Abstract
This review summarizes the current state of scientific understanding of the apoptosis pathway, with a focus on the proteins involved in the pathway, their interactions and functions. This forms the rationale for detailing the preclinical and clinical pharmacology of drugs that modulate the pivotal proteins in this pathway, with emphasis on drugs that are furthest advanced in clinical development as anticancer agents. There is a focus on describing drugs that modulate three of the most promising targets in the apoptosis pathway, namely antibodies that bind and activate the death receptors, small molecules that inhibit the anti-apoptotic Bcl-2 family proteins, and small molecules and antisense oligonucleotides that inactivate the inhibitors of apoptosis, all of which drive the equilibrium of the apoptotic pathway towards apoptosis. These structurally different yet functionally related groups of drugs represent a promising novel approach to anticancer therapeutics whether used as monotherapy or in combination with either classical cytotoxic or other molecularly targeted anticancer agents.
Keywords: apoptosis, Bcl-2 protein family, death domain receptors, inhibitors of apoptosis proteins
Introduction
The scientific understanding of the biology of cancer has led to the recognition that it is the unusual properties of cancer cells plus the complementarity of the stroma which supports them that provides the optimal milieu for cancer cell proliferation [1, 2]. One of the unique properties of cancer cells is their ability to evade programmed cell death; this cell death pathway is termed apoptosis. Apoptosis is derived from the 5th Century BC Greek, meaning ‘falling off’, and is a term that was applied scientifically in the 1970s [3, 4].
Many chemotherapeutic drugs cause apoptosis indirectly, mostly via targets and pathways that ultimately modulate the intrinsic apoptotic pathway machinery. The primary objective of this review is to focus on the clinical pharmacology of drugs that directly target different steps in the apoptotic pathways, via direct activation of the death receptors, inactivation of anti-apoptotic Bcl-2 proteins or inactivation of inhibitor of apoptosis proteins (IAPs). This collection of therapeutics has the potential to overcome apoptosis resistance in cancers and enhance the rate of cancer cell death, thus providing a potential new approach to improve cancer treatment.
Overview of apoptosis
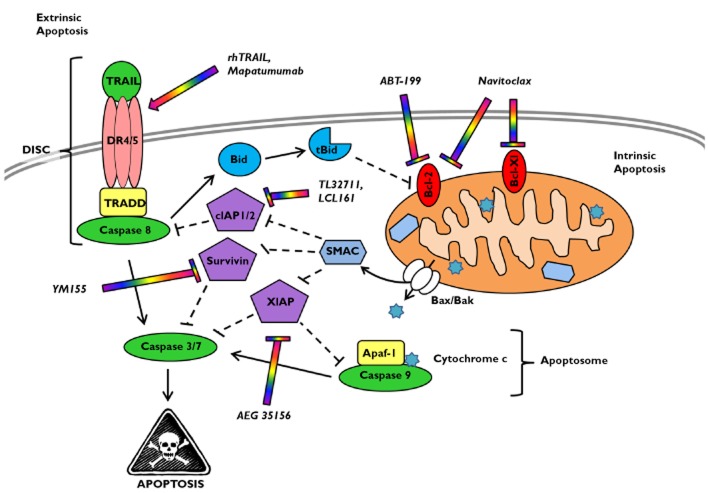
Apoptosis can be initiated by stress signals from within the cell or by external environmental stressors or toxins (Figure 1). This death signal then involves widespread proteolysis by caspases, nucleosomal fragmentation by endonucleases, and cell surface tagging for phagocyte engulfment [5]. Extrinsic apoptosis is regulated by members of the tumour necrosis factor (TNF) receptor protein family [death receptor (DR) family], which contain an extracellular ligand-binding domain and an intracellular death domain that signals for apoptosis. Intrinsic apoptosis is regulated at the mitochondrial membrane by members of the Bcl-2 protein family, in which the complex interaction between anti-apoptotic and pro-apoptotic members dictates whether apoptosis is triggered. Extrinsic apoptosis activates caspases 8 and 10 in the death-inducing signalling complex (DISC), while intrinsic apoptosis activates caspase 9 within the apoptosome. These initiator caspases go on to activate the effector caspases 3 and 7 that amplify the proteolytic caspase cascade, committing the cells to die. However, this cascade can be blocked by inhibitors of apoptosis (IAPs), which bind active caspases and prevent further proteolysis.
Figure 1.
