Gastric adenocarcinoma of the intestinal type is believed to develop through a series of histopathologic stages, originally described by Correa, that include chronic gastritis, gastric atrophy, intestinal metaplasia, dysplasia and finally cancer (1). Intestinal metaplasia has been the focus of great fascination by many pathologists, since it was unexpected to find intestinal type mucosa containing goblet cells in the body or antrum of the stomach. Numerous studies have implicated intestinal metaplasia as a useful biomarker for gastric cancer risk, and even possibly a precursor to gastric malignancy. However, this evidence has been largely correlative. In patients with gastric cancer, intestinal metaplasia is frequently found in the stomach, even with early gastric cancer. Intestinal metaplasia is much more prevalent in high-risk areas for gastric cancer compared to low risk areas. Finally, in several studies where serial endoscopies and biopsies were performed, patients with intestinal metaplasia, particularly type III, frequently developed gastric cancer (2). Intestinal metaplasia has been used as the key biomarker in studies of H. pylori eradication or gastric cancer prevention, defining the preneoplastic lesion often considered the “point-of-no-return” (3). Indeed, since the gastric cancers that developed in this setting also showed intestinal differentiation, it seemed logical to conclude that the cancers arose directly from these intestinal metaplastic cells. Neverthless, in contrast with other neoplasms, little genetic mutational evidence exists to implicate intestinal metaplastic lineages as true direct precursors of gastric neoplasia.
Recent investigations have also highlighted the existence of a second metaplastic lineage, Spasmolytic Polypeptide-Expressing Metaplasia (SPEM) (4). SPEM is a metaplastic mucous cell lineage with phenotypic characteristics of deep antral gland cells including strong expression of Trefoil Factor 2 (TFF2, previously designated as spasmolytic polypeptide). In the past, this underappreciated mucous lineage was described with various names including pseudopyloric metaplasia or mucous metaplasia or antralization of the corpus, but mostly the lineage was ignored. Indeed, it is now appreciated that atrophy of the corpus and body of the stomach is always associated with the development of SPEM. In addition, SPEM is at least as strongly associated with the development of gastric cancer as intestinal metaplasia (4). Moreover, while intestinal metaplasia tends to be spotty or multifocal, SPEM typically appears diffusely throughout the body and corpus of the stomach in patients that progress to gastric cancer (5). SPEM is not simply an expansion of antral type mucosa, since gastrin cells are not present and SPEM cells express a number of proteins, such as HE-4, that are not expressed in the antrum (6). However, both the precise connection between intestinal metaplasia and SPEM and the relationship between SPEM and gastric cancer remain unclear.
Recent studies have examined the cellular origin of SPEM in mice. These investigations have demonstrated that SPEM develops in the fundus in the setting of parietal cell loss following chronic Helicobacter infection (7, 8). acute parietal cell loss due to treatment with DMP-777 (9), or chronic genetic parietal cell ablation by transgenically expressed toxin (10). These studies have led to accumulating evidence that SPEM may originate from transdifferentiation of mature chief cells into SPEM (6). In the case of H. felis infection, gastritis cystica profunda and dysplasia evolve from preexisting regions of SPEM (11). Still these studies have not been able to address intestinal metaplasia because Helicobacter-infected mice do not typically develop intestinal metaplasia. Intestinal goblet cells do develop in the fundus of transgenic mice where cdx2, a transcription factor, which is a master developmental regulator of intestinal differentiation, is targeted to the stomach using elements of the H+/K+-ATPase β subunit promoter (12, 13). Interestingly, amphiregulin-deficient mice developed SPEM after 6 months of age, and a subset of mice did go on to develop goblet cell intestinal metaplasia (14). In that case, compound glands containing both SPEM and intestinal metaplasia were clearly present. While murine studies have not been able to clarify the relationship between intestinal metaplasia and SPEM, studies in Mongolian gerbils have provided additional insights. Mongolian gerbils develop both SPEM and intestinal metaplasia in the fundus following infection with Helicobacter pylori (15). In Mongolian gerbils, intestinal metaplasia developed in pre-existing SPEM glands and mixed glands expressing both SPEM and intestinal metaplasia were clearly present (15). All of these studies have supported the notion that intestinal metaplasia may develop from pre-existing SPEM.
