Abstract
Purpose
At present, information regarding periodontal disease in geriatric patients is scarce. The purpose of this study was to quantify the periodontal pathogens present in the saliva of Korean geriatric patients and assess the relationship between the bacterial levels and the periodontal condition.
Methods
Six putative periodontal pathogens were quantified by using a real-time polymerase chain reaction assay in geriatric patient groups (>60 years) with mild chronic periodontitis (MCP), moderate chronic periodontitis (MoCP), and severe chronic periodontitis (SCP). The copy numbers of Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, and Prevotella intermedia were measured.
Results
It was found that the bacterial copy numbers increased as the severity of the disease increased from MCP to SCP, except for P. intermedia. For P. intermedia, it was found that samples in the MCP group yielded the largest amount. It was also found that the quantities of P. gingivalis, T. forsythia, and T. denticola, the so-called "red complex" bacteria, were lower than those of F. nucleatum, A. actinomycetemcomitans, and P. intermedia in all of the samples.
Conclusions
Collectively, the results of this study suggest that the levels of P. gingivalis, T. forsythia, F. nucleatum, and T. denticola present in saliva are associated with the severity of periodontal disease in geriatric patients.
Keywords: Chronic periodontitis, Disease progression, Geriatrics, Microbiology, Real-time polymerase chain reaction, Saliva
INTRODUCTION
With the advancement of medical science and prolonged life expectancy, the number of geriatric people around the world is increasing rapidly. At present, there are around 600 million people aged 60 years and over, and it is expected that the total will double by 2025 and will reach virtually two billion by 2050, which will make up 22 percent of the world's population [1]. As people age, they become more susceptible to chronic and life-threatening diseases in addition to increases in acute infections, exacerbated by compromised immune systems. Multifactorial chronic diseases such as cancer, cardiovascular diseases, diabetes, and oral diseases are more prevalent in this age group. Poor oral health, accompanied by unstable periodontal conditions and tooth loss, can ultimately lead to impaired oral functions including chewing. These can further lead to disabilities and reduced quality of life of this population. In addition, poor oral health can increase the risks of systemic disease, given the evidence for an interrelationship between oral and general health [2,3]. However, oral health conditions in the elderly have not yet been extensively studied.
Periodontitis is a chronic inflammatory disease that results from a complex polymicrobial infection, leading to tissue destruction as a consequence of the perturbation of the homeostasis between the subgingival microflora and the host defenses in susceptible individuals [4,5]. It has been established that dental plaque is an etiological agent of periodontal disease. Dental plaque is a biofilm consisting of more than 700 different bacterial species and their products [6,7]. Immunological and inflammatory responses by the host to dental plaque biofilm via host-parasite interaction are manifested by signs and symptoms of periodontal disease. It is generally accepted that periodontal disease is more prevalent in the elderly. Numerous data suggest that clinical attachment loss (CAL) increases with age as a result of lifetime disease accumulation rather than an age-specific condition [8,9]. In the U.S. population aged 55 to 64, the prevalence of periodontitis, when the case is defined as having at least one site with CAL of more than 4 mm, is around 50% [10]. In addition, in the elderly, periodontal disease may lead to root caries, eating disabilities, and an increase patients' risk of developing systemic diseases such as diabetes mellitus, cardiovascular disease, and respiratory disease [11]. Therefore, it is crucial to maintain a stable periodontal condition free of active inflammation in the periodontium to promote and maintain adequate oral and general systemic health. For this purpose, evaluating the current periodontal condition of elderly people in order to formulate an effective preventive and maintenance program along with a specific treatment regimen for promoting periodontal health is necessary. This will help ensure the quality of life of the elderly population [12].
Human saliva is an attractive body fluid for disease diagnosis and monitoring because saliva testing is simple, safe, low-cost, and noninvasive. The use of saliva for diagnosis and monitoring of periodontal disease is highly desirable because periodontal pathogens, host antibacterial products, and other host-related proteins are readily detectable in saliva by new and highly sensitive technologies, such as real-time polymerase chain reaction (RT-PCR) analysis [13,14]. It has been determined that RT-PCR provides a new rapid diagnostic tool and opens the opportunity to detect small numbers of oral pathogens in clinical specimens, that are under the detection limit by the culture technique [15,16]. Therefore, a novel rapid method of RT-PCR can be used for identification and quantification of periodontopathic bacteria in saliva samples [15-17].
At present, microbiological information regarding the quantity of periodontal pathogens present in the geriatric population is scarce. The purpose of this study was to quantify six major periodontal pathogens present in saliva by real-time PCR analysis and assess the relationship between the bacterial levels and the status of the periodontal condition.
