Abstract
Aim
A growing body of evidence suggests that bisphosphonates may have chemopreventive potential against colorectal cancer. Our aim was to examine this association through a meta-analysis of observational studies.
Methods
A comprehensive search for relevant articles published up to October 2012 was performed, reviews of each study were conducted and data were abstracted. Prior to meta-analysis, the studies were evaluated for publication bias and heterogeneity. Pooled relative risk (RR) estimates and 95% confidence intervals (CIs) were calculated using the random effects and the fixed effects models. Subgroup and sensitivity analyses were also performed.
Results
Eight large population-based epidemiological studies (one case-control, two nested case-control analyses within a cohort and five cohort studies), involving more than 630 000 participants, contributed to the analysis. We found no evidence of publication bias. However, significant heterogeneity was detected among the cohort studies. The analysis revealed a significant protective association between bisphosphonate use and colorectal cancer risk (fixed RR = 0.85, 95% CI 0.80, 0.90, random RR = 0.85, 95% CI 0.75, 0.96). When the analysis was stratified into subgroups according to study design, the association was inverse in both case-control and cohort studies, but only in the former was it statistically significant. The sensitivity analysis confirmed the stability of our results. Furthermore, we found evidence for a dose effect; Long term bisphosphonate use was associated with a 27% decrease in the risk of developing colorectal cancer as compared with non-use (RR = 0.73, 95% CI 0.57, 0.93).
Conclusion
Our findings support a protective effect of bisphosphonates against colorectal cancer. However, further evidence is warranted.
Keywords: bisphosphonates, colorectal cancer, meta-analysis
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Bisphosphonates are prescribed for the prevention or treatment of osteoporosis, and the treatment of bone metastases in patients with breast cancer
Their use is rapidly increasing worldwide
A growing body of evidence suggests that bisphosphonates may have chemopreventive potential against colorectal cancer
WHAT THIS STUDY ADDS
Our findings support a protective effect of bisphosphonates against colorectal cancer
They also provide evidence for a dose effect, an indication that the observed association is likely to be causal
Further prospective research is needed
Introduction
Bisphosphonates (BPs) comprise a class of drugs that inhibit osteoclast-mediated bone resorption and reduce the release of calcium into the blood stream [1]. They are prescribed for the prevention or treatment of osteoporosis [2, 3] and the treatment of bone metastases in patients with breast cancer [4]. Their use is rapidly increasing worldwide [5, 6].
A growing body of preclinical data suggests that BPs may have chemopreventive potential against colorectal cancer. They have been shown to inhibit colorectal carcinogenesis, reduce proliferation and induce apoptosis of colon cancer cells [7–10]. However, the clinical relevance of these findings remains unclear. The results of experimental studies are difficult to extrapolate to humans, but they cannot be dismissed. At a minimum, a further insight into the role of BPs in human populations is needed.
Recently, some epidemiological studies examined the relationship between BPs and colorectal cancer. The publication by Rennert et al. [11], which was a population-based case-control study conducted in Israel, attracted the most attention with a 59% reduction in the risk of colorectal cancer among post-menopausal women. In contrast to the results derived from this particular work, other observational studies did not support an association between the use of bisphosphonates and colorectal cancer risk [12, 13].
Thus, the effect of BPs on the risk of colorectal cancer remains undetermined. To address this issue, we conducted a detailed meta-analysis of observational studies published in the peer-reviewed literature.
Methods
Search strategy
To identify the studies of interest, we systematically searched MEDLINE and SCOPUS databases for research reports published up to 8 October 2012. Search terms included: {bisphosphonate(s), diphosphonate(s), alendronate, clodronate, etidronate, ibandronate, pamidronate, risedronate or zoledronate} combined with {cancer(s), carcinoma(s), malignancy(ies), neoplasm(s) or tumour(s)} and {colorectal, colon or rectal}. We scanned titles and abstracts of the studies identified in the initial search and excluded those that were clearly irrelevant. The full text of the remaining articles was read to determine whether it contained information on the topic of interest. The reference lists of articles that included relevant information were also reviewed for additional studies. Language restrictions were not used.