The extrinsic and intrinsic apoptotic pathways. The extrinsic apoptotic pathway entails death ligands such as tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) binding to and activating its cognate receptors (TRAIL-R1/DR4, TRAIL-R2/DR5). This complex then recruits tumour necrosis factor-associated death domain protein (TRADD) and the initiator caspase 8. In this death-inducing signalling complex (DISC), caspase 8 is auto-activated by proteolysis and released into the cytosol. This leads to activation of caspases 3/7 and subsequently apoptosis. The intrinsic apoptotic pathway can be triggered by internal stress signals (e.g. radiation/chemotherapy damage to DNA) or via death receptor activation and caspase 8-mediated cleavage of Bid. Cleaved Bid (tBid) and/or other pro-apoptotic Bcl-2 proteins induce translocation of Bax/Bak into the mitochondrial membrane, leading to cytosolic release of cytochrome c and the second mitochondria-derived activator of caspase (SMAC). Cytochrome c binds the adaptor proteins Apaf-1 and caspase 9 to form the apoptosome which activates caspase 9. This caspase further activates caspases 3/7, resulting in apoptosis. Bcl-2 and Bcl-XL can inhibit apoptosis by preventing release of cytochrome c from the mitochondria. The inhibitor of apoptosis (IAP) proteins (e.g. cIAP1/2, XIAP and survivin) block caspase activation further downstream. SMAC displaces these IAP proteins, thus promoting apoptosis. The lead clinical drugs for each target in the apoptotic pathway are shown (multicoloured)
One of the hallmarks of cancer cells is their ability to evade apoptosis. This can occur by upregulation of anti-apoptotic proteins, by downregulation or loss of pro-apoptotic proteins or by defective functioning of pro-apoptotic proteins [6]. Thus, the apoptotic machinery is a pivotal potential target for cancer therapeutics.
Role of the death receptor family in apoptosis
The TNF receptor superfamily [TNFR, Fas (CD95/Apo1), death receptor 4 (DR4/TRAIL-R1) and death receptor 5 (DR5/TRAIL-R2)] manages many functions, including cell death/survival, differentiation and immune regulation [7]. Upon binding their respective ligands, these death receptors are activated to form homotrimers, clustering the receptor death domains, leading to recruitment of intracellular adaptor molecules (e.g. TRADD and FADD). These adaptor molecules recruit caspase 8 or 10 to the DISC, causing caspase self-cleavage and activation, which then goes on to activate the apoptotic caspase cascade [6]. Internalization of Fas and TNFR, but not DR4 or DR5, is required for DISC formation.
Death receptor-triggered apoptosis can be either dependent on or independent of the mitochondria, creating crossover between the extrinsic and the intrinsic apoptotic pathway (see Figure 1). Type 1 cells activate sufficient amounts of caspase 8 so that apoptosis occurs independent of the mitochondrial pathway. However, type 2 cells activate little caspase 8 and therefore require the activation of the mitochondrial apoptotic pathway, via caspase cleavage and activation of the pro-apoptotic protein Bid, in order to activate the full apoptotic caspase cascade. Additional intracellular control points in death receptor signalling include cellular FLICE (FADD-like interleukin-1β-converting enzyme)-inhibitory protein (c-FLIP), a catalytically inactive caspase 8/10 homologue that can bind and block signalling of FADD or caspase 8/10, and IAP family proteins which bind caspases, blocking their signalling.
Role of the Bcl-2 apoptotic protein family in apoptosis
Intrinsic apoptosis is regulated by the Bcl-2 family of proteins, which maintains the integrity of the mitochondrial membrane. The anti-apoptotic members of this protein family are Bcl-2, Bcl-Xl, Bcl-w, Bcl-B, Bfl-1 and Mcl-1, which contain four Bcl-2 homology domains (BH1–4) allowing them to lie within the outer mitochondrial membrane and bind/sequester pro-apoptotic proteins [8]. The pro-apoptotic family members include Bax and Bak, which contain domains BH1–3, and the BH3-only members Bad, Bid, Bim, Noxa, Puma, Bik, Bmf and Hrk. The BH3-only members can act as apoptosis sensitizers by binding to anti-apoptotic proteins and releasing Bax/Bak. Furthermore, Bid and Bim can operate as activators of Bax/Bak, stimulating Bax/Bak to oligomerize and form pores in the mitochondrial membrane.