MATERIALS AND METHODS
Subjects
The protocol of this study was approved by the Institutional Review Board (Approval number: 1-2008-05-226) of Chonnam National University Hospital, Gwangju, Korea. This study population included 89 geriatric subjects (34 male and 55 female subjects), 60-83 years old, who were residents of Gwangju and recruited for a public health survey performed jointly by the Chonnam National University Colleges of Medicine and Dentistry, Gwangju, Korea. Subjects who had been previously exposed to antibiotics in the past four months and had chronic inflammatory or immune disorders were excluded from this study. Each subject received a standard periodontal examination by a periodontist and was assigned to one of the three groups based upon the severity of periodontitis according to the criteria proposed above [18]. These groups are the 1) mild chronic periodontitis group (MCP; n=29; mean age, 64.1 years; range, 60 to 70 years) with CAL less than 3 mm, and the 2) moderate chronic periodontitis group (MoCP; n=29; mean age, 67.6 years; range, 60.8 to 83.0) with a CAL of 3-4 mm and the 3) severe chronic periodontitis group (SCP; n=30; mean age, 68.5 years; range, 60.9 to 82 years) with a CAL of more than 4 mm.
Saliva collection
Fifteen milliliters of unstimulated saliva were collected and kept at -80℃ until used. Each saliva sample was subjected to centrifugation at 8,000×g for 10 minutes, and the pellets were washed twice with phosphate buffered saline to remove all of the debris. Genomic DNA was extracted from a 1-mL saliva sample according to the manufacturer's protocol (G-spin, iN-tRON biotechnology, Seongnam, Korea). Purified DNA was kept in -20℃ for subsequent use.
Bacterial strains and culture
Aggregatibacter actinomycetemcomitans ATCC 33384 was cultured in 3% tryptic soy broth (BD Biosciences Immunocytometry Systems, San Jose, CA, USA) including 1 mg/mL yeast extract (BD Biosciences Immunocytometry Systems) and 10% horse serum (Hyclone, Seongnam, Korea), and Porphyromonas gingivalis ATCC 33277 was cultured in Brucella broth (BD Biosciences Immunocytometry Systems) adding 1 mg/mL yeast extract (Sigma-Aldrich Co., St. Louis, MO, USA), 5 µg/mL hemin (Sigma-Aldrich Co.), and 1 µg/mL menadione (Sigma-Aldrich Co.). Fusobacterium nucleatum ATCC 10953 and Prevotella intermedia ATCC 25611 were cultured in Brucella broth with added yeast extract (1 mg/mL), haemin (10 µg/mL), and menadione (5 µg/mL). Treponema denticola ATCC 35405 was cultured in TYGVS medium including tryptone (10 mg/mL), brain heart infusion broth (5 mg/mL), yeast extract (10 mg/mL), gelatin (10 mg/mL), (NH4)2SO4 (0.5 mg/mL), MgSO4 (0.1 mg/mL), K2HPO4 (1.13 mg/mL), KH2PO4 (0.9 mg/mL), NaCl (1 mg/mL), glucose (1 mg/mL), cysteine hydrochloride (1 mg/mL), thiamine pyrophosphate (12.5 µg/mL), sodium pyruvate (0.25 mg/mL), 0.027% acetic acid, 0.01% propionic acid, 0.0064% n-butyric acid, 0.0016% n-valeric acid, 0.0016% isobutyric acid, 0.0016% isovaleric acid, 0.0016% DL-methylbutyric acid, 10% heat-inactivated rabbit serum at 37℃ in anaerobic conditions (85% N2, 10% H2, 5% CO2) for 48 hours. Tannerella forsythia 43037 (American Type Culture Collection, Manassas, VA, USA) was grown anaerobically on 2% (w/v) tryptic soy agar (BD Biosciences Immunocytometry Systems) supplemented with 0.4% (w/v) yeast extract (Difco Laboratories, Livonia, MI, USA), 0.4% (w/v) phytone (BD Biosciences Immunocytometry Systems), 0.001% (w/v) N-acetylmuramic acid (Sigma-Aldrich Co.), 5 µg/mL hemin (Sigma-Aldrich Co.), 1 µg/mL menadione (Sigma-Aldrich Co.), and 5% (v/v) defibrinated sheep blood. Each strain was cultured twice prior to the experiments.
Extraction of DNA from the bacterial culture
Genomic bacterial DNA was extracted from each individual bacterial species and used as standard DNA templates in quantitative RT-PCR assay, according to the manufacturer's protocol by using a G-spin Genomic DNA Extraction Kit (iNtRON Biotechnology). The isolated DNA was quantified using NanoDrop2000 (Thermo Scientific, Wilmington, DE, USA) and 10× serially diluted DNA was used as standard DNA in RT-PCR analysis.
