped by the addition of 200 µL of dimethyl sulfoxide (DMSO) and 50 µL of glycine buffer to each well. The solution was then transferred to new wells, and the optical density of the solution in each well was measured at a wavelength of 570 nm using an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Menlo Park, CA, USA).
Measurement of ALP activity
hPDL cells were seeded at a density of 105 cells/mm2 in DMEM containing 10% FBS. When the cells had grown to confluence, it was designated day 0 (after culture for 3 days) and 50 µg/µL AA and 10 mM β-GP were added with Dex (0, 1, 10, 100, and 1,000 nM) to the medium.
On days 5 and 10, briefly, after washing twice with PBS, the cells were homogenized by sonication in 1 µL of 0.9% NaCl and 0.02% Triton X-100 at 4℃ and then centrifuged for 15 minutes at 12,000 rpm. The ALP activity in the supernatant was determined by measuring the amount of p-nitrophenol produced using p-nitrophenol phosphate substrate. Subsequently, the supernatant mixed with 0.5 M Tris-HCl buffer (pH 9.0) containing 0.5 mM p-nitrophenol phosphate and 0.5 mM MgCl2 was incubated at 37℃ for 30 minutes. The reaction was stopped by the addition of 250 µL of 1 N NaOH. Using a DU530 UV spectrometer (Beckman, New Brunswick, NJ, USA), hydrolysis of p-nitrophenyl phosphate was measured at a wavelength of 410 nm. The enzyme activity was normalized to the protein concentration of cells in each well. The ALP activity of the hPDL cells was determined by counting 3 wells per group on days 5 and 10.
Mineralized nodule formation
The hPDL cells were seeded at a density of 105 cells/mm2. When cells had grown to confluence, it was designated day 0 (after culture for 3 days) and 10% FBS, 50 µg/µL AA, and 10 mM β-GP were added with Dex (0, 1, 10, 100, and 1,000 nM) to DMEM. On days 7, 14, 21, and 28, the cells were fixed in 2% paraformaldehyde neutral buffer solution; the plates were then stained with Alizarin Red S for observation of mineralization nodules. The samples were observed using a light microscope.
Real-time PCR
The hPDL cells were seeded at a density of 105 cells/mm2. When the cells had grown to confluence, it was designated day 0 (after culture for 3 days) and 10% FBS, 50 µg/µL AA, and 10 mM β-GP were added with Dex (0, 1, 10, 100, and 1,000 nM) to the medium.
On day 7, the total RNAs were reverse-transcribed to produce cDNA using the ImProm-II reverse transcription system (Promega Inc., Madison, WI, USA) according to the manufacturer's protocol. SYBR Green I-based real-time polymerase chain reaction (PCR) was performed on the MJ Research DNA Engine Opticon Monitor II continuous fluorescence detection system (MJ Research Inc., Waltham, MA, USA). All of the PCR mixtures contained the following: PCR buffer (final concentration 10 mM Tris-Hcl [pH 9.0], 50 mM KCL, 2 mM MgCl2, and 0.1% Triton X-100), 250 mM deoxy-NTP (Roche, Mannheim, Germany), 0.5 mM of each PCR primer, 0.5X SYBR Green I (Molecular Probes, Eugene, OR, USA), 5% DMSO, and 1 U taq DNA polymerase (Promega Inc.) with 2 mL cDNA in a 25 mL final volume reaction mix. The samples were loaded into wells of low profile 96-well microplates. After an initial denaturation step for 1 minute at 94℃, the conditions for cycling were 35 cycles of 30 seconds at 94℃, 30 seconds at 53℃, and 1 minute at 72℃. The fluorescence signal was measured immediately after incubation for 5 seconds at 78℃, followed by the extension step, which eliminates the possible primer dimer. At the end of the PCR cycles, a melting curve was generated for identification of specificity of the PCR product. For each run, serial dilutions of human GAPDH plasmids were used as standards for quantitative measurement of the amount of amplified DNA. In addition, for normalization of each sample, mGAPDH primers were used to measure the amount of mGAPDH cDNA. All of the samples were run three times, the mean data for each group were measured, and the ratio of osteogenesis-related genes (ALP, Cbfa-1, OCN, and OSX) to GAPDH, and apoptosis-related genes (Bcl-2, BAX) to GAPDH were obtained. The sequences of primers used for the real-time PCR analysis are shown in Table 1.
Table 1.
Primer sequences used in reverse transcriptase polymerase chain reaction.