To trigger apoptosis, the balance of anti-apoptotic and pro-apoptotic Bcl-2 proteins must be shifted so that there is an excess of pro-apoptotic proteins at the mitochondria and/or neutralization of anti-apoptotic proteins. The crucial step in triggering intrinsic apoptosis is mitochondrial outer membrane permeabilization by Bax/Bak, releasing pro-death factors (i.e. cytochrome c) that commit the cell to die. Thus, true intrinsic apoptosis is considered to be Bax/Bak dependent. Once released into the cytosol, cytochrome c forms the apoptosome with Apaf-1 and caspase 9, initiating the caspase cascade [9]. Mitochondrial outer membrane permeabilization also releases second mitochondria-derived activator of caspases (SMAC), which binds and inhibits IAPs. Furthermore, mitochondrial outer membrane permeabilization releases apoptosis-inducing factor and endonuclease G, which activate caspase-independent apoptosis, causing chromatin condensation and large-scale DNA fragmentation. Thus, even in the absence of caspase activity, mitochondrial outer membrane permeabilization can commit the cell to die via a back-up cell death programme [10].
Alterations in the expression of Bcl-2 family members contribute to neoplastic transformation and cancer cell chemoresistance, with the anti-apoptotic members serving as oncogenes. Initially, the BCL-2 gene was identified in chromosomal translocations t(14;18), causing excessive Bcl-2 expression in follicular lymphoma [11]. A survey of 68 cancer cell lines revealed that Bcl-2 and Bfl-1 expression was highest in leukaemia, lymphoma and melanoma cell lines, while Mcl-1 expression was predominant in glioma, lung, prostate, breast, ovarian and renal cancers [12]. Clinically, Bcl-2 expression in B cells from acute myeloid leukaemia (AML)/acute lymphoblastic leukaemia (ALL) patients was high in comparison to normal B cells and yielded a survival advantage against chemotherapy [13, 14]. Furthermore, high expression levels of Bcl-2, Bcl-Xl and Mcl-1 have all been reported to protect a wide spectrum of malignancies, causing resistance to various chemotherapeutic drugs and making them strong candidates for drug intervention.
Role of the inhibitors of apoptosis protein family in apoptosis
The IAP family contains eight members, including XIAP, cIAP1, cIAP2 and survivin. All IAPs have baculoviral IAP repeat (BIR) domains that allow them to bind active caspases directly and either suppress or target the IAP–caspase complex for degradation [15], serving as brakes of the final common pathway for intrinsically or extrinsically triggered apoptosis. However, IAPs can be regulated negatively by XAF1, HTRA2 and SMAC to release the apoptotic brakes. XIAP is considered to be the most potent of the IAPs, with a Ki (inhibition constant) in the high picomolar range [16, 17], showing the greatest protection from apoptotic events [18, 19]. Expression of XIAP in tissues is ubiquitous, and the loss of XIAP is thought to be a prerequisite for cell death and results in immune rejection and tumour regression more so than other apoptosis modulators [20, 21]. Overexpression of IAPs has been reported to contribute to initiation of haematological malignancy, chemoresistance in both solid and haematopoietic tumours, and poor clinical prognosis [22–24].
Agents that target and activate the death receptors
Pro-apoptotic receptor agonists (PARAs)
Pro-apoptotic receptor agonists are proteins or small molecules that target the death receptors DR4, DR5, Fas or TNFR. The PARAs work from outside the cell and directly trigger apoptosis via ligand-mediated receptor agonism. TNFR signalling is complex because it can activate caspases but can also stimulate pro-inflammatory pathways, which lead to induction of nuclear factor-κB and expression of anti-apoptotic genes (e.g. cIAP2, c-FLIP, Bcl-Xl and Bfl-1), thus driving cell survival and proliferation. Tumour necrosis factor signalling in normal tissues has greatly impeded its clinical development due to hepatic and cardiovascular toxicity [15]. However, TNFR has been successfully targeted to treat autoimmune diseases with antagonists such as anti-TNFR antibodies and soft tissue sarcomas of the extremities by isolated limb perfusion using nanoparticles tagged with TNF. Fas receptor targeting results in massive hepatocyte apoptosis and lethal liver damage in animal models [25], and is generally unsuitable for clinical development. However, FasL fusion proteins based on a collagen trimerization platform (including APO010) may be better tolerated and are in early clinical safety evaluation. The lead clinical PARAs are discussed below, while a detailed list of current clinical trials for all death receptor agonists can be found in Table 1.
Table 1.