No published studies in humans have addressed directly the question of the relationship of SPEM to intestinal metaplasia in humans. We have therefore examined the morphological characteristics of SPEM and intestinal metaplasia in gastric resections specimens exclusively from the fundus. These studies have uncovered several critical observations about the induction of metaplasias in the stomach. First, SPEM can develop as a very localized phenomenon. Figure 1 demonstrates that SPEM can develop in single or groups of glands surrounded by regions with normal appearing mucous neck cells in the fundic mucosa. At times these SPEM gland groups are associated with adjacent areas with the appearance of mucous neck cell hyperplasia. These regions do not appear to interact with regions of intestinal metaplasia. The focal phenotype of SPEM glands suggests that they may act as part of a normal local reparative mechanism for the gastric mucosa.
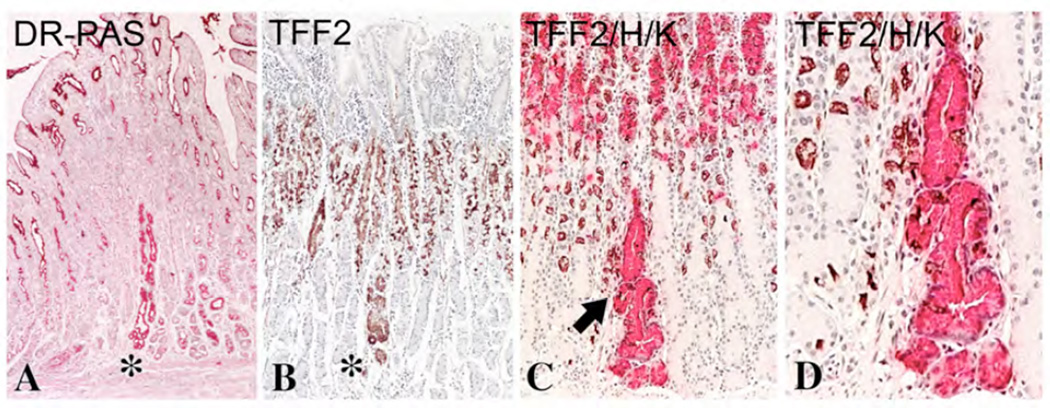
Figure 1. Focal early lesions for SPEM induction.
A. Diastase resistant-PAS (DR-PAS) staining of a section of human fundic mucosa showing the focal development of SPEM in a single gland unit (star). Note that in comparison with the carmine staining of surface cells, SPEM staining with DR-PAS is characteristically more reddish pink. B. TFF2 immunostaining staining with horseradish peroxidase secondary antibody staining and DAB (brown) chromagen showing a single gland unit with SPEM (star) surrounded by normal glands with TFF2-staining of mucous neck cells. C and D. Dual staining for TFF2 (red, alkaline phosphatase secondary antibody and Vector Red chromagen) and H/K-ATPase (brown DAB staining) staining of parietal cells showing the presence of one SPEM gland in the fundic mucosa.
We have also sought to determine if there is a relationship between SPEM glands and intestinal metaplasia in gastric resections. Examination of resection sections containing regions of both SPEM and intestinal metaplasia led to identification of regions with compound glands where SPEM cells were observed in the deep portions of the glands with intestinal metaplastic lineages in the luminal portions of the glands (Figure 2). Thus, goblet cells staining with either Alcian Blue or Muc2 were observed on the luminal aspects of glands that contain PAS-positive and TFF2-staining SPEM at their bases (Figure 2). It is important to emphasise that these were not residual pyloric type glands, since these sections were taken from areas surrounded by corpus mucosa. While we did observe scattered proliferative Ki67-positive cells within SPEM, in regions with intestinal metaplasia immediately adjacent to or overlying SPEM, we observed strong staining for Ki67 in cells within the region demarcating the zone between SPEM and intestinal metaplasia (Figure 2). Many of the Ki67-positive cells were also stained for MUC2. Thus, we observed clear evidence for the existence of intestinal metaplasia emanating from SPEM. The elevated proliferation in intestinal metaplasia adjacent to SPEM may indicate a transition or secondary differentiation of SPEM into intestinal metaplasia.