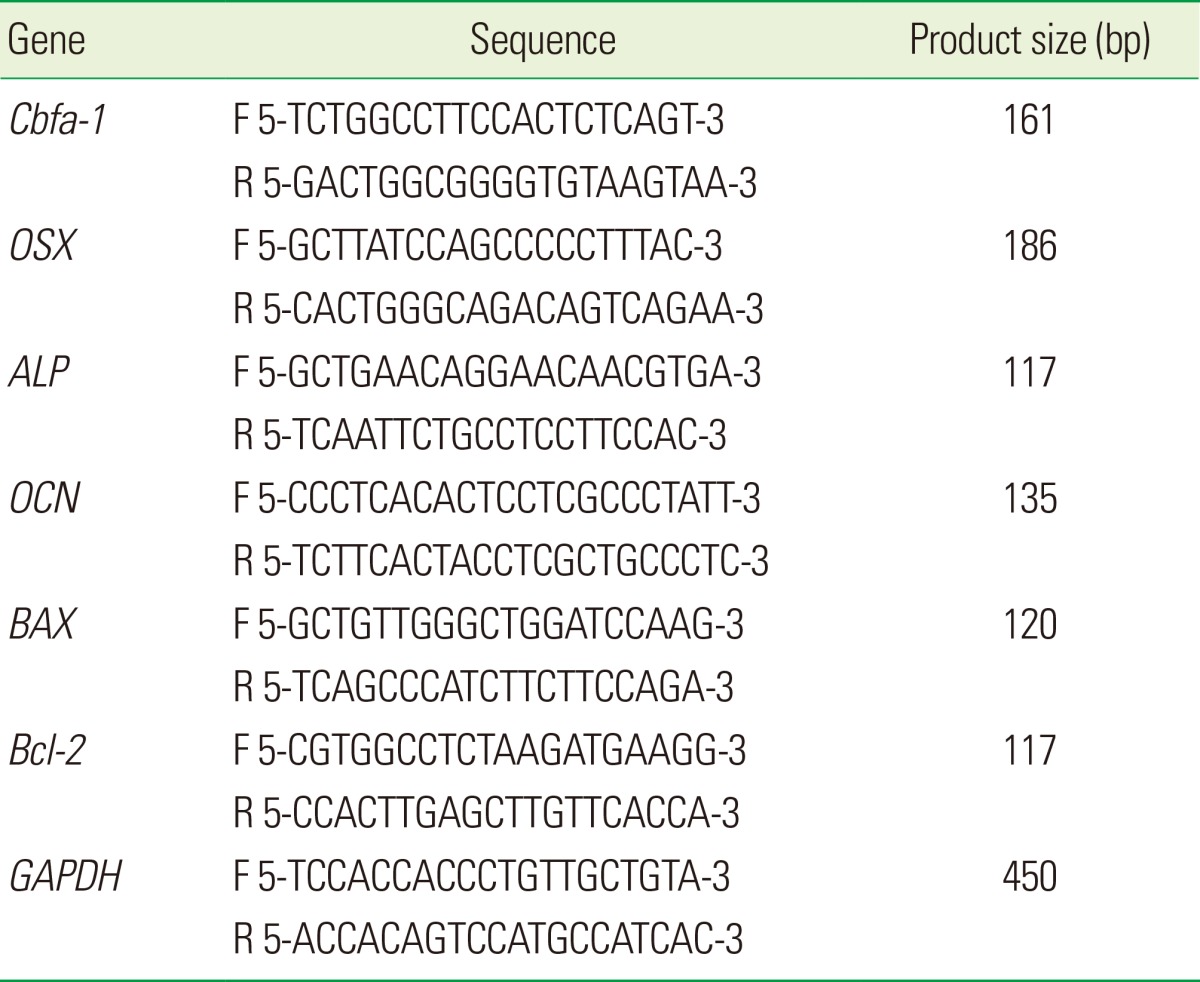
F: forward, R: reverse.
Statistical analysis
The results pertaining to the cellular viability, ALP activity, and real-time PCR were calculated as the mean±standard deviation. One-way analysis of variance was performed for determination of the differences within and between groups using Tukey test as a post hoc test among the tested groups. SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for analysis and a P-value of 0.05 or less was considered statistically significant.
