Abstract
Purpose
Nitric oxide (NO) is a short-lived bioactive molecule that is known to play an important role in the pathogenesis of periodontal disease. In the current study, we investigated the effect of the flavonoid quercetin on the production of NO in murine macrophages activated with lipopolysaccharide (LPS) from Prevotella intermedia, a pathogen related to inflammatory periodontal disease, and tried to elucidate the underlying mechanisms of action.
Methods
LPS was isolated from P. intermedia ATCC 25611 cells by the standard hot phenol-water method. The concentration of NO in cell culture supernatants was determined by measuring the accumulation of nitrite. Inducible NO synthase (iNOS) and heme oxygenase-1 (HO-1) protein expression, phosphorylation of c-Jun N-terminal kinase (JNK) and p38, inhibitory κB (IκB)-α degradation, and signal transducer and activator of transcription 1 (STAT1) phosphorylation were analyzed via immunoblotting.
Results
Quercetin significantly attenuated iNOS-derived NO production in RAW246.7 cells activated by P. intermedia LPS. In addition, quercetin induced HO-1 protein expression in cells activated with P. intermedia LPS. Tin protoporphyrin IX (SnPP), a competitive inhibitor of HO-1, abolished the inhibitory effect of quercetin on LPS-induced NO production. Quercetin did not affect the phosphorylation of JNK and p38 induced by P. intermedia LPS. The degradation of IκB-α induced by P. intermedia LPS was inhibited when the cells were treated with quercetin. Quercetin also inhibited LPS-induced STAT1 signaling.
Conclusions
Quercetin significantly inhibits iNOS-derived NO production in murine macrophages activated by P. intermedia LPS via anti-inflammatory HO-1 induction and inhibition of the nuclear factor-κB and STAT1 signaling pathways. Our study suggests that quercetin may contribute to the modulation of host-destructive responses mediated by NO and appears to have potential as a novel therapeutic agent for treating inflammatory periodontal disease.
Keywords: Lipopolysaccharides, Nitric oxide, Periodontal diseases, Prevotella intermedia, Quercetin
INTRODUCTION
Periodontal disease is a chronic bacterial infection of tooth-supporting structures that gives rise to destruction of gingival connective tissue and supporting alveolar bone, causing exfoliation of the teeth [1]. It is clear that periodontal disease is a significant risk factor for some systemic disorders such as coronary heart disease, stroke, and low-birth weight complications in pregnancy, and therefore, the control of periodontal disease contributes to effective prevention and management of these systemic diseases [2,3].
The main etiology of periodontal disease is a specific group of gram-negative anaerobic bacteria that colonize the periodontal pocket. Prevotella intermedia is a key periodontal pathogen that is frequently recovered from the subgingival plaque of patients with chronic periodontitis [4,5], necrotizing ulcerative gingivitis [6], and pregnancy gingivitis [7]. Lipopolysaccharide (LPS) is a principal ingredient of the cell wall of gram-negative bacteria, including P. intermedia. P. intermedia LPS has unique chemical and immunobiological characteristics considerably different from those of the classical LPSs from Escherichia coli and Salmonella species [8-10].
Evidence indicates that host responses to certain periodontopathogens and their products mediate periodontal tissue destruction in periodontitis [11,12]. LPSs from periodontal pathogens can activate host immune cells to produce and release diverse inflammatory mediators such as nitric oxide (NO) and cytokines, and thereby initiate the host inflammatory responses related to periodontal disease [13-16]. Therefore, besides bacterial control, host modulatory agents targeting these proinflammatory mediators induced by LPS appear to be beneficial in attenuating periodontal disease.
NO is a short-lived bioactive molecule generated by immunocompetent cells such as macrophages that acts as a messenger molecule for various physiological and pathological procedures [17]. NO is thought to play an important role in the pathogenesis of periodontal disease, as it does in other inflammatory diseases [18-22]. LPSs from periodontal pathogens, including Aggregatibacter actinomycetemcomitans, P. intermedia, and P. nigrescens, provoked significant production of NO in macrophages [13-15].
Quercetin (3,5,7,3',4'-pentahydroxyflavone) is a flavonoid found in apples, onions, tea, and red wine, and has been reported to possess a wide variety of biological effects, including antioxidative and anti-inflammatory activities [23-25]. The development and progression of inflammatory periodontal disease is associated with oxidative stress, and thus quercetin may have potential as a therapeutic agent for treating periodontal disease. Therefore, in the current study we investigated the effect of quercetin on the production of NO by murine macrophages activated with LPS from P. intermedia, a pathogen related to inflammatory periodontal disease, and tried to elucidate the underlying mechanisms of its action.