PCR primer design
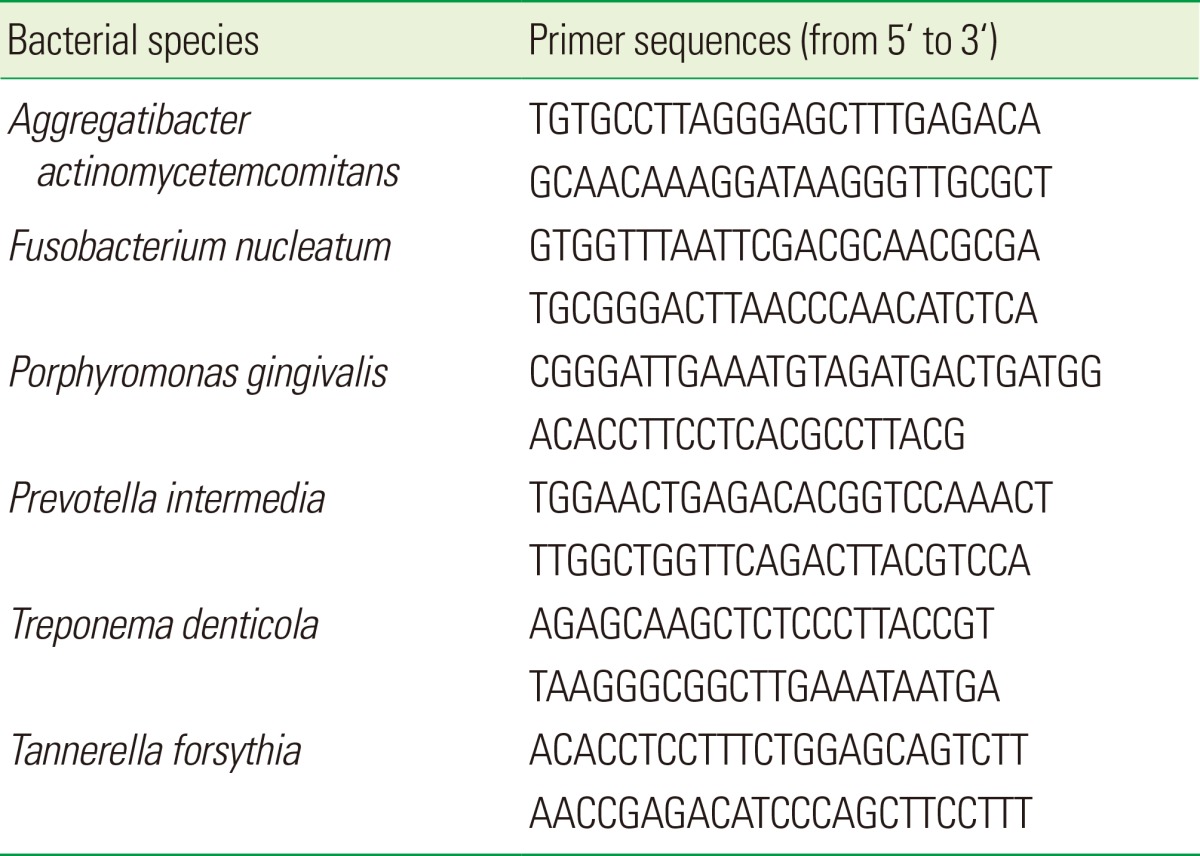
Primers for detecting 16S rDNA of A. actinomycetemcomitans, F. nucleatum, P. gingivalis, P. intermedia, and T. forsythia were designed using a software program (http://eu.idtdna.com) after the 16S rDNA sequences were retrieved from the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov). Primer sequences for 16S rDNA from T. denticola were obtained as previously described [19]. The designed primer sequences are shown in Table 1.
Table 1.
Target bacteria and their species-specific primers used in real-time polymerase chain reaction.
Real-time PCR
RT-PCR and data analysis were performed following instructions for the MiniOpticon RT-PCR system (Bio-Rad Laboratories Inc., Hercules, CA, USA). Duplicated samples were used for data accuracy. The total volume (25 µL) included SYBR Green (iQ SYBR Green Supermix, Bio-Rad Laboratories Inc.), genomic DNA, the primers (10 pmol of each), and sterile water. 40 thermo-cycles of 95℃, 15 seconds; 60℃, 30 seconds; and 72℃, 30 seconds were run after the denaturation of the DNA at 95℃ for 3 minutes. The melting curve analysis was conducted in 0.2 secodns intervals from 65℃ to 95℃.