Inclusion and exclusion criteria
This meta-analysis considered prospective or retrospective epidemiological studies that evaluated exposure to BPs and risk of colorectal cancer. Articles were excluded from the analyses for any of the following reasons: (i) they did not evaluate BP use as a risk factor for colorectal cancer, (ii) they did not provide a description of BP exposure and (iii) there was insufficient published data for calculating a relative risk (RR) estimate or a confidence interval (CI). We did not assess the methodological quality of the primary studies because quality assessment in meta-analysis is controversial and results can be highly misleading [14, 15]. Instead, we performed detailed subgroup and sensitivity analyses. We included in this synthesis studies reporting different measures of relative risk (odds ratio, hazard ratio). In practice, these measures of effect yield very similar estimates of relative risk, since the absolute risk of colorectal cancer is very low [16].
Data extraction
Two reviewers (SB, GN) abstracted the data independently. The following information was sought from each study: (i) publication data: first author's last name, year of publication and geographical location of the study, (ii) study design, (iii) number of participants, (iv) population characteristics, (v) types of BPs used, (vi) RR estimates with their 95% CIs and (vii) control for confounding factors by matching or adjustments. In studies where more than one estimate of effect (RR) was presented, we extracted the estimates that reflected the greatest degree of control for potential confounders. Differences in data extraction were settled by consensus, referring back to the original article.
Quantitative data synthesis
We used inverse-variance weighting to calculate fixed and random effects summary estimates [17]. Publication bias was evaluated using the Begg's adjusted rank correlation test [18] and the Egger's regression asymmetry test [19]. To examine whether the results of the studies were homogeneous, we used the Cochran's Q test [20] with a 0.10 level of significance. We also calculated the quantity I2 that describes the percentage variation across studies that is due to heterogeneity rather than chance [21]. We regarded an I2 value less than 40% as indicative of ‘not important heterogeneity’ and a value higher than 75% as indicative of ‘considerable heterogeneity’ [22].
Data were also stratified into subgroups on the basis of study design aiming to examine consistency across varying study designs with different potential biases. Homogeneity was assessed overall and within this stratification. To evaluate the stability of the results, we also performed a ‘leave-one-out’ sensitivity analysis. The scope of this approach was to evaluate the influence of individual studies, by estimating the summary relative risk in the absence of each study [23].
Because the documentation of a dose–response relation in a study lends support to a causal explanation of a disease–exposure association [24], we attempted to explore the relationship between duration of bisphosphonate use and risk of colorectal cancer. Therefore, we grouped drug exposure as short term and long term. Short term use included the RR estimates corresponding to the shortest durations, and long term use included the RR estimates for the longer durations of drug exposure, as reported in the individual studies. The respective pooled effect-estimates were calculated.
This work was conducted according to the MOOSE guidelines [25] and the PRISMA statement [26]. For all tests (except for heterogeneity), a probability level lower than 0.05 was considered statistically significant. All statistical tests were two-sided. Stata 12 software was used for the statistical analyses (Stata Corp., College Station, Texas, USA).
Results
Search results
The initial computerized search yielded 377 literature citations (Figure 1). However, most abstracts were duplicates or irrelevant to the topic of our analysis and were excluded from full-text review. We retrieved 46 potentially eligible publications. The full text was read and the reference lists were carefully checked. Finally, we identified eight population-based observational studies examining the association between bisphosphonate use and risk of colorectal cancer [11–13, 27–31]. Among them, one had a case-control design [11], two were nested case-control analyses within a cohort [13, 30] and five were cohort studies [12, 27–29, 31]. Four studies included only women [11, 12, 27, 28], while the other four included both genders [13, 29–31].
Figure 1.