MATERIALS AND METHODS
Reagents
Quercetin was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against c-Jun N-terminal kinase (JNK), phospho-JNK, p38, phospho-p38, inhibitory κB (IκB)-α, signal transducer and activator of transcription 1 (STAT1), and phospho-STAT1 were obtained from Cell Signaling Technology (Beverly, MA, USA), while antibodies against heme oxygenase-1 (HO-1) and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Tin protoporphyrin IX (SnPP) was obtained from Frontier Scientific, Inc. (Logan, UT, USA).
Bacterial culture and LPS isolation
P. intermedia ATCC 25611 was cultivated anaerobically on the surface of enriched Trypticase soy agar supplemented with 5% (v/v) sheep blood, or in GAM broth (Nissui, Tokyo, Japan) containing 1 µg/mL menadione and 5 µg/mL hemin. LPS was isolated from lyophilized bacterial cells by the standard hot phenol-water method, as described previously [26]. Briefly, 90% phenol was added to a bacterial suspension, and the mixture was extracted twice at 68℃ for 20 minutes. The aqueous phase was separated by centrifugation, and the pooled aqueous extract was dialyzed against water. The dialyzed extract was treated with DNase (25 µg/mL) and RNase (25 µg/mL) at 37℃ overnight. Contaminating protein was then hydrolyzed with proteinase K (50 µg/mL). The protein content of the purified LPS was less than 0.1%. Coomassie blue staining of overloaded sodium dodecyl sulfate (SDS)-polyacrylamide gels did not show any contaminating protein bands in the prepared LPS, confirming the pureness of the preparation (data not shown).
Cell cultures and assay of cellular toxicity
The murine macrophage cell line RAW264.7 (American Type Culture Collection, Rockville, MD, USA) was cultured in Dulbecco's modified Eagle's medium containing 10% (v/v) heat-inactivated fetal bovine serum and antibiotics (100 U/mL of penicillin and 100 µg/mL of streptomycin) at 37℃ in a humidified incubator under 5% CO2 as described previously [26]. The cells were plated in 24-well culture plates at a density of 5×105 cells/well and cultured for at least 12 hours to allow them to adhere to the plates. After washing three times with medium, different concentrations of P. intermedia LPS and quercetin were added and the cells were cultured for an additional 24 hours, after which the culture supernatants were collected for the NO assay. The cellular toxicity of the quercetin was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously [26].
Measurement of NO production
The concentration of NO in the cell culture supernatants was determined by measuring the accumulation of nitrite (NO2-) [27], as we have described in detail [26]. Briefly, 100 µL of Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, and 2.5% phosphoric acid) (Sigma-Aldrich Co.) was mixed with equal volumes of culture supernatants in 96-well flat-bottomed microplates and incubated at room temperature for 10 minutes. The absorbance at 540 nm was read with a Spectra Max 250 enzyme-linked immunosorbent assay Reader (Molecular Devices, Sunnyvale, CA, USA). Nitrite concentrations were determined using a standard curve established with serial dilutions of NaNO2 (Sigma-Aldrich Co.).
Immunoblotting analysis
Cells were seeded into 60-mm tissue culture dishes, at a density of 4×106 cells per dish, and treated with different concentrations of P. intermedia LPS and quercetin for the indicated periods of time. Cell lysates were prepared and analyzed as described previously [26]. Briefly, the extracted proteins (30 µg) were separated by standard SDS-polyacrylamide gel electrophoresis, electrophoretically transferred onto a nitrocellulose membrane, and subsequently incubated with specific primary antibodies. The membranes were then incubated with secondary antibodies conjugated to horseradish peroxidase and visualized by using a chemiluminescent substrate (Cell Signaling Technology).
Statistical analysis
Data are expressed as means±standard deviation, and statistical significance was determined by the Student t-test, using the Microsoft Excel program. P<0.05 was considered statistically significant.