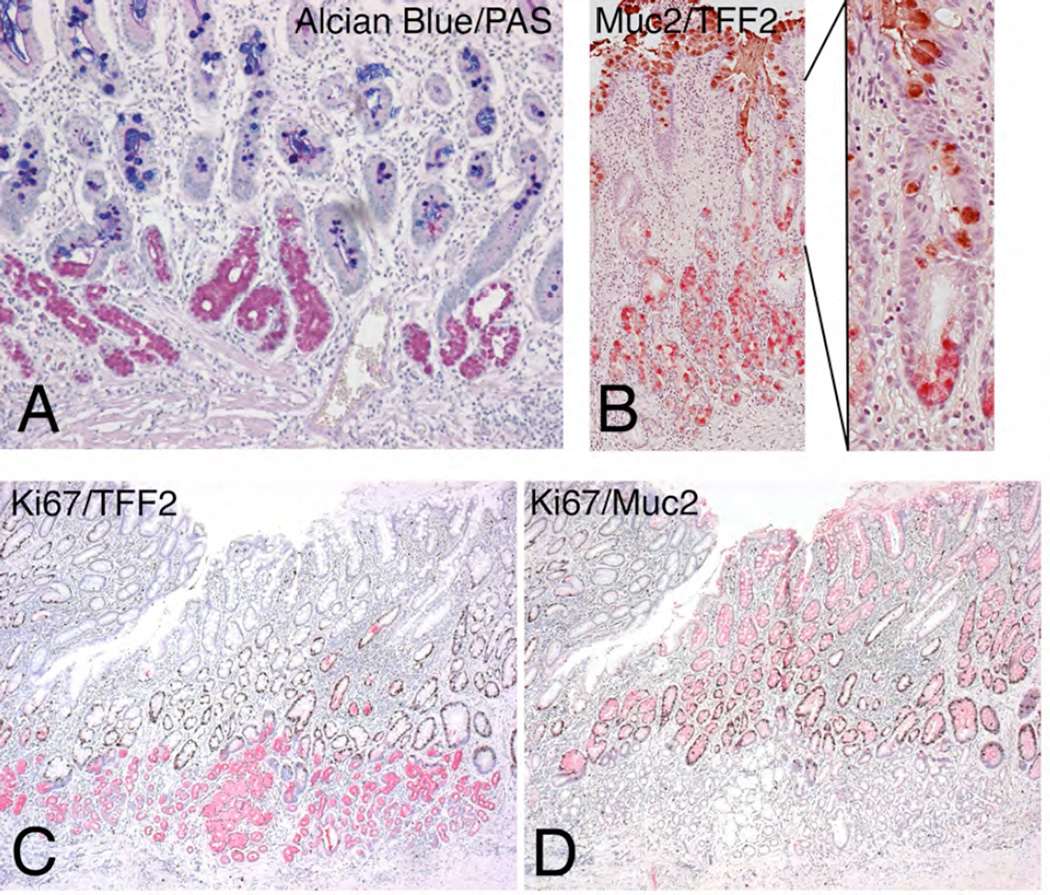
Figure 2. Compound glands containing SPEM and Intestinal Metaplasia.
A. Dual Alcian Blue and PAS staining of a human fundic specimen showing compound glands with Alcian Blue staining intestinal metaplasia in more luminal cells and PAS-staining SPEM at the bases of glands. B. Dual Muc2 (brown) and TFF2 (red) immunostaining of a section of fundic mucosa showing glands with Muc2-immunoreactive intestinal metaplasia surmounting TFF2-staining SPEM. C and D. Serial sections of fundic mucosa from a resection specimen showing in C dual immunostaining for Ki67 (brown nuclei) and TFF2 (red) and in D dual immunostaining for Ki67 and Muc2 (red). While Ki67 staining nuclei can be seen in scattered SPEM cells, the majority of Ki67-staining cells nuclei are seen in Muc2-immunoreactive cells at the interface between SPEM and intestinal metaplasia.
These data indicate that the standard concept proposed by Professor Correa now merits further expansion or modification (Figure 3). Work in mouse models and human tissue suggests that loss of parietal cells leads initially to the induction of SPEM. With chronic inflammation in the setting of parietal cell loss, SPEM may give rise to a further differentiation into intestinal metaplasia. This evolution of mucous cell metaplastic lineages has been noted previously for the Ulcer Associated Lineages (UACL) identified in patients with inflammatory bowel disease (16). While these findings create a strategy for the induction and progression of metaplastic lineages in humans, they do not address the actual origin of gastric adenocarcinoma. It is particularly exciting to consider that if SPEM is derived from chief cell transdifferentiation, then chief cells themselves, or a subset of chief cells, represent an unrecognized progenitor population that can be induced in the pathological stomach. Given the more differentiated nature of intestinal metaplasia, it now seems somewhat less likely that gastric cancer arises from a goblet cell-containing epithelium. Still, we must acknowledge that at present it remains uncertain whether either SPEM or intestinal metaplasia is a true precursor for cancer. Nevertheless, it seems likely that evolution of intestinal metaplasia from SPEM may lead to a hyperproliferative state in which the infected and inflamed mucosa may be more susceptible to establishment of deleterious mutations in stem or progenitor populations. Thus, SPEM and intestinal metaplasia may be commensals for the neoplastic process rather than true direct precursors. Taken together, this work suggests that a broader view of metaplastic initiation and pre-neoplastic progression is merited in evaluating gastric carcinogenesis.