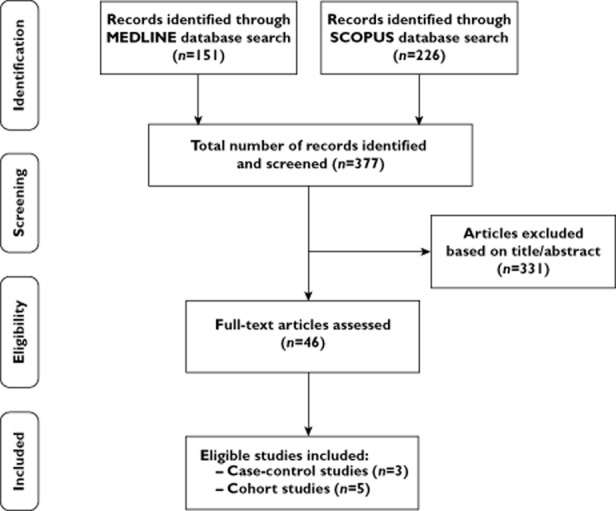
Flow diagram
In one case, two publications using data from the same Danish population significantly overlapped. The first study [31] examined the occurrence of all gastrointestinal cancers in users of bisphosphonates and other antiresorptive drugs against osteoporosis, while the second study [28] specifically examined colorectal cancer risk among post-menopausal women treated with alendronate. In an attempt to choose the most appropriate report, we decided to include the second study [28] in the meta-analysis, because it was more recent and based on a larger number of colorectal cancer cases (1683 [28] vs. 437 [31]) among alendronate users. In addition, we extracted from the first study [31] and included in the meta-analysis, the particular sub-cohort examining the occurrence of colon cancer in users of etidronate.
It is also possible that some individuals within the General Practice Research Database (GPRD) are included in both the Green et al. [13] and the Cardwell et al. [29] analyses. However, given the large size of the particular database, even if there is an overlap between the two analyses it should be minimal. Nevertheless, we also report the results of the sensitivity analysis, in which case, each study is completely removed from the analysis.
For another study [30], where RR for colorectal cancer was reported separately for the different types of bisphosphonates used, we calculated the study's combined effect estimate before inclusion in the meta-analysis.
The number of colorectal cancer cases ranged from 933 to 10 641 in the case-control analyses, and from 518 to 1683 in the cohort studies. In six publications [11, 27–31], alendronate was the most commonly used bisphosphonate. The other two studies [12, 13] did not report in detail the specific BP types that were used. All studies were controlled for potential confounding factors by matching or adjustments.
Five studies [11, 13, 28–30] reported a negative association (RR < 1.0), while three studies [12, 27, 31] found a positive relationship (RR > 1.0) between BP use and colorectal cancer. Four of the five studies reporting relative risks lower than 1.0 had confidence intervals that did not include unity [11, 28–30].
The publication dates of the studies included in the meta-analysis ranged between 2010 and 2012. The study designs, along with the RR estimates and the 95% CIs, are shown in Table 1.
Table 1.
Studies included in the meta-analysis
| Study | Study location | Study design | Bisphosphonate (BP) type | Number of subjects | CRC cases | RR | (95% CI) | Control for potential confounding factors* |
|---|---|---|---|---|---|---|---|---|
| Rennert et al. [11], 2011 | Israel | Case-control | Any BP; Alendronate 94.7% | 1 866 | 933 | 0.67 | (0.51, 0.88) | 1–13 |
| Khalili et al. [12], 2012 | USA | Cohort | Any BP | 86 277 | 801 | 1.04 | (0.82, 1.33) | 1, 2, 5, 8–10, 13–24 |
| Green et al. [13], 2010 | UK | Nested case-control | Any BP | 63 663 | 10,641 | 0.87 | (0.77, 1.00) | 1, 2, 8, 15, 24–26 |
| Chiang et al. [27], 2012 | Taiwan | Cohort | Alendronate | 27 603 | 518 | 1.02 | (0.83, 1.26) | 1, 2 |
| Pazianas et al. [28], 2012 | Denmark | Cohort | Alendronate | 153 030 | 1,683 | 0.69 | (0.60, 0.79) | 1, 2, 13, 27–31 |
| Cardwell et al. [29], 2012 | UK | Cohort | Any BP; Alendronate 70% | 83 652 | 608 | 0.74 | (0.60, 0.91) | 1, 2, 8, 15, 24, 25, 32–35 |
| Singh et al. [30], 2012 | Canada | Nested case-control | Alendronate 79.2%; Etidronate 11.5%; Risedronate 9.2% | 59 667 | 5,425 | 0.81 | (0.72, 0.92) | 1, 2, 27, 35–38 |
| Vestergaard [31], 2011 | Denmark | Cohort | Etidronate | 155 844 | 1,230 | 1.05 | (0.92, 1.20) | 1, 2, 39–46 |
1, age; 2, gender; 3, residence; 4, ethnic group; 5, family history of CRC; 6, engagement in sports activity; 7, vegetable consumption; 8, body mass index; 9, statin use; 10, aspirin use; 11, use of calcium supplements; 12, use of vitamin D supplements; 13, hormone replacement therapy; 14, race; 15, smoking status; 16, history of osteoporosis; 17, daily calcium intake; 18, vitamin D intake; 19, folate intake; 20, red meat intake; 21, level of physical activity; 22, history of polyps; 23, history of screening; 24, alcohol intake; 25, participating general practice; 26, observation period in the database; 27, Charlson comorbidity index score; 28, known ulcerative colitis; 29, known Crohn's disease; 30, known coeliac disease; 31, amount of prednisolone, NSAID and ASA used in the last 12 months; 32, glucocorticoid steroid prescription; 33, vitamin D prescription; 34, calcium prescription; 35, NSAID prescription; 36, length of stay in the province; 37, number of ambulatory care physician visits; 38, history of previous exposure to lower gastrointestinal endoscopy; 39, alcoholism; 40, use of inhaled bronchodilator or corticosteroid drug (proxy for smoking); 41, antacid drugs; 42, ASA or NSAID drugs; 43, marital status; 44, employment status; 45, income level; 46, history of gastric surgery.
