Abstract
Animal and in vitro studies suggest that the use of bisphosphonates (BPs) may be associated with reduced risk for colorectal cancer (CRC). However, results from these studies have been inconsistent. The aim of our study was to review and summarize the evidence provided by longitudinal studies on the association between BP use and CRC risk A comprehensive literature search for articles published up to October 2012 was performed. Prior to performing a meta-analysis, the studies were evaluated for publication bias and heterogeneity. Relative risks (RRs) or odds ratios were calculated. Six reports (four case–control studies and two cohort studies) published between 2010 and 2012 were identified. There was evidence of an association between any use of BPs and CRC risk using a fixed-effects model (RR = 0.80, 95% confidence interval = 0.74, 0.85) and a random-effects model (RR = 0.80, 95% confidence interval = 0.71, 0.90). However, we did not observe any evidence of a trend with increasing duration of use. Our findings indicate that there is evidence of an association between any use of BP and reduced CRC risk. However, this subject deserves further investigation.
Keywords: bisphosphonates, colorectal cancer
Introduction
Colorectal cancer (CRC) continues to be the second-most common cause of cancer-related deaths. A potential alternative strategy to reducing the incidence of CRC would be the use of pharmaceutical agents to prevent development of CRC (chemoprevention). The slow and long natural history of development of CRC provides a window of opportunity for implementing effective chemopreventive strategies. Chemopreventive agents may act in the preclinical phase and reduce the risk of CRC or delay the development of CRC by suppressing the growth of cancer cells or their precursors. Although several chemopreventive agents for CRC have been studied, none is currently recommended for use.
Bisphosphonates (BPs) are inhibitors of bone resorption, more than a dozen of which have been used clinically [1]; they are commonly used for the prevention and treatment of osteoporosis and other skeletal conditions, such as bone metastases [2–6]. Preclinical studies suggest that BPs can inhibit angiogenesis, invasion and adhesion of tumour cells and overall tumour progression and can stimulate adaptive and innate immunity [7]. Recently, reduced proliferation and induction of apoptosis of colon cancer cells have been demonstrated [8, 9]. In an experimental model of ulcerative colitis, BP therapy reportedly inhibited colorectal carcinogenesis [10].
Some clinical studies have demonstrated that BP use correlates with a significant reduction in CRC risk [11–14]. Based on these interesting findings, several observational studies have explored whether the use of BPs decreases the risk of CRC and found that women who received BP had a significant risk reduction of CRC compared with non-users. Analysis of the results of several studies suggests that the duration of BP use was also partly correlated with the risk reduction of CRC. However, those findings were inconsistent. Khalili et al. [15] showed that, compared with non-users, the multivariate-adjusted hazard ratios of CRC were 1.24 [95% confidence interval (CI) = 0.94, 1.64] for women with 1–2 years of use, 1.16 (95% CI = 0.79, 1.69) for 3–4 years of use, and 0.97 (95% CI = 0.60, 1.56) for 5 years of use. There was no association between BP use and CRC within strata of other risk factors. Green et al. [16] showed that there was a borderline association between prescription of BPs and colorectal cancer; relative risk (RR) for one or more vs. no prescriptions was 0.87 (95% CI = 0.77, 1.00).
We conducted a meta-analysis to quantify the risk reduction of CRC that is associated with BP use and to explore whether a threshold effect that is based on the duration of BP use exists.
Materials and methods
Retrieval of published studies
In order to identify the studies of interest, we conducted a computerized literature search. Sources included Pubmed, Web of Science, Medline and Embase (updated to 2 October 2012). Search terms included the following: bisphosphonates combined with colorectal cancer, or colorectal carcinoma. The titles and abstracts of the studies identified in the computerized search were scanned to exclude any studies that were clearly irrelevant. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. The reference lists of articles with information on the topic were reviewed to identify citations to other studies on the same topic. Reference lists of review articles were also inspected to determine relevant publications for completeness of our list of publications.
Inclusion and exclusion criteria
A study was included if it fulfilled the following criteria: (i) it was designed as a cohort study, case–control study or clinical trial; (ii) it evaluated the exposure factor of BP; and (iii) it had an outcome of colorectal cancer incidence. Studies without raw data about exposure and measurements were excluded. In the subgroup analyses, studies that did not provide more detailed information about dose–response effects were eliminated. Inclusion was not restricted by study size.