RESULTS
MTT assay for cellular viability
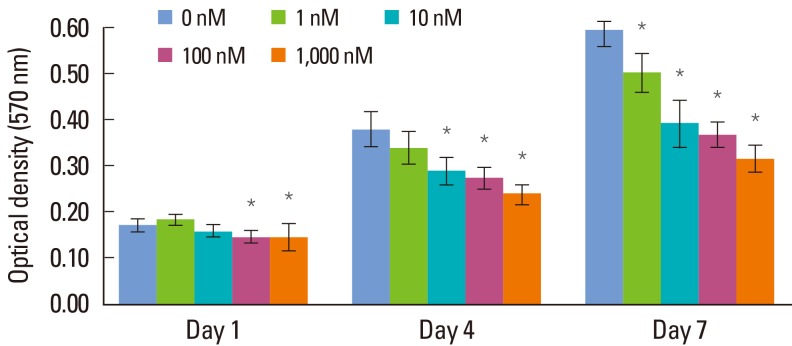
A MTT assay was performed for measurement of cell viability. The cellular viability showed a gradual increase in a time-dependent manner and decreased with an increasing concentration of Dex (Fig. 1). On day 1, the 1 nM group showed the highest cellular viability among the groups and the difference between the control and over-1-nM concentration groups was statistically significant (P<0.05). On days 4 and 7, the cellular viability decreased in a manner inversely proportional to the Dex concentration. On day 4, the control group showed the highest cellular viability and the difference in cellular viability between the control group and Dex-added groups was statistically significant, except for that of the 1 nM group (P<0.05). On day 7, the 1,000 nM group showed the lowest cellular viability, while the control group showed the highest outcome among the five groups. In an intergroup comparison, statistically significant differences were found, except between the 10 nM and 100 nM groups (P<0.05).
Figure 1.
Results of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (optical density) of human periodontal ligament cells supplemented with different concentrations of dexamethasone (Dex) (0, 1, 10, 100, and 1,000 nM) on days 1, 4, and 7. Similar results were obtained in three separate experiments, and the data are presented as the mean±standard deviation. *Statistically significant difference, compared to the group without Dex treatment (P<0.05).
ALP activity
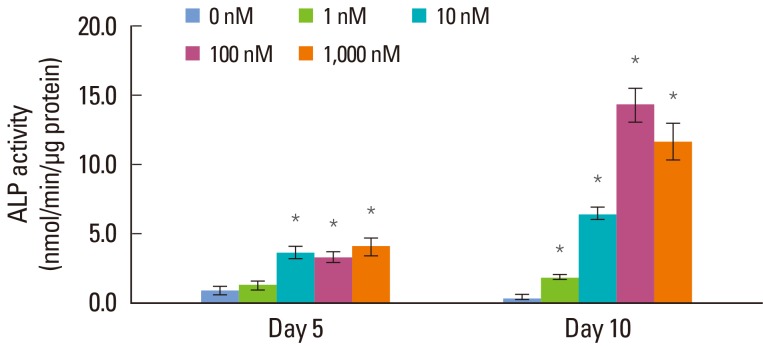
The ALP activity increased from days 5 to 10 in all of the groups, and the amount of the increase tended to rise in a concentration-dependent manner (Fig. 2). On day 5, the ALP activity increased in a Dex concentration-dependent manner, except in the 100 nM group. On day 10, a marked increase of ALP activity by administration of Dex was observed, compared with day 5. This increase was in proportion to the concentration of Dex up to 100 nM, and the ALP activity showed a slight decrease in the 1,000 nM group (P<0.05). A statistically significant difference was observed among each of the groups in all of the comparisons (P<0.05).
Figure 2.
Alkaline phosphatase (ALP) activity of periodontal ligament cells supplemented with different concentrations of dexamethasone (Dex) (0, 1, 10, 100, and 1,000 nM) on days 5 and 10. Similar results were obtained in three separate experiments, and the data are presented as the mean±standard deviation. *Statistically significant difference, compared to the group without Dex treatment (P<0.05).