CI, confidence interval; CRC, colorectal cancer; RR, relative risk.
Meta-analysis of exposure to bisphosphonates and colorectal cancer risk
Three case-control analyses [11, 13, 30] and five cohort studies [12, 27–29, 31] evaluated exposure to BPs and colorectal cancer risk. The meta-analysis encompassed these eight population-based epidemiological studies, which involved 631 602 individuals in total. Among them, 21 839 were colorectal cancer cases. The bisphosphonate use was significantly associated with a 15% decrease in the risk of colorectal cancer (fixed effects model: RR = 0.85, 95% CI 0.80, 0.90 and random effects model: RR = 0.85, 95% CI 0.75, 0.96). Figure 2 presents a Forest plot of the relative risks and their 95% CIs from the primary studies, along with the random-effects summary estimate. The P values for the Begg's and the Egger's test were P = 0.90 and P = 0.98, respectively, suggesting the absence of publication bias. In contrast, the Cochran's Q test had a P value <0.001 (Q = 29.49 on 7 d.f.) and the corresponding quantity I2 was 76%, indicating considerable heterogeneity among the studies (Table 2).
Figure 2.
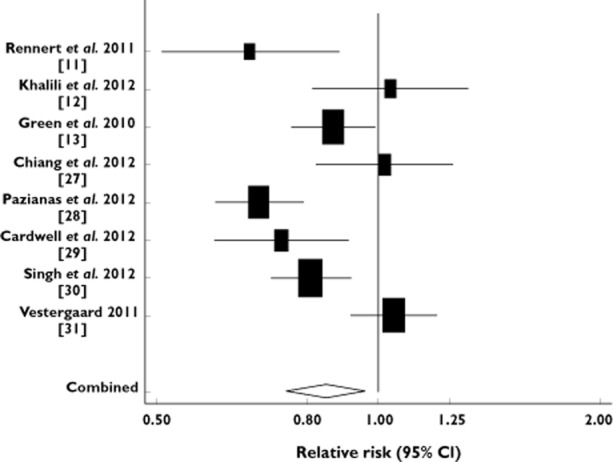
Forest plot: meta-analysis of bisphosphonate use and risk of colorectal cancer. The relative risks and their 95% confidence intervals are displayed on a logarithmic scale. Pooled estimate is from a random effects model
Table 2.
Meta-analysis results
| Fixed effects model | Random effects model | Tests of homogeneity | Tests of publication bias | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | RR | (95% CI) | RR | (95% CI) | Q value (d.f.) | P value | I2 | Begg's P value | Egger's P value | |
| – Case-control analyses | 3 | 0.82 | (0.75, 0.89) | 0.81 | (0.73, 0.91) | 2.93 (2) | 0.23 | 32% | 0.99 | 0.36 |
| – Cohort studies | 5 | 0.88 | (0.81, 0.95) | 0.89 | (0.73, 1.09) | 25.21 (4) | <0.001 | 84% | 0.99 | 0.79 |
| All studies combined | 8 | 0.85 | (0.80, 0.90) | 0.85 | (0.75, 0.96) | 29.49 (7) | <0.001 | 76% | 0.90 | 0.98 |
| – Short term use | 6 | 0.91 | (0.82, 1.00) | 0.93 | (0.80, 1.08) | 11.29 (5) | 0.046 | 56% | 0.26 | 0.24 |
| – Long term use | 6 | 0.78 | (0.66, 0.91) | 0.73 | (0.57, 0.93) | 9.47 (5) | 0.092 | 47% | 0.060 | 0.11 |
CI, confidence interval; d.f., degrees of freedom; RR, relative risk.