Data extraction
Data were extracted by two independent reviewers using the same standardized form. Discrepancies were settled through additional reviews until a consensus was reached. Information obtained from each study included the first author, year of publication, study design, the number of subjects in the exposure groups, the sample size, types of BPs, the definition of exposure, hazard ratio/odds ratio (OR) or with 95% CI, and the adjusted potential confounding factors.
Statistical analysis
Two techniques were used to estimate the pooled relative risk estimates, namely the Mantel–Haenszel method [17] assuming a fixed-effects model and the DerSimonian–Laird method [18] assuming a random-effects model. The fixed-effects model leads to valid inferences about the particular studies that have been assembled, and the random-effects model assumes that the particular study samples were drawn from a larger pool of potential studies and leads to inferences about all studies in the hypothetical population of studies. If heterogeneity is not present (P > 0.05), the fixed-effects models may be biased. When heterogeneity is found (P ≤ 0.05), the random-effects models may be biased [17, 18].
The between-study heterogeneity was evaluated by using the Q statistic and the I2 statistic [19]. The potential publication bias was examined by using a funnel plot for asymmetry and was further examined quantitatively by using the Begg rank correlation test and the Egger linear regression test [20]. The Begg and Mazumdar test is a statistical analogue of the visual funnel graph. It determines whether there is a significant correlation between the effect estimates and their variances. The absence of significant correlation suggests that the studies have been selected in an unbiased manner. The Egger regression asymmetry test tends to indicate the presence of a publication bias more frequently than the Begg approach. It detects funnel plot asymmetry by determining whether the intercept deviates significantly from zero in a regression of the standardized-effect estimates against their precision [21].
Data were stratified into subgroups based on study design to examine the consistency across varying study designs with different potential biases. Homogeneity was assessed overall and within this stratification.
In order to assess any association between duration of BP use and the risk of CRC, we used the available data from studies with ‘duration <1 year’ and ‘duration ≥1 year’.
All P values are two tailed. For all tests, a probability level <0.05 was considered statistically significant. Stata 11.0 software was used for the statistical analyses (StataCorp., College Station, TX, USA).
Results
The literature search and the study characteristics
The flow diagram that depicts the detailed literature searches and the selection process is shown in Figure 1. After screening the title, the abstracts and the full-text articles, six eligible articles met the inclusion criteria from 108 identified studies. The main characteristics of the six included studies are shown in Table 1. All of these studies were published after 2010. One study originated from the USA, one from Israel, one from Canada, one from Denmark and two from the UK. Two studies were prospective cohort studies, and the other four were retrospective population-based case–control studies. Overall, we enrolled 18 670 cases. Two studies [11, 12] reported in detail the types of BPs that were used, while the other studies did not.
Figure 1.
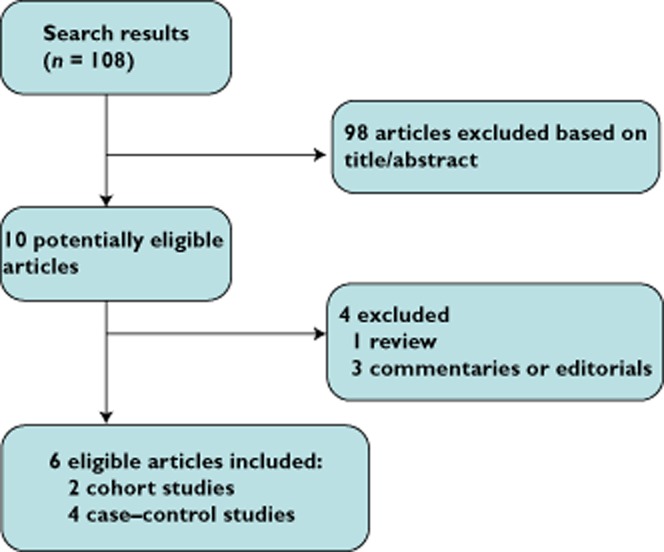
Selection process
Table 1.