Statistical analysis
All of the experiments were performed in triplicate to ensure data accuracy and the average of the triplicate data was used for the analysis. For the MCP and MoCP groups, the experimental data for one sample could not be obtained. Therefore, for subsequent statistical analysis, it was decided that a parametric method would be adopted when the data presented a normal distribution and a nonparametric method without a normal distribution. All of the statistical analyses were performed by using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). A one-way analysis of variance test was performed for the data distributed normally. A nonparametric Kruskal-Wallis test was used for the data that were not distributed normally. In addition, a Mann-Whitney U test with Bonferroni correction (α=0.05/3=0.0167) and Tukey Post hoc test were used for multiple comparisons among the groups.
RESULTS
Preparation of the reference DNA and calculation of the bacterial cell copy numbers
To determine assay validation, specificity, and the low detection limit in the PCR reactions, genomic DNA of 6 different bacterial strains was used as a reference standard. A standard curve was generated using the CFX Manager software (Bio-Rad Laboratories Inc.). Relative values for the DNA amount present in saliva samples were extrapolated from the standard curve generated from the reference standard.
Bacterial cell copy numbers were calculated as described previously [20]. It was found that there were 4 copies/genome in P. gingivalis, 2 copies/genome in T. forsythia and T. denticola, 5 copies/genome in F. nucleatum, 7 copies/genome in A. actinomycetemcomitans, and 3 copies/genome in P. intermedia, as the 16S rRNA gene is a multicopy gene, from the Oral Pathogen Sequence Databases (http://www.oralgen.iland.gov/). The number of cell copies was divided by these values to adjust to 1 genome=1 bacterium. Then, the DNA amount present in one bacterial cell was obtained using a formula, DNA weight (pg)=genome size (bp)×1.023E-9, where it is assumed that one genome exists in one bacterial cell, as described before [20]. For example, in the case of P. gingivalis, the DNA weight contained in one P. gingivalis cell was calculated as 0.0024 pg using the above formula, since the genome size of P. gingivalis is 2.354E6 bp (http://www.brop.org). The DNA weight contained in one bacterial cell for the other five periodontal pathogens was calculated using the same method. Finally, the bacterial cell copy numbers present in the saliva samples were calculated based on the DNA amount present in each sample.
Quantification of periodontal pathogens in different groups
A total of 88 saliva samples were analyzed in this study. Twenty-nine subjects were diagnosed with MCP, 29 subjects with MoCP, and 30 subjects with SCP. Based upon the Central Limit Theorem, it was determined through power analysis that if the sample size were greater than 30, the distribution could be treated as a normal distribution. Initially, 30 subjects were selected for each group. However, for the MCP and MoCP groups, experimental data for one sample could not be obtained.
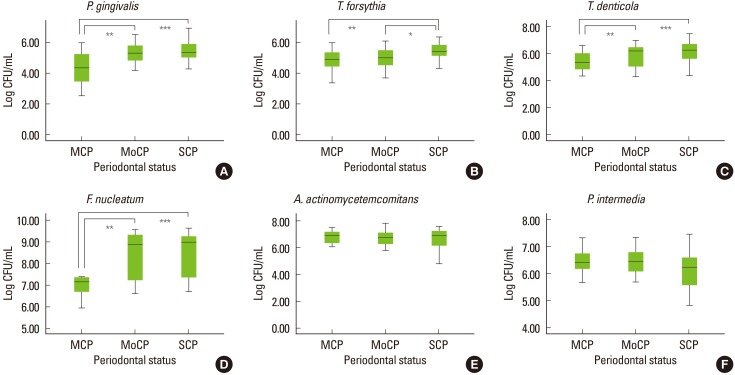
Table 2 shows the amount of six periodontal pathogens present in the saliva samples identified by using RT-PCR. It was observed that there existed more F. nucleatum in the saliva samples than any other bacteria in all three groups (Fig. 1). The second most detected pathogen was A. actinomycetemcomitans, followed by P. intermedia. The amount of P. gingivalis, T. forsythia, and T. denticola exhibited almost the same values. However, the levels of these three bacteria were lower than those of F. nucleatum, A. actinomycetemcomitans, and P. intermedia.
Table 2.
Cell copy numbers (log10 CFU/mL) of each bacterium present in saliva as analyzed by real-time polymerase chain reaction.