To examine the consistency of meta-analytic findings across varying study designs with different potential biases, we stratified data into subgroups on the basis of study design. When the analysis was restricted to the cohort studies, the inverse association between bisphosphonate use and risk of colorectal cancer was significant assuming a fixed effects model (RR = 0.88, 95% CI 0.81, 0.95), but lacked significance in the random effects approach (RR = 0.89, 95% CI 0.73, 1.09). However, the random-effects model is generally thought to be more appropriate, because it provides a more conservative estimate of the pooled effect size. The P values for the Begg's and the Egger's tests for publication bias were P = 0.99 and P = 0.79, respectively. In contrast, the Cochran's Q test had a P value <0.001, and the quantity I2 was 84%, indicating a large degree of heterogeneity among the cohort studies (Table 2).
On the other hand, when the analysis included only the case-control studies, the Cochran's Q test had a P value of 0.23 (Q = 2.93 on 2 d.f.) and the quantity I2 was 32%, indicating the homogeneity of the case-control estimates. The Begg's and the Egger's test were P = 0.99 and P = 0.36, respectively, suggesting a low probability of publication bias. The inverse association between BP use and colorectal cancer was significant assuming either a fixed effects (RR = 0.82, 95% CI 0.75, 0.89) or a random-effects model (RR = 0.81, 95% CI 0.73, 0.91) (Table 2). Furthermore, it should be noted that the stratified analysis on the basis of study design (case-control analyses vs. cohort studies) showed no between-groups heterogeneity (P = 0.25).
To explore whether the results were dominated by a single study, we performed a ‘leave-one-out’ sensitivity analysis. In this analysis, the overall effect size was calculated, removing one study at a time. This approach confirmed the stability of our results (Figure 3). Of particular importance here, is the fact that excluding either the study of Green et al. [13] or the study of Cardwell et al. [29], that may contain a small number of overlapped individuals, the significance of the combined estimate was not altered.
Figure 3.
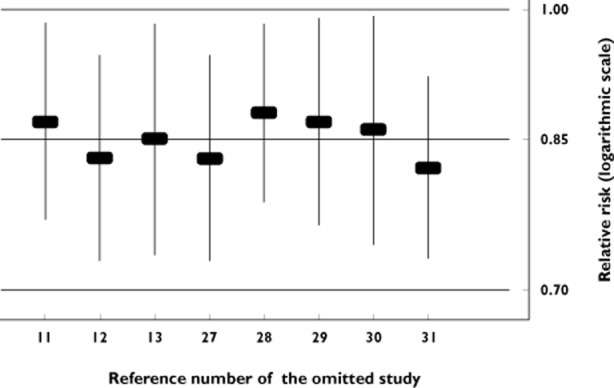
‘Leave-one-out’ sensitivity analysis. Pooled relative risk and 95% confidence interval omitting each study
To analyze any association between duration of BP use and risk of colorectal cancer, we grouped drug exposure as short term and long term use. Short term included the shortest durations, while long term included the longer durations of drug use, as reported in the individual studies (Table 3). It is notable that the relative risk estimates for long term use were lower than the respective estimates for short term use in all six primary studies [11–13, 29–31] contributing to this analysis (Table 3). The pooled results provided evidence for a dose effect; Long term bisphosphonate use was associated with a significant 27% reduction in the risk of developing colorectal cancer as compared with non-use (random effects model: RR = 0.73, 95% CI 0.57, 0.93, Cochran's P = 0.092, I2 = 47%) (Table 2). On the contrary, short term use did not cause any significant reduction in the risk of colorectal cancer (random effects model: RR = 0.93, 95% CI 0.80, 1.08, Cochran's P = 0.046, I2 = 56%) (Table 2).