Studies included in the meta-analysis
| Study (year) | Design | No. of cases | Total no. of subjects | Exposure | Exposure definition | RR/OR (95% CI) | Adjustments | |
|---|---|---|---|---|---|---|---|---|
| Singh et al. (2012) [11] | Case–control | 5425 | 59 667 | Any bisphosphonate | 2–13 prescriptions | 0.84 (0.71, 1.00) | 1, 2, 3, 4, 5, 6, 7 | |
| ≥14 prescriptions | 0.78 (0.65, 0.94) | |||||||
| Alendronic acid | ≥2 prescriptions | 0.87 (0.74, 1.01) | ||||||
| Etidronic acid | 0.72 (0.50, 1.03) | |||||||
| Risedronic acid | 0.50 (0.30, 0.85) | |||||||
| Multiple agents | 0.83 (0.60, 1.15) | |||||||
| Khalili et al. (2012) [15] | Case–control | 801 | 86 277 | Any bisphosphonate | Regular use | 1.04 (0.82, 1.33) | 1, 6, 8, 10, 11, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 | |
| 1–2 years of use | 1.24 (0.94, 1.64) | |||||||
| 3–4 years of use | 1.16 (0.79, 1.69) | |||||||
| ≥5 years of use | 0.97 (0.60, 1.56) | |||||||
| Cardwell et al. (2012) [14] | Cohort | 608 | 41 826 | Any bisphosphonate | Any use | 0.74 (0.60, 0.91) | 6, 8, 10, 17, 27, 28, 29 | |
| After 1 year of DDDs | 0.72 (0.53, 0.98) | |||||||
| Pazianas et al. (2012) [12] | Cohort | 262 | 153 030 | Alendronate | 10 mg day–1 | 0.69 (0.60, 0.79) | 1, 6, 6, 13, 24, 25, 26 | |
| Rennert et al. (2011) [13] | Case–control | 933 | 1866 | Any bisphosphonate | Any use | 0.67 (0.51, 0.88) | NA | |
| <1 year of use | 1.10 (0.71, 1.72) | |||||||
| ≥1 year of use | 0.50 (0.36, 0.71) | |||||||
| >2 years of use | 0.51 (0.34, 0.79) | |||||||
| >3 years of use | 0.39 (0.22, 0.68) | |||||||
| Green et al. (2010) [16] | Case–control | 10 641 | 63 663 | Any bisphosphonate | Prescribed | 0.87 (0.77, 1.00) | 8, 10, 20 | |
| No. of prescriptions | 1–9 | 0.92 (0.77, 1.09) | ||||||
| ≥10 | 0.82 (0.67, 1.00) | |||||||
| Duration of use | ≤1 year | 0.91 (0.75, 1.11) | ||||||
| 1–3 years | 0.83 (0.66, 1.04) | |||||||
| ≥3 years | 0.88 (0.67, 1.15) | |||||||
Adjustments are as follows: 1, age; 2, sex; 3, duration of coverage with Manitoba Health; 4, number of ambulatory care physician visits; 5, previous exposure to lower gastrointestinal endoscopy; 6, exposure to nonsteroidal anti-inflammatory drugs; 7, Charlson Comorbidity Index score; 8, body mass index; 9, race; 10, smoking status; 11, family history of colon cancer; 12, history of osteoporosis; 13, known ulcerative colitis; 14, hormone replacement therapy; 15, regular statin use; 16, total daily calcium intake; 17, vitamin D intake; 18, folate intake; 19, red meat intake; 20, total daily alcohol intake; 21, level of physical activity; 22, history of polyps; 23, history of screening; 24, known Crohn's disease; 25, known coeliac disease; 26, amount of prednisolone; 27, calcium in the male and female combined analyses and male-only analyses; 28, additionally adjusted for hormone replacement therapy prescription in the female-only analyses; 29, glucocorticoid steroid. Abbreviations are as follows: CI, confidence interval; DDDs, defined daily doses; NA, not available; OR, odds ratio; and RR, relative risk.
Bisphosphonate use and the risk of coloretal cancer
Four case–control studies and two cohort studies evaluated exposure to BPs and CRC risk.
The funnel plot had the expected funnel shape (Figure 2). The P values for the Begg and Mazumdar test and the Egger test were P = 0.851 and P = 0.410, respectively, both suggesting a very low probability of publication bias. In contrast, the Cochran's Q test had a P value of 0.023 (Q = 13 on five degrees of freedom) and the quantity I2 was 61.6%, both providing evidence of heterogeneity among the studies (Table 2).
Figure 2.