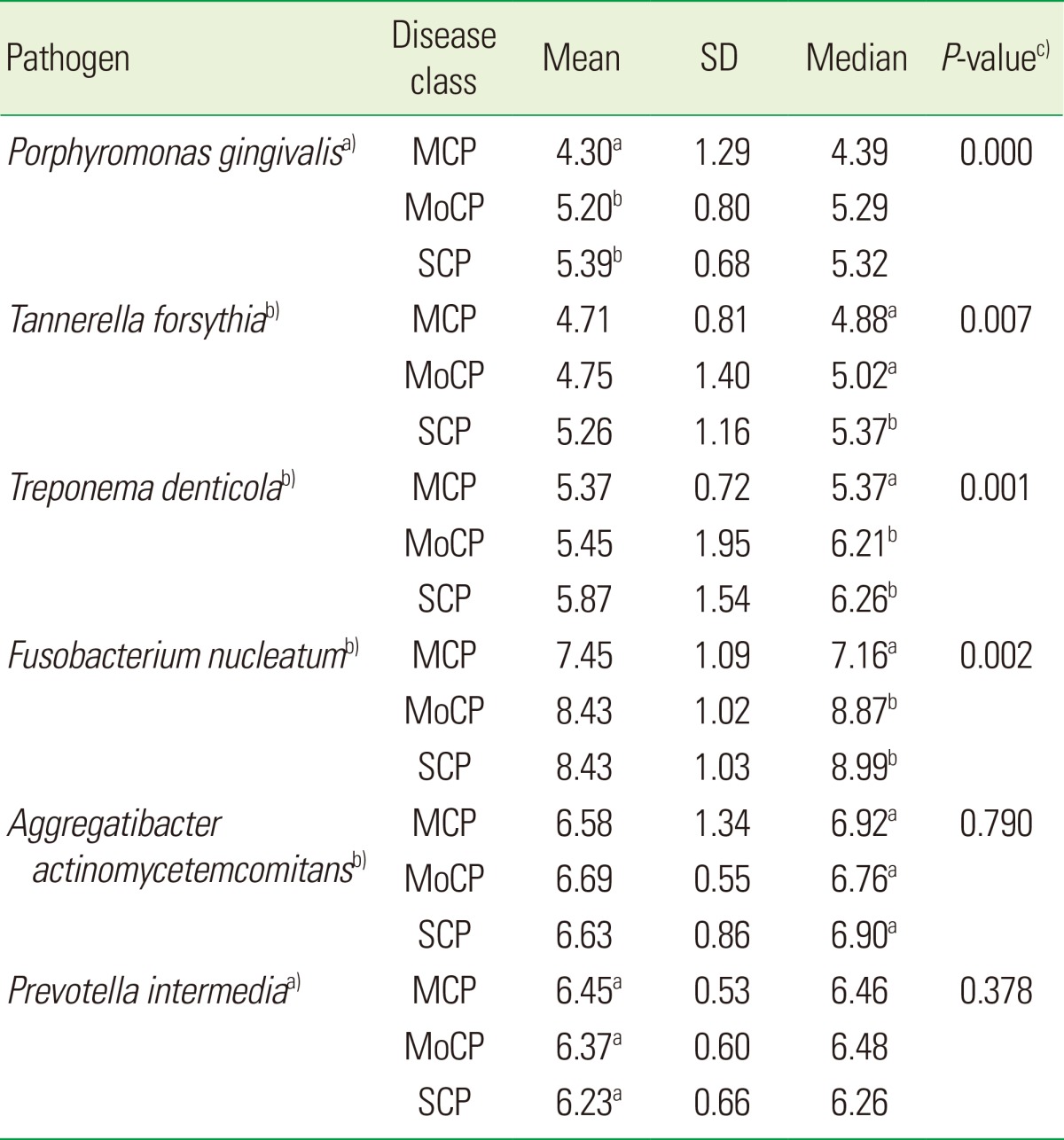
SD: standard deviation; MCP: mild chronic periodontitis; MoCP: moderate chronic periodontitis, SCP: severe chronic periodontitis.
a)One-way analysis of variance (ANOVA) was used to compare three groups, since the outcome variable was normally distributed. Post hoc test with Bonferroni correction was performed for multiple comparisons. b)Kruskal-Wallis test was used to compare three groups, since the outcome variable was not normally distributed. Mann-Whitney U test with Bonferroni correction (α=0.05/3=0.0167) was performed for multiple comparisons between the groups. c)P-value was shown for Kruskal-Wallis test and one-way ANOVA.
Different alphabets (a),b)) means that there is a significant difference between two groups and the same alphabet (a),a)) means that there is no difference between two groups.
Figure 1.
Box plot to describe multiple comparisons between the groups for Log10 copy cell numbers/mL of (A) Porphyromonas gingivalis, (B) Tannerella forsythia, (C) Treponema denticola, (D) Fusobacterium nucleatum, (E) Aggregatibacter actinomycetemcomitans, and (F) Prevotella intermedia. Mild chronic periodontitis (MCP, n=29), moderate chronic periodontitis (MoCP, n=29), and severe chronic periodontitis (SCP, n=30). The box represents the first and third quartiles. The line within the box represents the median value. *Significant difference (P≤0.05). **Significant difference (P≤0.01). ***Significant difference (P≤0.001). CFU: colony forming unit.