Mineralized nodule formation
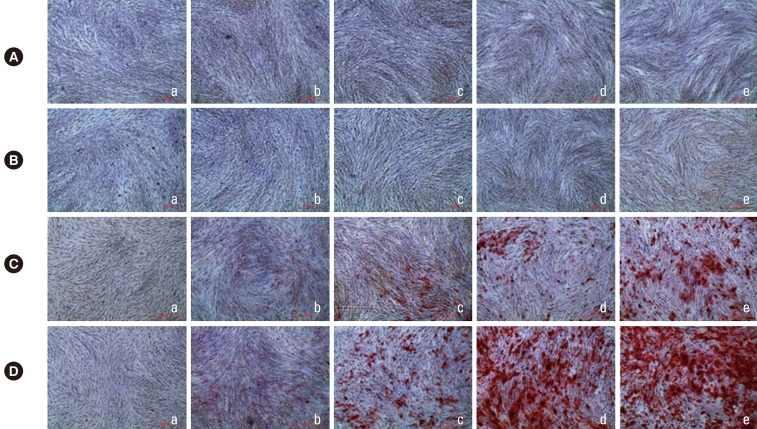
The culture dishes were fixed with 2% paraformaldehyde solution, and then stained with Alizarin red S on days 7, 14, 21, and 28 (Fig. 3). For 7 and 14 days, hPDL cells cultured in all of the groups exhibited a fibroblast-like spindle shape and exhibited few mineralized nodules (Fig. 3A and B). After osteogenic induction of 21 days, a prominent increase in the mineralization was observed in the 10, 100, and 1,000 nM groups. The magnitude of mineralization was high in the 100 and 1,000 nM groups, while no mineralized nodules were observed in the control group or the 1 nM group (Fig. 3C). After an osteogenic induction period of 28 days, the aspect of mineralization was similar to that of 21 days; however, the amount of mineralization was higher compared to that at 21 days (Fig. 3D).
Figure 3.
Macroscopic and microscopic features of mineralization in the control group (a) and the dexamethasone group with concentrations of of 1 nM (b), 10 nM (c), 100 nM (d), and 1,000 nM (e) after an osteogenic induction period of 7 (A), 14 (B), 21 (C), and 28 (D) days (Alizarin Red S staining, ×40).
Real-time PCR
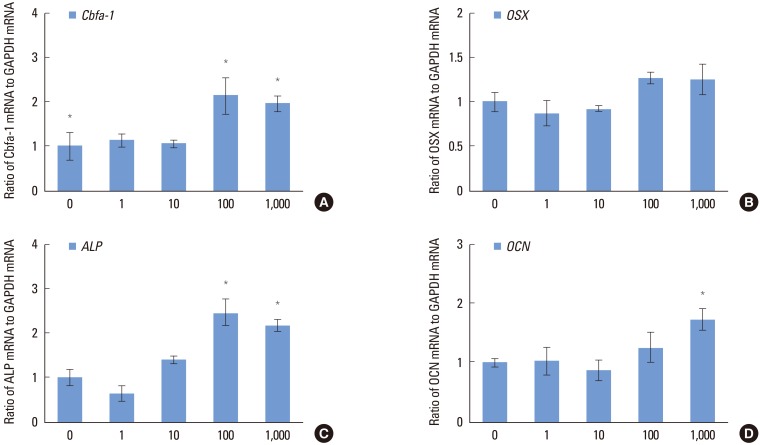
During observation of osteoblastic markers, a dose-dependent increase in the osteogenesis-related genes was found (Fig. 4). The Cbfa-1 expression level of the 100 and 1,000 nM groups was statistically higher compared to the control group (P<0.05). The 100 nM group showed the highest value, and the level of expression was rather decreased in the 1,000 nM group (Fig. 4A). The OSX mRNA level showed a rather steady trend according to the Dex concentration; however, compared to the control group, a higher expression level was detected in the 100 and 1,000 nM groups, which was not statistically significant (Fig. 4B). The ALP gene expression level of the 1 nM group was lower than that of the control group, and the highest value among the tested groups was detected in the 100 nM group, which was similar to the result for the ALP activity (Fig. 4C). The OCN mRNA level showed an increasing tendency according to the concentration of Dex, and the highest value was detected in the 1,000 nM group (P<0.05) (Fig. 4D).
Figure 4.
Dose-dependent effects of dexamethasone (Dex) on the osteogenesis-related genes of human periodontal ligament cells cultured in osteogenic medium: (A) Cbfa-1, (B) OSX, (C) ALP, and (D) OCN. The cells were exposed to various concentrations (0, 1, 10, 100, and 1,000 nM) of Dex for seven days. Similar results were obtained in three separate experiments, and the data are presented as the mean±standard deviation. The graphs show the ratio of selected gene mRNAs to GAPDH mRNA from the real-time polymerase chain reaction results. *Statistically significant difference, compared to the group without Dex treatment (P<0.05).