Clinical studies of pro-apoptotic (death) receptor agonists (PARAs)
| Clinical drug | Drug type | Target | Phase | Treatment | Patients | Status* |
|---|---|---|---|---|---|---|
| Mapatumumab | mAb | DR4 | I/II | +Cisplatin and radiotherapy | Advanced cervical cancer | Active |
| I | +Sorafenib | Advanced hepatocellular carcinoma | Completed | |||
| II | +Sorafenib | Advanced hepatocellular carcinoma | Closed | |||
| II | +Bortezomib | Relapsed or refractory MM | Completed | |||
| II | +Carboplatin and paclitaxel | NSCLC | Completed | |||
| II | Monotherapy | Relapsed or refractory NHL | Completed | |||
| II | Monotherapy | Relapsed or refractory NSCLC | Completed | |||
| Lexatumumab | mAb | DR5 | I | ±Interferon γ | Paediatric relapsed or refractory solid tumours and lymphomas | Completed |
| Conatumumab | mAb | DR5 | I/II | +Capecitabine, radiotherapy and gemcitabine | Locally advanced pancreatic cancer | Terminated |
| I | +Bortezomib or vorinostat | Lymphoma | Suspended | |||
| I/II | +Carboplatin and paclitaxel | Untreated NSCLC | Completed | |||
| I/II | +mFOLFOX6 and bevacizumab | Untreated metastatic CRC | Closed | |||
| I/II | +Doxorubicin | Untreated unresectable soft tissue sarcoma | Closed | |||
| I/II | +Gemcitabine | Metastatic pancreatic cancer | Completed | |||
| I/II | +Panitumumab | Metastatic CRC | Closed | |||
| I/II | +AMG479 | Advanced refractory solid tumours | Terminated | |||
| II | +FOLFIRI | Metastatic KRas mutant CRC | Closed | |||
| II | ±FOLFOX6, ganitumab, bevacizumab | Advanced tumours | Closed | |||
| Tigatuzumab | mAb | DR5 | I | Monotherapy | Metastatic CRC | Active |
| I | Monotherapy | Advanced solid tumours and lymphomas | Completed | |||
| I | +FOLFIRI | Metastatic CRC | Closed | |||
| II | +Abraxane | Metastatic triple negative breast cancer | Closed | |||
| II | +Sorafenib | Advanced liver cancer | Closed | |||
| II | +Carboplatin and paclitaxel | Metastatic or unresectable NSCLC | Completed | |||
| II | +Carboplatin and paclitaxel | Locally advanced or metastatic ovarian cancer | Completed | |||
| II | Monotherapy | Untreated, unresectable pancreatic cancer | Completed | |||
| Apomab | mAb | DR5 | I | Monotherapy | Advanced recurrent or metastatic disease | Completed |
| I | +Irinotecan and cetuximab or +FOLFIRI | Metastatic CRC | Completed | |||
| I | +FOLFOX and bevacizumab | Untreated and metastatic CRC | Completed | |||
| II | +Rituximab | NHL | Completed | |||
| II | +Paclitaxel, carboplatin and bevacizumab | Untreated or recurrent NSCLC | Completed | |||
| II | Monotherapy | Advanced chondrosarcoma | Terminated | |||
| LBY-135 | mAb | DR5 | I | ±Capecitabine | Advanced solid tumours | Completed |
| Dulanermin | rhTRAIL | DR4, DR5 | I | +Camptosar and erbitux or +FOLFIRI ±bevacizumab | Metastatic CRC | Closed |
| I | +FOLFOX and bevacizumab | Untreated, locally advanced, recurrent or metastatic CRC | Closed | |||
| II | +Carboplatin and paclitaxel ±bevacizumab | Untreated NSCLC | Completed | |||
| I/II | ±Rituximab | NHL | Terminated | |||
| APO010 | Recombinant protein | FasR | I | Monotherapy | Solid tumours | Completed |
Abbreviations are as follows: CRC, colorectal cancer; DR4, death receptor 4; DR5, death receptor 5; FasR, Fas receptor; FOLFIRI, folinic acid, 5-fluorouracil, irinotecan regimen; FOLFOX, folinic acid, 5-fluorouracil, oxaliplatin regimen; mAb, monoclonal antibody; MM, multiple myeloma; NHL, non-Hodgkin's lymphoma; NSCLC, nonsmall cell lung cancer; rhTRAIL, recombinant human TRAIL.
Status according to http://www.clinicaltrials.gov as of 3 January 2013: closed = active, not recruiting; and suspended = temporarily halted patient recruitment.