Table 3.
Definitions of short term and long term bisphosphonate use
| Study | Short term use: | RR (95% CI) | Long term use: | RR (95% CI) |
|---|---|---|---|---|
| Rennert et al. [11], 2011 | <1 year of use | 1.10 (0.71, 1.72) | >3 years of use | 0.39 (0.22, 0.68) |
| Khalili et al. [12], 2012 | 1–2 years of use | 1.24 (0.94, 1.64) | ≥ 5 years of use | 0.97 (0.60, 1.56) |
| Green et al. [13], 2010 | ≤ 1 year of use | 0.91 (0.75, 1.11) | ≥ 3 years of use | 0.88 (0.67, 1.15) |
| Cardwell et al. [29], 2012 | >1 year of use | 0.74 (0.58, 0.95) | >4 years of use | 0.48 (0.22, 1.01) |
| Singh et al. [30], 2012 | <210 days of use | 1.04 (0.80, 1.33) | >1250 days of use | 0.83 (0.63, 1.10) |
| Vestergaard [31], 2011 | ≤ 2 years of use | 0.80 (0.67, 0.95) | >5 years of use | 0.66 (0.38, 1.13) |
CI, confidence interval; RR, relative risk.
Discussion and conclusion
Meta-analysis is a systematic and quantitative integration of the results of a set of independent studies. By combining the findings of primary research, it allows for an objective appraisal of the epidemiological evidence, which may lead to resolution of uncertainty and disagreement [32]. To the best of our knowledge, this is the first meta-analysis to explore the association between bisphosphonate use and risk of colorectal cancer. Having encompassed eight large population-based observational studies with more than 630 000 participants, of whom 21 839 were colorectal cancer cases, it revealed a significant inverse association between BP use and colorectal cancer, possibly suggesting a protective effect of BPs against colorectal cancer development.
Overall, BP use was associated with a significant 15% reduction in the risk of colorectal cancer (Figure 2). In the subgroup analysis by study design, this effect was evident among the case-control analyses but weaker (along with considerable heterogeneity) among the cohort studies. However, the absence of between-groups heterogeneity and the remarkable robustness of the overall effect size in the sensitivity analysis (Figure 3) reinforced our confidence in the validity of the results. Furthermore, our analysis provided evidence for a dose effect, an indication that the observed association was likely to be causal [24]. Long term use was associated with a statistically significant 27% decrease in the risk of developing colorectal cancer compared with non-use, while there was no difference in the risk between short term users and non-users.
Doubtless, study biases might influence the results of meta-analyses. It is well documented that in biomedical literature there is a publication bias in favour of statistically significant results [33, 34]. However, the likelihood of a substantial publication bias that affected our results is small. The Begg's and Egger's tests revealed no relation between the estimates of relative risk and study size. Thus, we are confident that the presence of publication bias due to the preferential publication of large studies with significant findings is unlikely.
Nevertheless, our meta-analysis has several limitations. Despite the differences among the primary studies with respect to different drugs prescribed to the populations studied, all BPs have been regarded as being the same (as most of the primary studies did not provide drug-specific effect estimates). Pharmacologically, this is not correct and may, therefore, have different effects on risk. Second, our analysis revealed considerable heterogeneity, especially among the cohort studies. However, the variability diminished significantly when we analyzed by duration of drug exposure (Table 2) and subgroup and sensitivity analyses provided very stable effect-estimates. Nevertheless, the results are based on heterogeneous data and should be interpreted with caution. Third, the observational studies included in this meta-analysis lacked the experimental random allocation of the intervention, and may therefore suffer from selection and information biases. Results may have also been confounded by several factors, given that each one of the studies included in our meta-analysis controlled for somewhat different potential confounders (Table 1).
In addition, there is a discussion concerning the likelihood of confounding by low bone density [35] in studies exploring the associations between BP use and site-specific cancer risks. However, high bone density has been associated with reduced rather than increased colorectal cancer risk [36–38]. Thus, if such bias exists, it would imply that the reduction in colorectal cancer risk among BP users, shown in our meta-analysis, is conservatively underestimated. In other words, existence of confounding by low bone density should mask the protective effect of bisphosphonates.