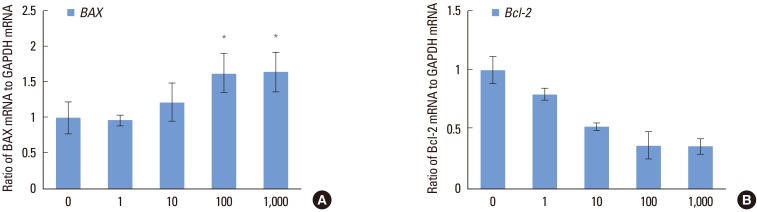
Regarding apoptosis-related genes, BAX showed an increasing tendency with the addition of Dex. A significantly higher level of expression was observed in the 100 and 1,000 nM groups, compared to the control group (P<0.05) (Fig. 5A). On the other hand, Bcl-2, which is known to be an antiapoptotic marker, was observed to be inversely proportional to the Dex concentration. The highest expression was observed in the control group and the lowest expression was observed in the 1,000 nM group (Fig. 5B).
Figure 5.
Dose-dependent effects of dexamethasone (Dex) on the apoptosis-related genes of human periodontal ligament cells cultured in osteogenic medium: (A) BAX and (B) Bcl-2. The cells were exposed to various concentrations (0, 1, 10, 100, and 1,000 nM) of Dex for seven days. Similar results were obtained in three separate experiments, and the data are presented as the mean±standard deviation. The graphs show the ratio of selected gene mRNAs to GAPDH mRNA from the real-time polymerase chain reaction results. *Statistically significant difference, compared to the group without Dex treatment (P<0.05).
DISCUSSION
To understand the regeneration of the destroyed periodontal attachment apparatus, knowledge of the factors that regulate proliferation and differentiation of PDL cells is critical. One of the regulators is glucocorticoid, and Dex is a member of the glucocorticoid class of hormones, which is required for osteoblastic differentiation [8].
Glucocorticoid exerts complex effects on the skeleton, which are dependent on the concentration and duration of exposure [24,25]. Regarding the dose-dependent effect of Dex, a maximal stimulatory effect on bone cell populations derived from fetal rat calvariae or adult rat vertebrae at a Dex of 10 nM has been reported, and the effects decreased at higher concentrations of Dex (100 to 1,000 nM) [24]. The maximally effective concentrations of glucocorticoids for stimulation of osteoblastic proliferation and differentiation are within the range of physiological circulating levels of glucocorticoids (100 to 1,000 nM of corticosterone in rats) [20-23]. Using Dex, which is known to stimulate osteogenesis and apoptosis, this study focused on the dose-dependent effect of Dex on hPDL cells (0 to 1,000 nM of Dex).
The number of viable cells was evaluated using the MTT assay, which measures cellular viability through the determination of mitochondrial dehydrogenase activity and is considered a sensitive assay for assessment of osteoblastic proliferation. This study found that with the passage of time from day 1 to day 7, cellular viability increased in all of the groups (Fig. 1). With supplementation of Dex, viable hPDL cells decreased in a manner inversely proportional to the Dex concentration. Both the inhibition and stimulation of bone cell proliferation have been reported when glucocorticoids were added to cultures of isolated bone and bone cells [25-27]. The results of this study are in accordance with those of a report showing that Dex inhibited the proliferation of osteoblasts and induced a reduction in the number of osteoblasts and osteocytes through enhanced apoptosis [25], but in contrast to a chick periosteal culture, which showed that Dex at 100 nM induced a significant increase in proliferation of osteogenic cells [26]. These divergent results may reflect differences in the level of cellular differentiation in various model systems [28].
Osteoblastic differentiation was assessed by ALP activity, an early marker of osteoblastic cell differentiation and a membrane-associated enzyme [29]. Previous studies have reported the maximal expression of ALP on day 7, prior to the initiation of nodule mineralization [30]. Therefore, days 5 and 10, around the day of maximal ALP expression were selected for the analysis of ALP activity. According to the results on ALP activity, Dex was shown to induce differentiation of hPDL cells to osteoblasts in a concentration-dependent manner (Fig. 2); however, the maximal effect of the concentration was not beyond 100 nM. That is, ALP activity was slightly inhibited in the 1,000 nM group, compared with the 100 nM group on day 10. The reduced ALP activity in the 1,000 nM group is in accordance with the results of a study showing that exposure to high dosages exerted negative effects on osteoblasts, including increased apoptosis, decreased osteoblast proliferation, and a later decrease in bone formation [31,32]. A study of human bone marrow stromal cells reported detection of maximal ALP activity at 10 nM [31]. In addition, when hPDL cells were treated with increasing concentrations of Dex, ranging from 10 nM to 500 nM, Dex induced a dose-dependent increase in ALP activity, which appeared to plateau at approximately 500 nM of Dex [32].