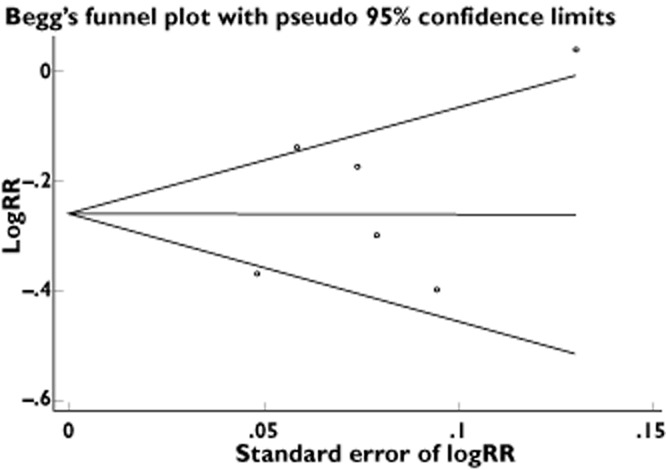
Funnel plots of the relative risk of developing colorectal cancer, with the standard error, for all studies included in the meta-analysis. Relative risks are displayed on a logarithmic scale. The x-axis represents standard error of log RR, and the y-axis represents log RR. For bisphosphonate use: P = 0.851 for the Begg–Mazumdar test; P = 0.410 for the Egger test
Table 2.
Meta-analysis results
| Fixed-effects model | Random-effects model | Tests of homogeneity | Tests of publication bias | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | RR | (95% CI) | RR | (95% CI) | Q value (d.f.) | P value | I2 | Begg's P value | Egger's P value | |
| Any bisphosphonate use and colorectal cancer risk | ||||||||||
| All studies | 6 | 0.80 | (0.74, 0.85) | 0.80 | (0.71, 0.90) | 13.00 (5) | 0.023 | 61.6% | 0.851 | 0.410 |
| Case–control studies | 4 | 0.86 | (0.78, 0.94) | 0.85 | (0.75, 0.98) | 5.69 (3) | 0.128 | 47.3% | 0.502 | 0.218 |
| Cohort studies | 2 | 0.70 | (0.63, 0.79) | 0.70 | (0.63, 0.79) | 0.30 (1) | 0.583 | 0% | NA | NA |
| Duration of bisphosphonate use and colorectal cancer risk | ||||||||||
| Duration <1 year | ||||||||||
| All studies | 3 | 0.86 | (0.76, 0.98) | 0.86 | (0.72, 1.04) | 2.93 (2) | 0.231 | 31.7% | 0.602 | 0.633 |
| Case–control studies | 2 | 0.88 | (0.75, 1.03) | 0.93 | (0.78, 1.12) | 0.59 (1) | 0.59 | 31.7% | NA | NA |
| Cohort studies | 1 | 0.82 | (0.64, 1.04) | 0.74 | (0.57, 0.94) | NA | NA | NA | NA | NA |
| Duration ≥1 year | ||||||||||
| All studies | 4 | 0.82 | (0.71, 0.94) | 0.79 | (0.56, 1.11) | 17.33 (3) | 0.001 | 82.7% | 0.051 | 0.125 |
| Case–control studies | 3 | 0.85 | (0.72, 0.99) | 0.81 | (0.51, 1.28) | 16.49 (2) | 0.000 | 87.9% | 0.174 | 0.001 |
| Cohort studies | 1 | 0.72 | (0.53, 0.98) | 0.72 | (0.52, 0.97) | NA | NA | NA | NA | NA |
We found that the any use of BPs was significantly associated with a 20% risk reduction in the incidence of colorectal cancer using the fixed-effects model (RR = 0.80, 95% CI = 0.74, 0.85) and the random-effects model (RR = 0.80, 95% CI = 0.71, 0.90; Table 2). However, the random-effects model is generally thought to be more appropriate, because it provides a more conservative estimate of the pooled effect size.
In order to examine consistency across varying study designs with different potential biases, we stratified data into subgroups on the basis of study design. The association was statistically significant, either among cohort studies (random-effects model, RR = 0.70, 95% CI = 0.63, 0.79; fixed-effects model, RR = 0.70, 95% CI = 0.63, 0.79) or among case–control studies (random-effects model, RR = 0.85, 95% CI = 0.75, 0.98; fixed-effects model, RR = 0.86, 95% CI = 0.78, 0.94; Table 2).