It was found that saliva samples in SCP yielded the highest levels of bacterial cells compared to those of MCP and MoCP, except A. actinomycetemcomitans and P. intermedia, which showed no significant difference between the groups. It was found that there were significant differences among the groups for P. gingivalis (P=0.000), T. forsythia (P=0.007), T. denticola (P=0.001), and F. nucleatum (P=0.002). However, there was no difference among the groups for A. actinomycetemcomitans (P=0.790) and P. intermedia (P=0.378).
When multiple comparisons were performed, as shown in Fig. 1, it was found that there was a significant increase in the amount of P. gingivalis from MCP to MoCP (P≤0.01) and from MCP to SCP (P≤ 0.001). For T. forsythia, there was a significant increase from MCP to SCP (P≤ 0.01) and also from MoCP to SCP (P≤ 0.05). It was also shown that the amount of T. denticola was significantly increased from MCP to MoCP (P≤ 0.01) and SCP (P≤ 0.001). In addition, F. nucleatum exhibited a significant increase in its amount from MCP to MoCP (P≤ 0.01) and SCP (P≤ 0.001). It was found that there was no significant difference among the groups for A. actinomycetemcomitans or P. intermedia. Therefore, analysis for multiple comparisons between groups was not conducted.
DISCUSSION
Periodontal disease is one of the most common infectious diseases affecting humans. For this reason, a better understanding of how bacterial pathogens are involved in the initiation and progression of periodontal disease is required to properly manage and monitor this affliction. Information on the microbiological aspects of the periodontal condition in geriatric subjects is not yet well established. The purpose of this study was to perform a quantitative analysis of the putative periodontal pathogens present in the saliva of geriatric patients and evaluate the relationship between the quantity of bacteria and the periodontal condition.
The results of the present study confirm that periodontal pathogens are commonly present in saliva, as the tested pathogens were detected by RT-PCR in more than 95% of the subjects (data not shown). It was found that F. nucleatum and P. intermedia were present in all of the subjects (data not shown).
Our results suggest that a combination of pathogenic bacteria in saliva may represent a disease marker or indicator for periodontal disease and predict periodontal disease progression. In this study, it was found that the quantity of three important periodontal pathogens, the so-called red complex bacteria consisting of P. gingivalis, T. forsythia, and T. denticola along with F. nucleatum significantly increased as the disease progressed from mild (MCP) to severe chronic periodontitis (SCP). It has been established that P. gingivalis, T. forsythia, and T. denticola are strong candidates for etiological agents of chronic periodontitis and considered to be associated with the progression of periodontal disease, since these bacteria are closely related to periodontal breakdown shown by deepening periodontal pockets and bleeding on probing [21,22]. It has become evident that no single bacterial species is responsible for periodontal disease, but a mixture or group of bacteria result in periodontal infection and further damage. It is mostly likely that gram-negative anaerobes are involved in the initiation and progression of periodontitis [23,24]. Among these, red-complex bacteria are thought to play a major role in microbial pathogenicity in the progression of chronic periodontitis [25-27]. In a prospective study, it was observed that the levels of P. gingivalis and T. denticola increased as the periodontal disease progressed [28]. However, these findings have not yet been confirmed in the study of geriatric patients. Therefore, the results of our present study provide one more piece of evidence that red-complex bacteria may be involved in the progression of periodontal disease in geriatric patients.
In this study, it was found that red-complex bacteria existed in a lower quantity than A. actinomycetemcomitans, F. nucleatum, and P. intermedia in all of the disease groups (Fig. 1). In another study, it was also observed that species considered to be most periodontopathic exhibited both relatively low salivary copy-counts, but exhibited good sensitivity and specificity values in the prediction of periodontal disease [29]. This finding is consistent with the results of this study, suggesting that periodontopathic bacteria does not necessarily exist in higher numbers in saliva than other bacteria that are not directly associated with the progression of periodontal disease. It was also found that the quantity of F. nucleatum present in saliva was the highest, followed by A. actinomycetemcomitans and P. intermedia in all disease groups. For P. intermedia, the quantity was the highest in MCP, and for A. actinomycetemcomitans the quantity was highest in SCP, although there was no statistical difference among the different disease groups (Fig. 1). This finding suggests that these two pathogens may not be involved in the progression of chronic periodontitis in the elderly.