As cells form multilayered clusters, the rate of proliferation is known to decrease and nodule cells begin to mineralize the extracellular matrix [33]. In this study, regarding previous reports examining mineralization [34], the magnitude of mineralization was confirmed by staining on days 7, 14, 21, and 28. On days 7 and 14, the spindle-shaped morphology of hPDL fibroblasts was observed in all groups, and no notable mineralized nodules were detected (Fig. 3A and B). No difference was observed among groups. On days 21 and 28, the Dex-treated groups showed increased mineralization, compared with the control group (Fig. 3C and D). Notably, the 100 and 1,000 nM Dex groups showed the most significant nodule formation. Fewer nodules were observed in the 10 nM groups and no nodules were detected in the control and 1 nM group. Although cellular viability was endangered by Dex, as mentioned above, Dex was essential for mineralization of hPDL cells.
Previous studies have also reported maximal mRNA expression of osteogenic differentiation genes, including ALP, BMP-2, and BMP-4 on day 7, which is the multilayer formation stage prior to initiation of mineralization [30]. Therefore, RNAs of hPDL cells were extracted on day 7 for real-time PCR. For analysis of gene expression during the osteogenic process, this study examined Cbfa-1, OSX, ALP, and OCN by real-time PCR. Cbfa-1 is a key transcription factor associated with osteoblast differentiation and skeletal morphogenesis and acts as a scaffold for nucleic acids and regulatory factors involved in skeletal gene expression. OSX, a recently identified zinc finger-containing transcription factor, is expressed in osteoblasts of all endochondral and membranous bones [35]. ALP is one of the earliest markers of osteoblastic cells, and mineralization is initiated by expression of the membrane-bound glycoprotein, ALP [36]. OCN is only expressed by fully differentiated osteoblasts and regulates their function [37]. Expression of Cbfa-1, OSX, ALP, and OCN showed an increasing tendency with the concentration of Dex (Fig. 4). These results appear to indicate that an increase in ALP activity and mineralization may contribute to osteogenesis-related genes, such as Cbfa-1, OSX, ALP, and OCN.
During the apoptotic process, mitochondrial outer membrane permeabilization is regulated by various proteins, such as those encoded by the mammalian Bcl-2 family of antiapoptotic genes [38]. Bcl-2 proteins are able to promote or inhibit apoptosis by direct action. BAX, a member of the Bcl-2 family, forms the pore, while Bcl-2 inhibits its formation. BAX promotes apoptosis by competing with Bcl-2 proper. Therefore, BAX and Bcl-2 were selected as apoptosis and antiapoptosis markers for real-time PCR.
Up-regulation of BAX and down-regulation of Bcl-2 was observed in a Dex concentration-dependent manner (Fig. 5A and B). The decrease in hPDL cellular viability caused by glucocorticoid may be explained in part by the increased prevalence of cell apoptosis, as demonstrated by BAX expression and decreased expression of antiapoptotic gene Bcl-2. Elevated expression of Bcl-2 at lower concentrations of Dex may inhibit differentiation of hPDL cells into mature osteoblasts. In addition, a dose-dependent increase of BAX, which is known to promote apoptosis, appears to indicate that cell apoptosis induced by Dex might play a role in the process of tissue mineralization, which is in accordance with the results of a study reporting that an apoptotic body may act as a nucleating body [17]. These data suggest that Dex likely promotes specific markers of the osteoblastic phenotype in primary PDL cells, at least in part, through suppression of antiapoptotic gene expression and an increase in apoptotic related genes, which presumably promote differentiation to the osteoblastic phenotype and elevation of bone-specific marker expression.
It has been reported that at the stage of mineralization of hPDL cells, apoptosis-inducing agents were up-regulated, and antiapoptosis activators were down-regulated [18]. It has also been reported that apoptosis is an important event in human dental pulp cells and that genes implicated in apoptosis are highly connected to the differentiation of dental pulp cells into odontoblasts [39]. These findings are in accordance with the findings of the present study. Apoptosis might play both passive and active roles in differentiation and mineralization of PDL cells. As for its active role, one paper reported that necrotic and apoptotic cell deaths were induced in an osteogenic culture of hMSCs and indicated that both necrotic and apoptotic cells of an osteoblast lineage served as nuclei for calcification on osteoblastic differentiation of hMSCs in vitro [40]. In this study, we were able to find a passive role for apoptosis, which was followed by differentiation, but we could not confirm the active role of apoptosis in the mineralization of hPDL cells and further study is needed. In summary, Dex was found to have variable effects on hPDL cells according to the Dex concentration. With the elevation of the Dex concentration, the hPDL cells were prone to osteogenic differentiation with increased apoptosis. Although cellular viability decreased with the Dex concentration, an increase in hPDL cell differentiation and mineralization up to 100 nM was noted. Therefore, hPDL cell differentiation followed by increased apoptosis, rather than their cellular viability at an early stage, is likely to be a key factor in glucocorticoid mineralization. In addition, apoptosis might play an important role in Dex-induced tissue regeneration; however, further study is needed for investigation of the precise mechanism.