RESULTS
Effects of quercetin on P. intermedia LPS-induced NO production and iNOS protein expression
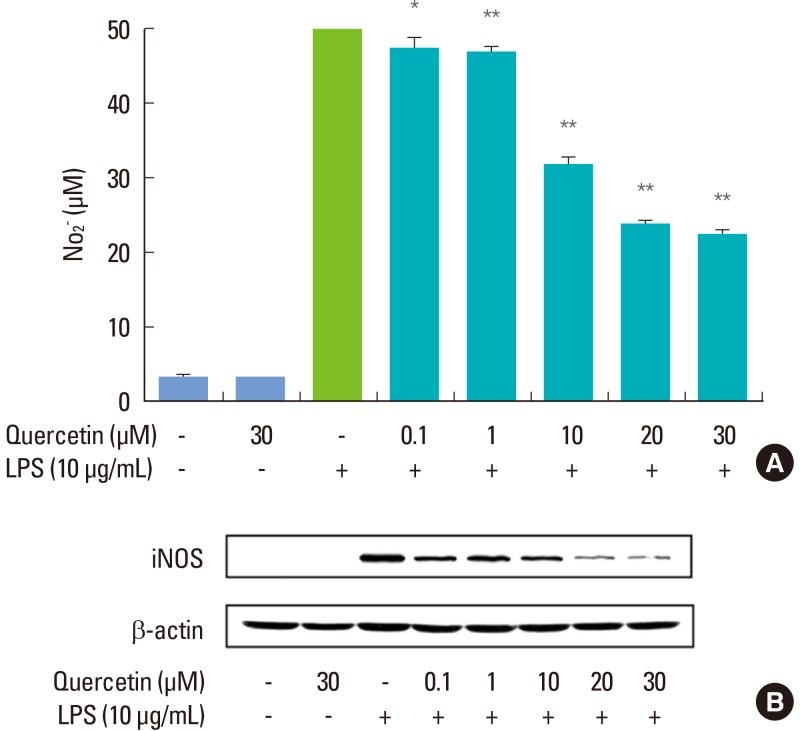
RAW264.7 cells stimulated with P. intermedia LPS produced a significant amount of NO, and quercetin attenuated the LPS-induced NO release in a dose-dependent manner (Fig. 1A). The cell viability was not affected by quercetin as determined by MTT assay (data not shown), suggesting that the inhibitory effect of quercetin on P. intermedia LPS-induced NO production was not due to its cytotoxicity. As shown in Fig. 1B, quercetin also dose-dependently suppressed LPS-induced expression of iNOS protein. At the highest concentration (30 µM), quercetin diminished the iNOS protein by approximately 98%.
Figure 1.
Effects of quercetin on Prevotella intermedia lipopolysaccharide (LPS)-induced nitric oxide (NO) production (A) and inducible NO synthase (iNOS) protein expression (B) in RAW264.7 cells. Cells were treated with different doses of quercetin (0, 0.1, 1, 10, 20, and 30 µM) in the absence or presence of P. intermedia LPS (10 µg/mL) for 24 hours. (A) NO production was assayed by measuring the accumulation of nitrite in culture supernatants. The results are mean±standard deviation of three independent experiments. *P<0.05 vs. P. intermedia LPS alone. **P<0.01 vs. P. intermedia LPS alone. (B) iNOS protein synthesis was measured by immunoblot analysis of cell lysates using an iNOS-specific antibody. A representative immunoblot from two separate experiments with similar results is shown.