Figure 3 shows the RRs and 95% CIs from the individual studies and the pooled results.
Figure 3.
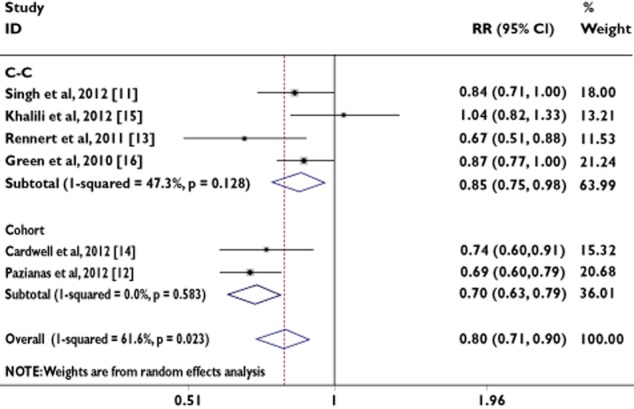
Analysis of studies, listed by first author and publication year, that examined colorectal cancer and its association with bisphosphonate use. The relative risk and 95% confidence interval (CI) for each study are displayed on a logarithmic scale. Pooled estimates are from a random-effects model
Analysis of ‘duration <1 year’ of bisphosphonate use and colorectal cancer
In order to assess any association between duration of BP use and the risk of CRC, we used the available data from studies that dichotomized to ‘duration <1 year’ and ‘duration ≥1 year’.
Two case–control studies and one cohort study evaluated exposure to ‘duration <1 year’ use of BPs and CRC risk. The P values for the Begg and Mazumdar test and the Egger test were P = 0.602 and P = 0.633, respectively, both suggesting a low probability of publication bias. In contrast, the Cochran's Q test had a P value of 0.231 (Q = 2.93 on two degrees of freedom) and the quantity I2 was 31.7%, providing little evidence of heterogeneity among the studies (Table 2).
We found that the use of ‘duration <1 year’ was associated with a 14% risk reduction in the incidence of CRC using the fixed-effects model (RR = 0.86, 95% CI = 0.76, 0.98) and the random-effects model (RR = 0.86, 95% CI = 0.72, 1.04; Table 2). However, the random-effects model is generally thought to be more appropriate, because it provides a more conservative estimate of the pooled effect size.
In order to examine consistency across varying study designs with different potential biases, we stratified data into subgroups on the basis of study design. The association was not statistically significant in case–control studies (random-effects model, RR = 0.93, 95% CI = 0.78, 1.12; fixed-effects model, RR = 0.88, 95% CI = 0.75, 1.03). The number of studies contributing to this analysis was very small (n = 3; Table 2).
Figure 4 shows the RRs and 95% CIs from the individual studies and the pooled results.
Figure 4.
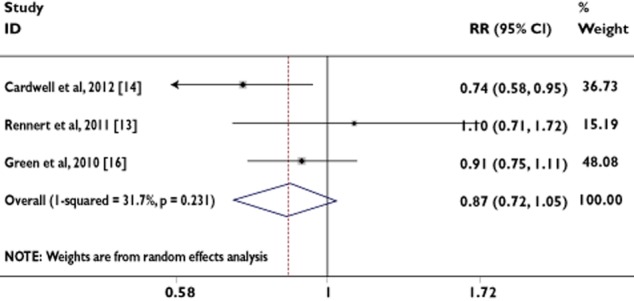
Analysis of studies, listed by first author and publication year, that examined colorectal cancer and its association with ‘duration <1 year’ of bisphosphonate use. The relative risk and 95% CI for each study are displayed on a logarithmic scale. Pooled estimates are from a random-effects model
Analysis of ‘duration ≥1 year’ of bisphosphonate use and colorectal cancer
Three case–control studies and one cohort study evaluated exposure to ‘duration ≥1 year’ use of BP and CRC risk. The number of studies contributing to this analysis was very small (n = 4; Table 2). The P values for the Begg and Mazumdar test and the Egger test were P = 0.051 and P = 0.125, respectively, both suggesting a low probability of publication bias. In contrast, the Cochran's Q test had a P value of 0.001 (Q = 17.33 on three degrees of freedom) and the quantity I2 was 82.7%, both providing evidence of heterogeneity among the studies (Table 2).