Though our knowledge of the possible mechanisms underlying this association is incomplete, our results may be biologically plausible. Bisphosphonates have been shown to exert direct antitumour effects in vitro and in cell lines, and have anti-angiogenic properties as evidenced by reduced vascular endothelial growth factor levels [39–41].
In addition, recent preclinical data suggest that BPs may have a site-specific potential for colorectal cancer chemoprevention [7–10]. This site-specific protective effect of oral BPs could be due to the local effects on the colonic mucosa, as only 1% of the orally administered dose is absorbed, while the remaining ∼99% moves slowly through the colorectal segments of the intestine in concentrations potentially in the millimolar range [42].
In conclusion, the synthesis of the existing epidemiological studies supports the hypothesis that exposure to BPs may reduce the risk of developing colorectal cancer. However, further prospective research is warranted to confirm or refute these findings and to explore the association for different types of BPs especially in the elderly, who comprise a population at risk for both osteoporosis and colorectal cancer. Until further evidence is available, this class of drugs remains a strong, but as yet unproven, candidate for colorectal cancer chemoprevention.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Source of funding
There was no specific funding source for this manuscript.
Data access and responsibility
Stefanos Bonovas had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis and acts as guarantor of the paper.
Authors' contributions
Study idea: SB.
Study design: SB, PB.
Literature search: SB, GN.
Data collection: SB, GN.
Statistical analysis: SB.
Data interpretation: SB, GN, PB.
First version of the manuscript: SB.
Critical revision for important intellectual content: SB, GN, PB.
Final approval of the version to be published: SB, GN, PB.
References
- 1.Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2005;353:595–603. doi: 10.1056/NEJMcp043801. [DOI] [PubMed] [Google Scholar]
- 2.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404–415. [PubMed] [Google Scholar]
- 3.Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399–428. doi: 10.1007/s00198-008-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS, Bosserman LD, Geoghegan C, Hillner BE, Theriault RL, Zuckerman DS, Von Roenn JH American Society of Clinical Oncology. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 5.Udell JA, Fischer MA, Brookhart MA, Solomon DH, Choudhry NK. Effect of the Women's Health Initiative on osteoporosis therapy and expenditure in Medicaid. J Bone Miner Res. 2006;21:765–771. doi: 10.1359/jbmr.060119. [DOI] [PubMed] [Google Scholar]
- 6.Watson J, Wise L, Green J. Prescribing of hormone therapy for menopause, tibolone, and bisphosphonates in women in the UK between 1991 and 2005. Eur J Clin Pharmacol. 2007;63:843–849. doi: 10.1007/s00228-007-0320-6. [DOI] [PubMed] [Google Scholar]
- 7.Suri S, Mönkkönen J, Taskinen M, Pesonen J, Blank MA, Phipps RJ, Rogers MJ. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone. 2001;29:336–343. doi: 10.1016/s8756-3282(01)00589-0. [DOI] [PubMed] [Google Scholar]
- 8.Sassa S, Okabe H, Nemoto N, Kikuchi H, Kudo H, Sakamoto S. Ibadronate may prevent colorectal carcinogenesis in mice with ulcerative colitis. Anticancer Res. 2009;29:4615–4619. [PubMed] [Google Scholar]
- 9.Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 10.Sewing L, Steinberg F, Schmidt H, Goke R. The bisphosphonate zoledronic acid inhibits the growth of HCT-116 colon carcinoma cells and induces tumor cell apoptosis. Apoptosis. 2008;13:782–789. doi: 10.1007/s10495-008-0211-z. [DOI] [PubMed] [Google Scholar]