F. nucleatum is a common human dental plaque microorganism that plays a crucial role in plaque development due to its ability to adhere to a wide range of other plaque microorganisms [23,30]. Therefore, it is not surprising to find that F. nucleatum exhibited its highest levels in saliva among the pathogens tested. A. actinomycetemcomitans is known for being prevalent in young adolescents and adults in more aggressive forms of periodontal disease [21]. However, its presence in geriatric subjects has not yet been adequately studied. It was found that A. actinomycetemcomitans can survive and colonize the oral mucosa in edentulous patients [31], suggesting that the levels of A. actinomycetemcomitans present in geriatric patients may be higher than previously expected. In a recent study [32], it was found that the detection rates of A. actinomycetemcomitans and F. nucleatum were higher than those of P. intermedia, P. gingivalis, T. denticola, and T. forsythia in the saliva of geriatric patients. These results suggest that the levels of A. actinomycetemcomitans and F. nucleatum present in the saliva of geriatric subjects are higher than those of other periodontal pathogens tested. This finding is consistent with the results of the present study. It is likely that A. actinomycetemcomitans and P. intermedia may be involved in the initiation of periodontal disease, but are not associated with the progression of the disease from mild to more advanced chronic periodontitis.
So far, the use of saliva in the diagnosis and management of periodontal disease has not yet been adequately applied in the study of the elderly. Although the analysis of subgingival dental plaque samples acquired from the disease site is warranted warranted for detecting and quantifying periodontal pathogens, this sampling technique requires specific skill and a dental visit. In addition, since bacterial samples are not usually acquired from all periodontal sites but from a few selected periodontal sites, this technique may not represent the whole periodontal condition. On the other hand, saliva sampling can be done by a dental hygienist or nurse, and is also less time-consuming and noninvasive. Therefore, saliva sampling may be an efficient tool for geriatric patients who do not have routine access to a periodontist or dentist, as saliva offers an excellent sample material for large population-based studies of periodontal health or carriage of periodontal pathogens [13,16]. In addition, it has been observed that there is a strong correlation between the levels of periodontal pathogens including P. gingivalis, T. denticola, T. forsythia, and P. intermedia in the subgingival plaque and saliva [29,33]. In addition, the results from this study have confirmed the validity of RT-PCR for identification and quantification of periodontal bacteria present in saliva, as other studies have already confirmed [15-17]. Collectively, these suggest that saliva can be used to monitor the management of periodontal disease of the elderly, especially in a large population in an institution or geriatric community.
Although it is not easy to determine the exact amount of particular pathogens required to cause a more advanced type of periodontitis, for example, going from MCP to MoCP or SCP, it is quite clear that the load of certain bacterial species like P. gingivalis, T. forsythia, and T. denticola increases as the severity of periodontitis increases [21,34,35]. In this study, the quantity of P. gingivalis, T. forsythia, and T. denticola were 4.9, 4.2, and 7 times higher in SCP than in MCP. This result suggests that P. gingivalis, T. forsythia, and T. denticola can be used to estimate the progression of chronic periodontitis in geriatric patients. Particularly, T. denticola, may be the best indicator for disease progression, since it showed a significant difference between MCP and SCP (7-fold difference).
In conclusion, within the limitations of this study, the results suggest that it is possible to use saliva instead of subgingival plaque to measure the levels of periodontal pathogens, and therefore, to assess the microbial aspects of periodontal health and the disease status. It is important to estimate the status of the periodontal condition in geriatric patients in order to evaluate and set up a more efficient tool for monitoring periodontal disease and ensuring periodontal stability via the maintenance program. In addition, oral bacteria may serve as a reservoir for other systemic diseases in geriatric patients. The results of this study indicated that six tested periodontal pathogens were abundantly present in saliva samples of geriatric patients with chronic periodontitis. The amount of red-complex bacteria consisting of P. gingivalis, T. forsythia, and T. denticola, along with that of F. nucleatum was significantly increased when the severity of disease progressed. Therefore, these bacteria may have the potential to be used as reliable markers for advanced periodontal disease in the elderly.