Involvement of HO-1 in the inhibitory effect of quercetin on P. intermedia LPS-induced NO production
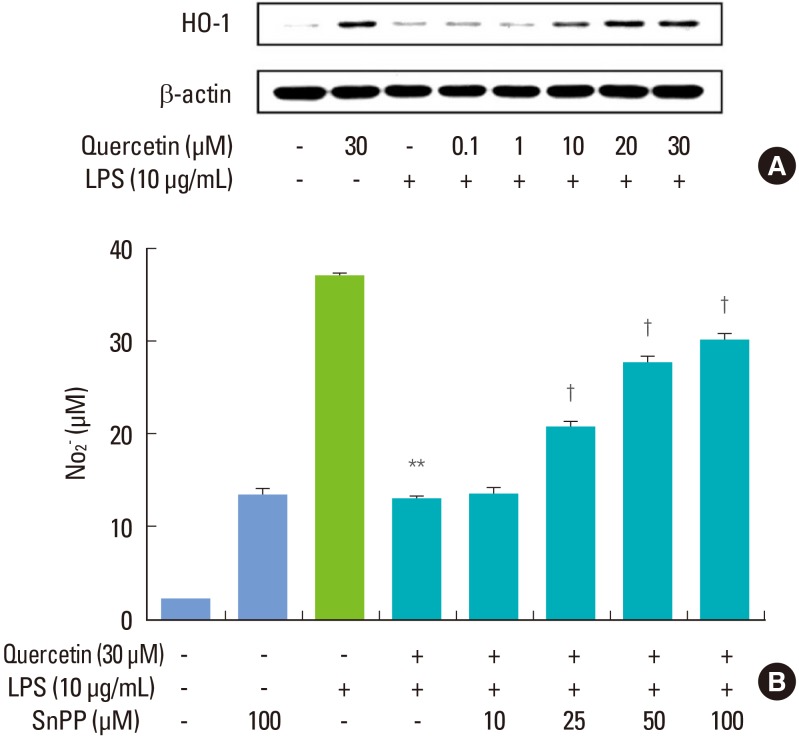
In cells activated with P. intermedia LPS, quercetin induced HO-1 protein in a dose-dependent manner (Fig. 2A). We next tested whether the suppressive effect of quercetin on P. intermedia LPS-induced NO production was related to quercetin-mediated HO-1 induction. Treatment SnPP, a competitive inhibitor of HO-1, abolished the inhibitory effect of quercetin on P. intermedia LPS-induced NO production concentration-dependently (Fig. 2B).
Figure 2.
Involvement of heme oxygenase-1 (HO-1) in the inhibitory effects of quercetin on Prevotella intermedia lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW264.7 cells. (A) Cells were treated with different doses of quercetin (0, 0.1, 1, 10, 20, and 30 µM) in the absence or presence of P. intermedia LPS (10 µg/mL) for 6 hours. HO-1 protein synthesis was measured by immunoblot analysis of cell lysates using HO-1-specific antibody. A representative immunoblot from two separate experiments with similar results is shown. (B) Cells were treated with quercetin (30 µM) and P. intermedia LPS (10 µg/mL) for 24 hours in the presence of different doses of tin protoporphyrin IX (SnPP) (0, 10, 25, 50, and 100 µM). Supernatants were removed after 24 hours and assayed for NO. The results are mean±standard deviation of three independent experiments. **P<0.01 vs. P. intermedia LPS alone. †P<0.01 vs. P. intermedia LPS plus quercetin.
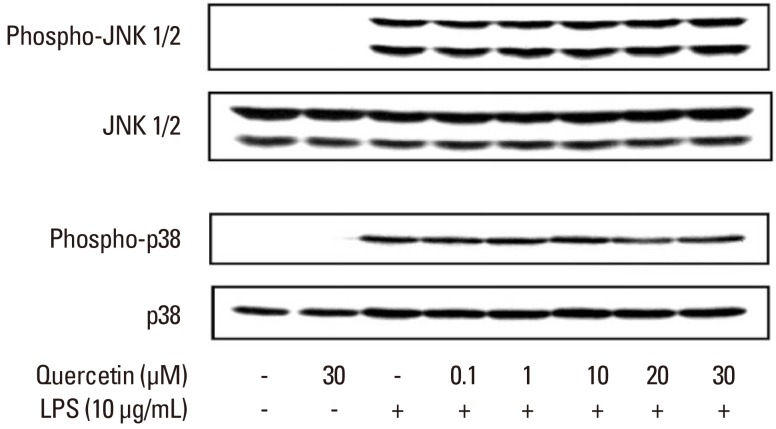
Effects of quercetin on P. intermedia LPS-induced phosphrylation of JNK and p38
Our previous study [26] demonstrated that P. intermedia LPS induces the production of NO in RAW264.7 cells via the JNK, p38, nuclear factor (NF)-κB, and JAK2/STAT1 pathways. We first examined the effect of quercetin on P. intermedia LPS-induced activation of JNK and p38. As shown in Fig. 3, quercetin did not inhibit the phosphorylation of JNK or p38 induced by LPS.
Figure 3.
Effects of quercetin on Prevotella intermedia lipopolysaccharide (LPS)-induced phosphorylation of c-Jun N-terminal kinase (JNK) and p38 in RAW264.7 cells. Cells were incubated with different doses of quercetin (0, 0.1, 1, 10, 20, and 30 µM) in the absence or presence of P. intermedia LPS (10 µg/mL) for 30 minutes (for JNK) or 15 minutes (for p38). The cell lysates were subjected to immunoblot analysis using specific antibodies. A representative immunoblot from two separate experiments with similar results is shown.