ACKNOWLEDGEMENTS
This research was supported by a Biomedical Research Institute grant from Kyungpook National University Hospital (2011).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 2.Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontol 2000. 2006;40:164–172. doi: 10.1111/j.1600-0757.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Gould TR, Melcher AH, Brunette DM. Migration and division of progenitor cell populations in periodontal ligament after wounding. J Periodontal Res. 1980;15:20–42. doi: 10.1111/j.1600-0765.1980.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch CA, Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res. 1991;26(3 Pt 1):144–154. doi: 10.1111/j.1600-0765.1991.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung CH, Golub EE, Forbes E, Tokuoka T, Shapiro IM. Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int. 1992;51:305–311. doi: 10.1007/BF00334492. [DOI] [PubMed] [Google Scholar]
- 6.Kodama HA, Amagai Y, Sudo H. Culture conditions affecting differentiation and calcification in the MC3T3-E1 osteogenic cell line. In: Ali SY, editor. Cell-mediated calcification and matrix vesicles. Amsterdam: Elsevier Science BV; 2006. pp. 297–302. [Google Scholar]
- 7.Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 1994;9:843–854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 8.Majeska RJ, Nair BC, Rodan GA. Glucocorticoid regulation of alkaline phosphatase in the osteoblastic osteosarcoma cell line ROS 17/2.8. Endocrinology. 1985;116:170–179. doi: 10.1210/endo-116-1-170. [DOI] [PubMed] [Google Scholar]
- 9.Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol. 1991;146:370–378. doi: 10.1002/jcp.1041460306. [DOI] [PubMed] [Google Scholar]
- 10.Shalhoub V, Conlon D, Tassinari M, Quinn C, Partridge N, Stein GS, et al. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J Cell Biochem. 1992;50:425–440. doi: 10.1002/jcb.240500411. [DOI] [PubMed] [Google Scholar]
- 11.Abe Y, Aida Y, Abe T, Hirofuji T, Anan H, Maeda K. Development of mineralized nodules in fetal rat mandibular osteogenic precursor cells: requirement for dexamethasone but not for beta-glycerophosphate. Calcif Tissue Int. 2000;66:66–69. doi: 10.1007/s002230050013. [DOI] [PubMed] [Google Scholar]
- 12.Bellows CG, Heersche JN, Aubin JE. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev Biol. 1990;140:132–138. doi: 10.1016/0012-1606(90)90060-v. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Pang B, Li Y, Zhu D, Pang T, Liu Y. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy. 2012;14:423–430. doi: 10.3109/14653249.2011.652735. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parfitt AM. Bone-forming cells in clinical conditions. In: Hall BK, editor. Bone. Caldwell: Telford Press and CRC Press; 1990. pp. 351–429. [Google Scholar]
- 16.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 17.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 18.Shin JH, Park JW, Yeo SI, Noh WC, Kim MK, Kim JC, et al. Identification of matrix mineralization-related genes in human periodontal ligament cells using cDNA microarray. J Korean Acad Periodontol. 2007;37(Suppl):447–463. [Google Scholar]
- 19.Kim HS, Park JW, Yeo SI, Choi BJ, Suh JY. Effects of high glucose on cellular activity of periodontal ligament cells in vitro. Diabetes Res Clin Pract. 2006;74:41–47. doi: 10.1016/j.diabres.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Bligh ME, Bhagwat SA, Castonguay TW. Aldosterone diurnal rhythm in the rat: a question of cross-reactivity? Physiol Behav. 1993;53:845–848. doi: 10.1016/0031-9384(93)90260-m. [DOI] [PubMed] [Google Scholar]
- 21.Malendowicz LK, Nussdorfer GG, Markowska A, Tortorella C, Nowak M, Warchol JB. Effects of neuromedin U (NMU)-8 on the rat hypothalamo-pituitary-adrenal axis. Evidence of a direct effect of NMU-8 on the adrenal gland. Neuropeptides. 1994;26:47–53. doi: 10.1016/0143-4179(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 22.Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology. 1995;136:5336–5342. doi: 10.1210/endo.136.12.7588279. [DOI] [PubMed] [Google Scholar]