- 11.Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. J Clin Oncol. 2011;29:1146–1150. doi: 10.1200/JCO.2010.33.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalili H, Huang ES, Ogino S, Fuchs CS, Chan AT. A prospective study of bisphosphonate use and risk of colorectal cancer. J Clin Oncol. 2012;30:3229–3233. doi: 10.1200/JCO.2011.39.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140:290–296. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 17.Petitti DB. Meta-Analysis, Decision Analysis and Cost-Effectiveness Analysis. New York: Oxford University Press; 1994. Statistical methods in meta-analysis; pp. 90–114. [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;8:101–129. [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley; 2008. [Google Scholar]
- 23.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Techn Bull. 1999;8:15–17. [Google Scholar]
- 24.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 27.Chiang CH, Huang CC, Chan WL, Huang PH, Chen TJ, Chung CM, Lin SJ, Chen JW, Leu HB. Oral alendronate use and risk of cancer in postmenopausal women with osteoporosis: a nationwide study. J Bone Miner Res. 2012;27:1951–1958. doi: 10.1002/jbmr.1645. [DOI] [PubMed] [Google Scholar]
- 28.Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RG. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate – Danish National Register Based Cohort Study. Osteoporos Int. 2012;23:2693–2701. doi: 10.1007/s00198-012-1902-4. [DOI] [PubMed] [Google Scholar]
- 29.Cardwell CR, Abnet CC, Veal P, Hughes CM, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of cancer. Int J Cancer. 2012;131:E717–725. doi: 10.1002/ijc.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh H, Nugent Z, Demers A, Mahmud S, Bernstein C. Exposure to bisphosphonates and risk of colorectal cancer: a population-based nested case-control study. Cancer. 2012;118:1236–1243. doi: 10.1002/cncr.26395. [DOI] [PubMed] [Google Scholar]
- 31.Vestergaard P. Occurrence of gastrointestinal cancer in users of bisphosphonates and other antiresorptive drugs against osteoporosis. Calcif Tissue Int. 2011;89:434–441. doi: 10.1007/s00223-011-9539-4. [DOI] [PubMed] [Google Scholar]
- 32.Nikolopoulos GK, Bagos PG, Bonovas S. Developing the evidence base for cancer chemoprevention: use of meta-analysis. Curr Drug Targets. 2011;12:1989–1997. doi: 10.2174/138945011798184191. [DOI] [PubMed] [Google Scholar]
- 33.Simes JR. Publication bias: the case for an international registry of trials. J Clin Oncol. 1986;4:1529–1541. doi: 10.1200/JCO.1986.4.10.1529. [DOI] [PubMed] [Google Scholar]
- 34.Sterling TD, Rosenbaum WL, Weinkam JJ. Publication decisions revisited: the effect of the outcome of statistical tests on the decision to publish and vice versa. Am Statist. 1995;49:108–112. [Google Scholar]
- 35.Schmidt C. New studies support case for bisphosphonates as possible chemopreventive agent. J Natl Cancer Inst. 2010;102:453–455. doi: 10.1093/jnci/djq114. [DOI] [PubMed] [Google Scholar]
- 36.Nelson RL, Turyk M, Kim J, Persky V. Bone mineral density and the subsequent risk of cancer in the NHANES I follow-up cohort. BMC Cancer. 2002;2:22. doi: 10.1186/1471-2407-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Felson DT, Ellison RC, Kreger BE, Schatzkin A, Dorgan JF, Cupples LA, Levy D, Kiel DP. Bone mass and the risk of colon cancer among postmenopausal women: the Framingham study. Am J Epidemiol. 2001;153:31–37. doi: 10.1093/aje/153.1.31. [DOI] [PubMed] [Google Scholar]
- 38.Ganry O, Lapotre-Ledoux B, Fardellone P, Dubreuil A. Bone mass density, subsequent risk of colon cancer and survival in postmenopausal women. Eur J Epidemiol. 2008;23:467–473. doi: 10.1007/s10654-008-9256-0. [DOI] [PubMed] [Google Scholar]
- 39.Stresing V, Daubiné F, Benzaid I, Mönkkönen H, Clézardin P. Bisphosphonates in cancer therapy. Cancer Lett. 2007;257:16–35. doi: 10.1016/j.canlet.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Clézardin P. The antitumor potential of bisphosphonates. Semin Oncol. 2002;29:33–42. doi: 10.1053/sonc.2002.37420. [DOI] [PubMed] [Google Scholar]
- 41.Wood J, Bonjean K, Ruetz S, Bellahcène A, Devy L, Foidart JM, Castronovo V, Green JR. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 42.Pazianas M, Russell RG. Potential therapeutic effects of oral bisphosphonates on the intestine. Ann N Y Acad Sci. 2011;1240:E19–25. doi: 10.1111/j.1749-6632.2011.06372.x. [DOI] [PubMed] [Google Scholar]