ACKNOWLEDGEMENTS
This study was partially supported by Chonnam National University (2010, 2011) and Chonnam National University Hospital Biomedical Research Institute (Grant CRI 11 031-1).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee R. The outlook for population growth. Science. 2011;333:569–573. doi: 10.1126/science.1208859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz J, Wallet S, Cha S. Periodontal disease and the oral-systemic connection: "is it all the RAGE?". Quintessence Int. 2010;41:229–237. [PubMed] [Google Scholar]
- 3.Dave S, Van Dyke T. The link between periodontal disease and cardiovascular disease is probably inflammation. Oral Dis. 2008;14:95–101. doi: 10.1111/j.1601-0825.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 5.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 6.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A. 1999;96:14547–14552. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt RJ, Levy SM, Beck JD. The prevalence of periodontal attachment loss in an Iowa population aged 70 and older. J Public Health Dent. 1990;50:251–256. doi: 10.1111/j.1752-7325.1990.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert GH, Heft MW. Periodontal status of older Floridians attending senior activity centers. J Clin Periodontol. 1992;19:249–255. doi: 10.1111/j.1600-051x.1992.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 10.Burt B Research, Science and Therapy Committee of the American Academy of Periodontology. Position paper: epidemiology of periodontal diseases. J Periodontol. 2005;76:1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 11.Boehm TK, Scannapieco FA. The epidemiology, consequences and management of periodontal disease in older adults. J Am Dent Assoc. 2007;138(Suppl):26S–33S. doi: 10.14219/jada.archive.2007.0360. [DOI] [PubMed] [Google Scholar]
- 12.Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol. 1990;61:521–528. doi: 10.1902/jop.1990.61.8.521. [DOI] [PubMed] [Google Scholar]
- 13.Kononen E, Jousimies-Somer H, Asikainen S. The most frequently isolated gram-negative anaerobes in saliva and subgingival samples taken from young women. Oral Microbiol Immunol. 1994;9:126–128. doi: 10.1111/j.1399-302x.1994.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 14.Umeda M, Contreras A, Chen C, Bakker I, Slots J. The utility of whole saliva to detect the oral presence of periodontopathic bacteria. J Periodontol. 1998;69:828–833. doi: 10.1902/jop.1998.69.7.828. [DOI] [PubMed] [Google Scholar]
- 15.Boutaga K, van Winkelhoff AJ, Vandenbroucke-Grauls CM, Savelkoul PH. The additional value of real-time PCR in the quantitative detection of periodontal pathogens. J Clin Periodontol. 2006;33:427–433. doi: 10.1111/j.1600-051X.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Boutaga K, Savelkoul PH, Winkel EG, van Winkelhoff AJ. Comparison of subgingival bacterial sampling with oral lavage for detection and quantification of periodontal pathogens by real-time polymerase chain reaction. J Periodontol. 2007;78:79–86. doi: 10.1902/jop.2007.060078. [DOI] [PubMed] [Google Scholar]
- 17.Morillo JM, Lau L, Sanz M, Herrera D, Silva A. Quantitative real-time PCR based on single copy gene sequence for detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. J Periodontal Res. 2003;38:518–524. doi: 10.1034/j.1600-0765.2003.00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Cogulu D, Oncag O, Kutukculer N, Uzel A, Eronat C. The correlation between serum immunoglobulin A and immunoglobulin G levels and the presence of Treponema denticola in human periapical lesions. J Endod. 2007;33:1413–1416. doi: 10.1016/j.joen.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Saito D, Coutinho LL, Borges Saito CP, Tsai SM, Hofling JF, Gonçalves RB. Real-time polymerase chain reaction quantification of Porphyromonas gingivalis and Tannerella forsythia in primary endodontic infections. J Endod. 2009;35:1518–1524. doi: 10.1016/j.joen.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 22.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 23.Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 24.Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(Suppl 6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanner AC, Kent R, Jr, Kanasi E, Lu SC, Paster BJ, Sonis ST, et al. Clinical characteristics and microbiota of progressing slight chronic periodontitis in adults. J Clin Periodontol. 2007;34:917–930. doi: 10.1111/j.1600-051X.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 26.van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002;29:1023–1028. doi: 10.1034/j.1600-051x.2002.291107.x. [DOI] [PubMed] [Google Scholar]
- 27.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 28.Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, Reynolds EC. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol Immunol. 2009;24:469–477. doi: 10.1111/j.1399-302X.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 29.Saygun I, Nizam N, Keskiner I, Bal V, Kubar A, Acikel C, et al. Salivary infectious agents and periodontal disease status. J Periodontal Res. 2011;46:235–239. doi: 10.1111/j.1600-0765.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- 30.Liljemark WF, Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7:180–198. doi: 10.1177/10454411960070020601. [DOI] [PubMed] [Google Scholar]
- 31.Quirynen M, Van Assche N. Microbial changes after full-mouth tooth extraction, followed by 2-stage implant placement. J Clin Periodontol. 2011;38:581–589. doi: 10.1111/j.1600-051X.2011.01728.x. [DOI] [PubMed] [Google Scholar]
- 32.Yasui M, Ryu M, Sakurai K, Ishihara K. Colonisation of the oral cavity by periodontopathic bacteria in complete denture wearers. Gerodontology. 2012;29:e494–e502. doi: 10.1111/j.1741-2358.2011.00506.x. [DOI] [PubMed] [Google Scholar]
- 33.Darout IA, Albandar JM, Skaug N. Correlations between bacterial levels in autologous subgingival plaque and saliva of adult Sudanese. Clin Oral Investig. 2002;6:210–216. doi: 10.1007/s00784-002-0177-0. [DOI] [PubMed] [Google Scholar]
- 34.Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 35.Socransky SS, Haffajee AD, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. J Clin Periodontol. 1991;18:766–775. doi: 10.1111/j.1600-051x.1991.tb00070.x. [DOI] [PubMed] [Google Scholar]