We found that the use of ‘duration ≥1 year’ was associated with a 21% risk reduction in the incidence of CRC, assuming the random-effects model (RR = 0.79, 95% CI = 0.56, 1.11; Table 2).
In order to examine consistency across varying study designs with different potential biases, we stratified data into subgroups on the basis of study design. The association was not statistically significant in case–control studies, assuming the random-effects model (RR = 0.81, 95% CI = 0.51, 1.28; Table 2).
Figure 5 shows the RRs and 95% CIs from the individual studies and the pooled results.
Figure 5.
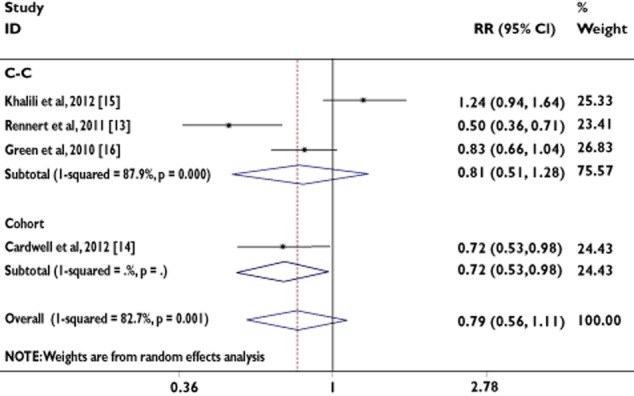
Analysis of studies, listed by first author and publication year, that examined colorectal cancer and its association with ‘duration ≥1 year’ of bisphosphonate use. The relative risk and 95% CI for each study are displayed on a logarithmic scale. Pooled estimates are from a random-effects model
Discussion
In the present study, which included 18 670 cases of CRC, we observe a protective role of any use of BPs on CRC risk. Although our results cannot exclude a protective effect of BP with longer follow-up, we did not observe any evidence of a trend with increasing duration of use, and more studies are needed to explore the dose response.
Our results are consistent with a previous case–control study [13] of 63 663 individuals nested in the UK General Practice Research Database, which showed a possible weak inverse association (RR = 0.87, 95% CI = 0.77, 1.00) between BP use and CRC risk. Although the UK study adjusted their analysis for lifestyle risk factors, such as smoking, alcohol intake and body mass index, there was no adjustment for other potential confounding factors, such as the use of lower gastrointestinal endoscopy and the frequency of healthcare visits. In addition, by considering prescriptions written rather than dispensed, the drug use may have been misclassified because of unfilled and lost prescriptions. Our results are also consistent with a recent case–control analysis nested in Manitoba's population database, in which Singh et al. [11] concluded that use of BPs was associated with a lower risk of CRC. However, a borderline statistically significant inverse association was observed only in a multivariate model, which adjusted for age, sex, socio-economic status, number of ambulatory care visits, prior exposure to lower endoscopy, nonsteroidal anti-inflammatory drug use and Charlson Comorbidity Index score (RR = 0.84, 95% CI = 0.71, 1.00) [11]. However, the analysis of the Manitoba database was unable to account for physical activity and vitamin D and calcium intake, which confounded the association between BP use and CRC.
Our findings differ notably from recent data from the Molecular Epidemiology of Colorectal Cancer (MECC) case–control study [13] conducted in Northern Israel, which observed that use of BPs for at least 1 year was associated with a 59% significant reduction in the risk of CRC. However, the MECC study had limited information on key potential confounders, including physical activity (assessed as participation in sports activities) and calcium or vitamin D intake (assessed only as use of supplements). Inadequate control for healthy behaviours associated with the primary indication for use of BPs (osteoporosis) could account for the inverse association observed in the MECC study. Studies by Green et al. [16] and Cardwell et al. [14] used data from the UK General Practice Research Database, although they used different methods and data from different (but overlapping) time periods. There is undoubtedly some overlap in the cases (and potentially controls) in these two studies; therefore, we performed the same meta-analyses with (Table 2) and without the two studies (Table 3). When we exclude the two studies, we can also observe a protective role of any use of BP on CRC risk (random-effects model: RR = 0.79, 95% CI = 0.66, 0.95).
Table 3.