- 23.Sharma AC, Bosmann HB, Motew SJ, Hales KH, Hales DB, Ferguson JL. Steroid hormone alterations following induction of chronic intraperitoneal sepsis in male rats. Shock. 1996;6:150–154. doi: 10.1097/00024382-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Ishida Y, Bellows CG, Tertinegg I, Heersche JN. Progesterone-mediated stimulation of osteoprogenitor proliferation and differentiation in cell populations derived from adult or fetal rat bone tissue depends on the serum component of the culture media. Osteoporos Int. 1997;7:323–330. doi: 10.1007/BF01623772. [DOI] [PubMed] [Google Scholar]
- 25.Tenenbaum HC, Heersche JN. Differentiation of osteoblasts and formation of mineralized bone in vitro. Calcif Tissue Int. 1982;34:76–79. doi: 10.1007/BF02411212. [DOI] [PubMed] [Google Scholar]
- 26.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 27.Beloti MM, Rosa AL. Osteoblast differentiation of human bone marrow cells under continuous and discontinuous treatment with dexamethasone. Braz Dent J. 2005;16:156–161. doi: 10.1590/s0103-64402005000200013. [DOI] [PubMed] [Google Scholar]
- 28.McCulloch CA, Tenenbaum HC. Dexamethasone induces proliferation and terminal differentiation of osteogenic cells in tissue culture. Anat Rec. 1986;215:397–402. doi: 10.1002/ar.1092150410. [DOI] [PubMed] [Google Scholar]
- 29.Shen Q, Zhu S, Hu J, Geng N, Zou S. Recombinant human bone morphogenetic protein-4 (BMP-4)-stimulated cell differentiation and bone formation within the expanding calvarial suture in rats. J Craniofac Surg. 2009;20:1561–1565. doi: 10.1097/SCS.0b013e3181b09cc1. [DOI] [PubMed] [Google Scholar]
- 30.Choi MH, Noh WC, Park JW, Lee JM, Suh JY. Gene expression pattern during osteogenic differentiation of human periodontal ligament cells in vitro. J Periodontal Implant Sci. 2011;41:167–175. doi: 10.5051/jpis.2011.41.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng SL, Zhang SF, Avioli LV. Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J Cell Biochem. 1996;61:182–193. doi: 10.1002/(sici)1097-4644(19960501)61:2<182::aid-jcb3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Hayami T, Zhang Q, Kapila Y, Kapila S. Dexamethasone's enhancement of osteoblastic markers in human periodontal ligament cells is associated with inhibition of collagenase expression. Bone. 2007;40:93–104. doi: 10.1016/j.bone.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 34.Porter RM, Huckle WR, Goldstein AS. Effect of dexamethasone withdrawal on osteoblastic differentiation of bone marrow stromal cells. J Cell Biochem. 2003;90:13–22. doi: 10.1002/jcb.10592. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 36.Lynch MP, Capparelli C, Stein JL, Stein GS, Lian JB. Apoptosis during bone-like tissue development in vitro. J Cell Biochem. 1998;68:31–49. [PubMed] [Google Scholar]
- 37.Ducy P, Geoffroy V, Karsenty G. Study of osteoblast-specific expression of one mouse osteocalcin gene: characterization of the factor binding to OSE2. Connect Tissue Res. 1996;35:7–14. doi: 10.3109/03008209609029169. [DOI] [PubMed] [Google Scholar]
- 38.Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta. 2006;1762:191–201. doi: 10.1016/j.bbadis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Seo MS, Hwang KG, Kim H, Baek SH. Analysis of gene expression during odontogenic differentiation of cultured human dental pulp cells. Restor Dent Endod. 2012;37:142–148. doi: 10.5395/rde.2012.37.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita H, Yamamoto M, Ogino T, Kobuchi H, Ohmoto N, Aoyama E, et al. Necrotic and apoptotic cells serve as nuclei for calcification on osteoblastic differentiation of human mesenchymal stem cells in vitro. Cell Biochem Funct. 2013 May 08; doi: 10.1002/cbf.2974. [Epub]. http://dx.doi.org/doi:10.1002/cbf.2974. [DOI] [PubMed] [Google Scholar]