Effect of quercetin on P. intermedia LPS-induced IκB-α degradation
We next determined whether quercetin affects the NF-κB signaling pathway that mediates P. intermedia LPS-induced production of NO. To examine the effect of quercetin on P. intermedia LPS-induced degradation of IκB-α, upstream signaling pathway of NF-κB, the levels of cytoplasmic IκB-α protein were evaluated. The degradation of IκB-α induced by LPS was inhibited when the cells were treated with quercetin (Fig. 4).
Figure 4.
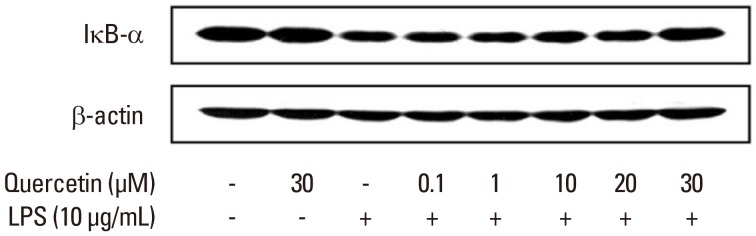
Effects of quercetin on Prevotella intermedia lipopolysaccharide (LPS)-induced inhibitory κB (IκB)-α degradation in RAW264.7 cells. Cells were incubated with different doses of quercetin (0, 0.1, 1, 10, 20, and 30 µM) in the absence or presence of P. intermedia LPS (10 µg/mL). After 30 minutes of incubation, IκB-α degradation was determined by immunoblot analysis of cell lysates using an antibody against IκB-α. A representative immunoblot from two separate experiments with similar results is shown.
Effect of quercetin on P. intermedia LPS-induced phosphorylation of STAT1
In addition, we investigated the effect of quercetin on P. intermedia LPS-induced phosphorylation of STAT1. The phosphorylation of STAT1 induced by LPS was dose-dependently inhibited when the cells were treated with quercetin (Fig. 5).
Figure 5.
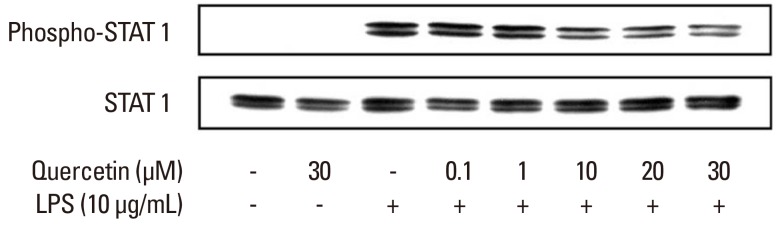
Effects of quercetin on Prevotella intermedia lipopolysaccharide (LPS)-induced phosphorylation of signal transducer and activator of transcription 1 (STAT1) in RAW264.7 cells. Cells were incubated with different doses of quercetin (0, 0.1, 1, 10, 20, and 30 µM) in the absence or presence of P. intermedia LPS (10 µg/mL) for 4 hours. Expression of phospho-STAT1 was measured by immunoblot analysis of cell lysates. Total STAT1 was used as an internal control. A representative immunoblot from two separate experiments with similar results is shown.
DISCUSSION
NO is known to play an important role in the pathogenesis of periodontal disease [18-22]. Thus, this molecule could be a potential therapeutic target for the host modulation of periodontal disease. The results of the present study indicate that quercetin significantly attenuates the release of iNOS-induced NO in P. intermedia LPS-activated murine macrophages, and hence this flavonoid may have a potential use in the treatment of periodontal disease.
HO-1 catalyzes the degradation of heme into carbon monoxide, free iron, and biliverdin [28,29]. Studies have shown that HO-1 plays an important role in the regulation of oxidative stress and inflammation [28-30]. In animal models, HO-1 deficient mice demonstrated an increased inflammatory state, but overexpression of HO-1 exerted anti-inflammatory effects [28,31]. Due to its anti-inflammatory and antioxidative effects, HO-1 is suggested to be a potential therapeutic target for the treatment of inflammatory diseases. In this study, we examined the possibility that the suppressive effect of quercetin against P. intermedia LPS-induced NO production is related to HO-1 expression induced by quercetin. The results indicated that quercetin-mediated HO-1 induction plays a role in modulating P. intermedia LPS-induced NO production. Evidence suggests that the inhibitory effect of quercetin against LPS-induced NO production is mediated by the enzymatic by-products of heme catabolism, such as CO and bilirubin [32-34]. Our previous study [35] also showed that HO-1 led to the effect of the flavonoid kaempferol on regulating P. intermedia LPS-induced NO release. Kaempferol inhibited NO production and iNOS protein expression in P. intermedia LPS-stimulated RAW264.7 cells at the translational level via HO-1-mediated reactive oxygen species reduction.