Meta-analysis results
| Fixed-effects model | Random-effects model | Tests of homogeneity | Tests of publication bias | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | RR | (95% CI) | RR | (95% CI) | Q value (d.f.) | P value | I2 | Begg's P value | Egger's P value | |
| Any bisphosphonate use and colorectal cancer risk | ||||||||||
| All studies | 4 | 0.77 | (0.71, 0.85) | 0.79 | (0.66, 0.95) | 10.36 (3) | 0.016 | 71.1% | 0.174 | 0.324 |
| Case–control studies | 3 | 0.84 | (0.74, 0.96) | 0.84 | (0.67, 1.04) | 5.61 (2) | 0.060 | 64.4% | 0.602 | 0.761 |
| Cohort studies | 1 | 0.69 | (0.60, 0.79) | 0.69 | (0.60, 0.79) | NA | NA | NA | NA | NA |
| Duration of bisphosphonate use and colorectal cancer risk | ||||||||||
| Duration <1 year | ||||||||||
| All studies (case–control studies) | 1 | 1.10 | (0.70, 1.71) | 1.10 | (0.70, 1.71) | NA | NA | NA | NA | NA |
| Duration ≥1 year | ||||||||||
| All studies (case–control studies) | 2 | 0.79 | (0.32, 1.92) | 0.79 | (0.32, 1.92) | 16.44 (1) | 0.000 | 93.9% | 0.317 | NA |
Although we report that the risk of CRC was reduced by approximately 20% with exposure to risedronic acid, this finding needs to be confirmed in additional studies. If corroborated in other studies, this may indicate that risedronic acid could be considered as an agent of choice when BPs are prescribed for osteoporosis to individuals who also have a higher risk of CRC (e.g. a family history of CRC or a personal past history of colorectal polyps or CRC). There are structural differences between the different BPs [22, 23]. Bisphosphonates can be classified into two groups based on the structure of R2 side-chains. Nitrogen-containing BPs (N-BPs; e.g. alendronic acid, risedronic acid and zoledronic acid) are much more potent with regard to their bone antiresorptive activity than the non-nitrogen-containing BPs (e.g. etidronic acid). Among the N-BPs, those with a tertiary nitrogen in a ring structure (hetrocyclic N-BPs; e.g. risedronic acid and zoledronic acid) are more similar to each other structurally and have the highest potency. In addition, the N-BPs, such as risedronic acid and zoledronic acid, inhibit the mevalonate pathway (i.e. the cholesterol synthesis pathway) and induce caspase activity [24]. Among all BPs, hetrocyclic N-BPs, such as risedronic acid and zoledronic acid, are the strongest inhibitors of farnesyl pyrophosphate synthase, which is a key branch-point enzyme in the mevalonate pathway [22], an effect that may explain, at least in part, their anticancer effect. Zoledronic acid is the BP that has been evaluated most extensively in vitro for its antitumour activity, including its effect on colon cancer cells [24]. Besides this, osteonecrosis of the jaw has been reported most commonly among long-term users of intravenous BPs [25, 26]. If the results from our study are confirmed by others, then the benefits of using BPs may outweigh the risks, especially for individuals with a higher risk of CRC.
As far as we know, this is the first meta-analysis to be carried out with the aim of investigating the relationship of use of BPs with CRC risk.
Several limitations should be considered in interpreting the results of this meta-analysis. First, our search was restricted to studies published in indexed journals. We did not search for unpublished studies or for original data. However, we did not impose any exclusion criteria regarding language, place of publication or quality. Second, the included studies were different in terms of study design and duration of drug exposure. We tried to explore sources of heterogeneity by conducting several subgroup analyses. However, the summary effect estimates are based on sparse and heterogeneous data. Likewise, we did not have specific information on the types or brands of BPs and the doses used. Thus, we cannot completely exclude the possibility that use of higher doses or specific types of BPs may have a differential association, as was suggested by the Manitoba case–control study, in which a strong inverse association was observed with risedronic acid, the least commonly used agent of the class. Fourth, observational studies lack the experimental random allocation of the intervention necessary to test exposure–outcome hypotheses optimally. Thus, results may have been confounded by several factors, given that each one of the studies included in our meta-analysis controlled for somewhat different confounding factors (Table 1). Fifth, we cannot exclude the possibility of a modest inverse association between BPs and CRC within the lower limit of our confidence bounds. Other limitations include the relatively high proportion of missing data on potential confounders, such as weight, potential for residual confounding and multiple comparisons. Our results should therefore be interpreted with caution.