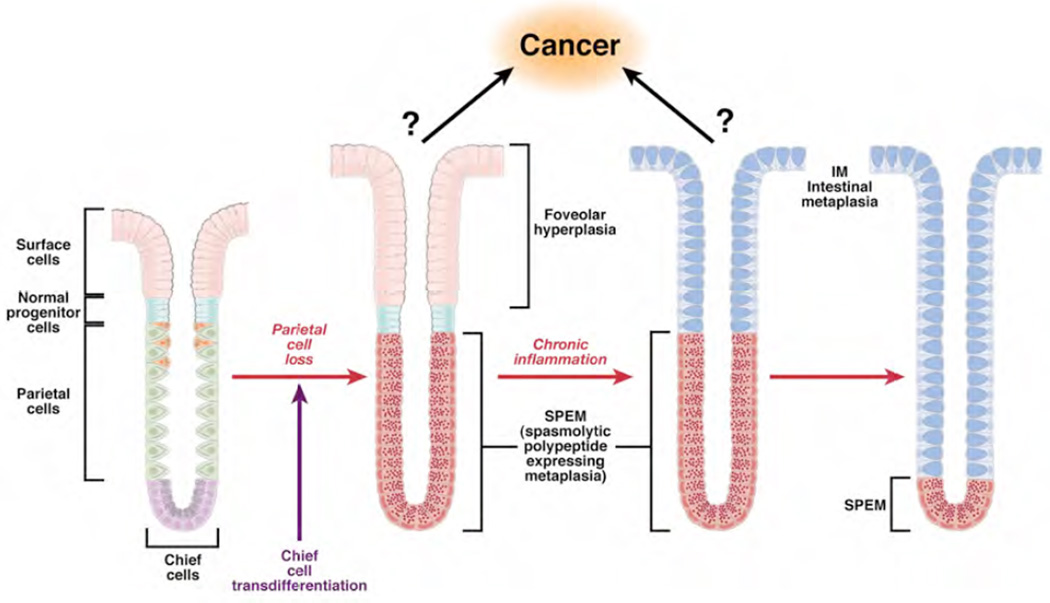
Figure 3. A revised model for the evolution of metaplasia in the stomach.
Loss of parietal cells leads to evolution of SPEM at the bases of glands from transdifferentiation of chief cells. With continuing chronic inflammation, intestinal metaplasia develops within the luminal aspect of SPEM glands. Over time, intestinal metaplasia comes to dominate over SPEM in metaplastic mucosa. In remains to be determined whether gastric cancer arises form SPEM or from proliferative intermediates generated during the further differentiation of SPEM into intestinal metaplasia.
References
- 1.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 2.Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32:1110–1113. doi: 10.1136/gut.32.10.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zivny J, Wang TC, Yantiss R, Kim KH, Houghton J. Role of therapy or monitoring in preventing progression to gastric cancer. J Clin Gastroenterol. 2003;36:S50–60. doi: 10.1097/00004836-200305001-00009. discussion S61–52. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab.Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 5.El-Zimaity HMT, Ota H, Graham DY, Akamatsu T, Katsuyama T. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428–1436. doi: 10.1002/cncr.10375. [DOI] [PubMed] [Google Scholar]
- 6.Nozaki K, Ogawa M, Williams JA, LaFleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008:511–521. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, Odze R, Wang TC. Hypertrophic gastropathy in Helicobacter felis-Infected wild type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 8.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 9.Nomura S, Yamaguchi H, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild type and gastrin deficient mice. Amer.J.Physiol. 2004;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 10.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houghton J, Stoicov C, Nomura S, Carlson J, Li H, Rogers AB, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 12.Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka I, Honda S, Osawa H, Kaneko Y, Sugano K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 13.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 14.Nam KT, Lee HJ, Mok H, Romero-Gallo J, Crowe JE, Jr, Peek RM, Jr, Goldenring JR. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizawa N, Takenaka Y, Yamaguchi H, Tetsuya T, Tanaka H, Tatematsu M, Nomura S, Goldenring JR, Kaminishi M. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 16.Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]