Mitogen-activated protein kinase signaling pathways are known to be involved in LPS-induced production of proinflammatory mediators in macrophages. However, quercetin did not affect the phosphorylation of JNK and p38 induced by P. intermedia LPS, suggesting that these pathways do not take part in the inhibition of LPS-induced NO release by quercetin.
NF-κB is a transcription factor that plays a critical role in LPS-induced activation of macrophages and the resultant production of proinflammatory cytokines and other mediators [36,37]. In resting cells, NF-κB is maintained in the cytoplasm in a latent form linked to the IκB proteins. In response to a variety of stimuli, including LPS, IκB becomes phosphorylated and degraded. Then, the free NF-κB is translocated into the nucleus, where it induces the transcription of a wide variety of target genes [38]. In this study, the degradation of IκB-α induced by P. intermedia LPS was inhibited when cells were treated with quercetin. Since NF-κB has been considered an attractive molecular target for the treatment of inflammatory diseases, inhibition of NF-κB activity by quercetin would be useful in inflammatory periodontal disease therapy.
Another transcription factor that is significant in the regulation of inflammatory responses is the STAT. Janus kinases (JAKs) activate the transcription factors of this family. STAT1, downstream of JAK2, has been reported to be important for gene expression in macrophages activated by LPS [39]. The results of the present study indicate that quercetin attenuates P. intermedia LPS-induced production of NO via inhibition of the STAT1 cascade. Thus, STAT1 could also be an attractive molecular target for treating inflammatory periodontal disease.
In conclusion, the present study showed that quercetin significantly inhibits iNOS-derived NO production in murine macrophages activated by P. intermedia LPS. The underlying mechanisms of the inhibitory effect of quercetin involve anti-inflammatory HO-1 induction and inhibition of the NF-κB and STAT1 signaling pathways. Although further studies are needed to delineate the detailed mechanism of action, our study suggests that quercetin may contribute to the modulation of host-destructive responses mediated by NO and appears to have potential as a novel therapeutic agent for treating inflammatory periodontal disease.
ACKNOWLEDGEMENTS
This work was supported by a 2-Year Research Grant of Pusan National University.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 2.Teng YT, Taylor GW, Scannapieco F, Kinane DF, Curtis M, Beck JD, et al. Periodontal health and systemic disorders. J Can Dent Assoc. 2002;68:188–192. [PubMed] [Google Scholar]
- 3.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanner AC, Haffer C, Bratthall GT, Visconti RA, Socransky SS. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979;6:278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 5.Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung CP, Nisengard RJ, Slots J, Genco RJ. Bacterial IgG and IgM antibody titers in acute necrotizing ulcerative gingivitis. J Periodontol. 1983;54:557–562. doi: 10.1902/jop.1983.54.9.557. [DOI] [PubMed] [Google Scholar]
- 7.Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980;15:111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamada S, Takada H, Ogawa T, Fujiwara T, Mihara J. Lipopolysaccharides of oral anaerobes associated with chronic inflammation: chemical and immunomodulating properties. Int Rev Immunol. 1990;6:247–261. doi: 10.3109/08830189009056635. [DOI] [PubMed] [Google Scholar]
- 9.Kirikae T, Nitta T, Kirikae F, Suda Y, Kusumoto S, Qureshi N, et al. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect Immun. 1999;67:1736–1742. doi: 10.1128/iai.67.4.1736-1742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto M, Asai Y, Tamai R, Jinno T, Umatani K, Ogawa T. Chemical structure and immunobiological activity of lipid A from Prevotella intermedia ATCC 25611 lipopolysaccharide. FEBS Lett. 2003;543:98–102. doi: 10.1016/s0014-5793(03)00414-9. [DOI] [PubMed] [Google Scholar]