Despite the limitations listed above, our analysis shows a protective role of any use of BPs on CRC risk. Further studies are required to examine the relationship between use of BPs and CRC incidence, but it is reassuring that cancer risk was not increased in users of these drugs. The finding in this study requires confirmation through further characterization of the association by frequency and duration of use, cumulative dose and timing of exposure. In addition, it will be important to evaluate the underlying conditions for medication use.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81172501).
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 2.Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crino L, Dirix L, Gnant M, Gralow J, Hadji P, Hortobagyi GN, Jonat W, Lipton A, Monnier A, Paterson AH, Rizzoli R, Saad F, Thurlimann B. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–432. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 3.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404–415. [PubMed] [Google Scholar]
- 4.Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, Cauley JA, Blumenstein BA, Albain KS, Lipton A, Brown S. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi GN, Theriault RL, Lipton A, Porter L, Blayney D, Sinoff C, Wheeler H, Simeone JF, Seaman JJ, Knight RD, Heffernan M, Mellars K, Reitsma DJ. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 6.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kassmann H, Piswanger-Solkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F, Bjelic-Radisic V, Steger G, Greil R, Marth C, Kubista E, Samonigg H, Wohlmuth P, Mittlbock M, Jakesz R. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 7.Santini D, Virzi V, Fratto ME, Bertoldo F, Sabbatini R, Berardi R, Calipari N, Ottaviani D, Ibrahim T. Can we consider zoledronic acid a new antitumor agent? Recent evidence in clinical setting. Curr Cancer Drug Targets. 2010;10:46–54. doi: 10.2174/156800910790980223. [DOI] [PubMed] [Google Scholar]
- 8.Sewing L, Steinberg F, Schmidt H, Goke R. The bisphosphonate zoledronic acid inhibits the growth of HCT-116 colon carcinoma cells and induces tumor cell apoptosis. Apoptosis. 2008;13:782–789. doi: 10.1007/s10495-008-0211-z. [DOI] [PubMed] [Google Scholar]
- 9.Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 10.Sassa S, Okabe H, Nemoto N, Kikuchi H, Kudo H, Sakamoto S. Ibadronate may prevent colorectal carcinogenesis in mice with ulcerative colitis. Anticancer Res. 2009;29:4615–4619. [PubMed] [Google Scholar]
- 11.Singh H, Nugent Z, Demers A, Mahmud S, Bernstein C. Exposure to bisphosphonates and risk of colorectal cancer. Cancer. 2012;118:1236–1243. doi: 10.1002/cncr.26395. [DOI] [PubMed] [Google Scholar]
- 12.Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RGG. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate – Danish National Register Based Cohort Study. Osteoporos Int. 2012;23:2693–2701. doi: 10.1007/s00198-012-1902-4. [DOI] [PubMed] [Google Scholar]
- 13.Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. J Clin Oncol. 2011;29:1146–1150. doi: 10.1200/JCO.2010.33.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardwell CR, Abnet CC, Veal P, Hughes CM, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of cancer. Int J Cancer. 2012;131:E717–725. doi: 10.1002/ijc.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalili H, Huang ES, Ogino S, Fuchs CS, Chan AT. A prospective study of bisphosphonate use and risk of colorectal cancer. J Clin Oncol. 2012;30:3229–3233. doi: 10.1200/JCO.2011.39.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonovas S, Filioussi K, Sitaras NM. Do nonsteroidal anti-inflammatory drugs affect the risk of developing ovarian cancer? A meta-analysis. Br J Clin Pharmacol. 2005;60:194–203. doi: 10.1111/j.1365-2125.2005.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 23.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34:453–475. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Neville-Webbe HL, Gnant M, Coleman RE. Potential anticancer properties of bisphosphonates. Semin Oncol. 2010;37(Suppl. 1):S53–65. doi: 10.1053/j.seminoncol.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamsen B. Bisphosphonate adverse effects, lessons from large databases. Curr Opin Rheumatol. 2010;22:404–409. doi: 10.1097/BOR.0b013e32833ad677. [DOI] [PubMed] [Google Scholar]
- 26.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]