- 11.Preshaw PM. Host response modulation in periodontics. Periodontol 2000. 2008;48:92–110. doi: 10.1111/j.1600-0757.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- 12.Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents: a systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Sosroseno W, Barid I, Herminajeng E, Susilowati H. Nitric oxide production by a murine macrophage cell line (RAW264.7) stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 2002;17:72–78. doi: 10.1046/j.0902-0055.2001.00091.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Ha MS, Choi EY, Choi JI, Choi IS. Prevotella intermedia lipopolysaccharide stimulates release of nitric oxide by inducing expression of inducible nitric oxide synthase. J Periodontal Res. 2004;39:424–431. doi: 10.1111/j.1600-0765.2004.00757.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Ha MS, Choi EY, Choi JI, Choi IS. Nitric oxide production and inducible nitric oxide synthase expression induced by Prevotella nigrescens lipopolysaccharide. FEMS Immunol Med Microbiol. 2005 Jan 1;43:51–58. doi: 10.1016/j.femsim.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Choi EY, Kim EG, Shin SH, Lee JY, Choi JI, et al. Prevotella intermedia lipopolysaccharide stimulates release of tumor necrosis factor-alpha through mitogen-activated protein kinase signaling pathways in monocyte-derived macrophages. FEMS Immunol Med Microbiol. 2007;51:407–413. doi: 10.1111/j.1574-695X.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 17.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 18.Matejka M, Partyka L, Ulm C, Solar P, Sinzinger H. Nitric oxide synthesis is increased in periodontal disease. J Periodontal Res. 1998;33:517–518. doi: 10.1111/j.1600-0765.1998.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 19.Kendall HK, Haase HR, Li H, Xiao Y, Bartold PM. Nitric oxide synthase type-II is synthesized by human gingival tissue and cultured human gingival fibroblasts. J Periodontal Res. 2000;35:194–200. doi: 10.1034/j.1600-0765.2000.035004194.x. [DOI] [PubMed] [Google Scholar]
- 20.Lappin DF, Kjeldsen M, Sander L, Kinane DF. Inducible nitric oxide synthase expression in periodontitis. J Periodontal Res. 2000;35:369–373. doi: 10.1034/j.1600-0765.2000.035006369.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirose M, Ishihara K, Saito A, Nakagawa T, Yamada S, Okuda K. Expression of cytokines and inducible nitric oxide synthase in inflamed gingival tissue. J Periodontol. 2001;72:590–597. doi: 10.1902/jop.2001.72.5.590. [DOI] [PubMed] [Google Scholar]
- 22.Batista AC, Silva TA, Chun JH, Lara VS. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 2002;8:254–260. doi: 10.1034/j.1601-0825.2002.02852.x. [DOI] [PubMed] [Google Scholar]
- 23.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Davis JM, Murphy EA, McClellan JL, Carmichael MD, Gangemi JD. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol Regul Integr Comp Physiol. 2008;295:R505–R509. doi: 10.1152/ajpregu.90319.2008. [DOI] [PubMed] [Google Scholar]
- 25.Utesch D, Feige K, Dasenbrock J, Broschard TH, Harwood M, Danielewska-Nikiel B, et al. Evaluation of the potential in vivo genotoxicity of quercetin. Mutat Res. 2008;654:38–44. doi: 10.1016/j.mrgentox.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Choi EY, Jin JY, Lee JY, Choi JI, Choi IS, Kim SJ. Melatonin inhibits Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-6 in murine macrophages by suppressing NF-κB and STAT1 activity. J Pineal Res. 2011;50:197–206. doi: 10.1111/j.1600-079X.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- 27.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 28.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 29.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 30.Morse D, Choi AM. Heme oxygenase-1: the "emerging molecule" has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 31.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 33.Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 34.Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1131–L1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- 35.Choi IS, Choi EY, Jin JY, Park HR, Choi JI, Kim SJ. Kaempferol inhibits P. intermedia lipopolysaccharide-induced production of nitric oxide through translational regulation in murine macrophages: critical role of heme oxygenase-1-mediated ROS reduction. J Periodontol. 2013;84:545–555. doi: 10.1902/jop.2012.120180. [DOI] [PubMed] [Google Scholar]
- 36.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 37.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 39.Gao JJ, Filla MB, Fultz MJ, Vogel SN, Russell SW, Murphy WJ. Autocrine/paracrine IFN-αβ mediates the lipopolysaccharide-induced activation of transcription factor Stat1α in mouse macrophages: pivotal role of Stat1alpha in induction of the inducible nitric oxide synthase gene. J Immunol. 1998;161:4803–4810. [PubMed] [Google Scholar]