Recombinant human TNF-related apoptosis-inducing ligand (rhTRAIL) – dulanermin
Preclinical studies
Early preclinical studies to activate the TRAIL receptors DR4 and DR5 used recombinant soluble fragments of murine TRAIL and showed antitumour activity in xenograft models. Soluble TRAIL was optimized for better stability and solubility, while eliminating early concerns of hepatotoxicity [26–28]. These studies ultimately led to the development of recombinant human TRAIL (rhTRAIL) which is composed of amino acids 114–281 of the natural ligand and activates both DR4 and DR5. Clinical grade soluble rhTRAIL showed antitumour activity in vitro in 16 of 39 cancer cell lines but not in several cell lines from normal tissues [26]. Recombinant human TRAIL showed promising antitumour efficacy in mouse xenografts of human cancers [colon [29], lung [30], pancreas [31], multiple myeloma (MM) [32], non-Hodgkin's lymphoma (NHL) [33] and glioma [34, 35]]. Combinations of rhTRAIL with proteasome inhibitors [36–38], HDAC inhibitors [39], the anti-CD20 antibody rituximab [33], antimetabolites, topoisomerase inhibitors, DNA-damaging agents or microtubule-targeting agents have shown additive or synergistic antitumour effects in preclinical models (reviewed in [40]). Preclinical studies of rhTRAIL included safety assessments in cynomolgus monkeys and chimpanzees, and revealed no liver or other major organ/tissue toxicity, but a limited half-life of approximately 25 min [26].
Clinical studies
In phase I and II studies, patients received rhTRAIL (dulanermin) doses up to 15 mg kg−1 intravenously for 5 days consecutively. The serum half-life was approximately 36 min at 8 mg kg−1, and rhTRAIL was well tolerated at this dose, with partial responses seen in two chondrosarcoma patients [41]. However, the antitumour benefit of rhTRAIL as part of combination therapy in phase II studies in solid tumours (e.g. colorectal cancer and nonsmall cell lung cancer) has not fulfilled its apparent early potential.
Monoclonal antibodies against the TRAIL receptors
Preclinical studies
Mapatumumab is a fully human IgG1 antibody that activates DR4 and has antitumour effects ex vivo and in vivo as a single agent in colon, nonsmall cell lung cancer (NSCLC) and renal cancer murine models. Mapatumumab also showed enhanced antitumour effects in combination with 5-fluorouracil, irinotecan, topotecan or irradiation in colon xenograft models [42, 43]. Of the numerous anti-DR5 antibodies in development, lexatumumab and conatumumab are fully human IgG1 antibodies, while tigatuzumab is a humanized IgG1. Lexatumumab showed preclinical efficacy in renal cell carcinoma and colorectal xenografts [43, 44]. Conatumumab was effective as a single agent and in combinations with gemcitabine and CPT-11 in colorectal, lung and pancreatic carcinoma models [45]. Likewise, tigatuzumab demonstrated preclinical antitumour activity in NSCLC, colorectal and pancreatic cancer in vitro and in vivo [46].
Clinical studies
Phase I studies have established that mapatumunab is well tolerated at doses between 10 and 30 mg kg−1 administered every 21 days. The pharmacokinetics showed a mean terminal elimination half-life of 18.8 days, which was dose independent and justified dosing every 2–3 weeks. Toxicities were all mild and included fatigue, fever and myalgias [47]. A randomized, controlled phase II trial to evaluate mapatumumab in combination with carboplatin (C) and paclitaxel (P) as first-line therapy in advanced NSCLC was conducted. Patients were randomly assigned to Arm A, paclitaxel 200 mg m−2 + carboplatin area under the curve (AUC) 6.0 (PC); Arm B, PC + mapatumumab 10 mg kg−1; or Arm C, PC + mapatumumab 30 mg kg−1. One hundred and eleven patients entered the study; no benefit from the addition of mapatumumab to PC was found. Adverse events were generally balanced across treatment groups; there was no evidence that mapatumumab exacerbated toxicities associated with PC. Further evaluation of mapatumumab in combination with PC in patients with advanced NSCLC was not supported by these study data [48]. The results of ongoing combination studies (see Table 1) are awaited.
The preliminary disappointing efficacy of these monoclonal antibodies targeting DR4 or DR5 has been attributed to a number of potential issues, namely inadequate tissue penetration to compete for binding with the endogenous ligand, lack of tumour expression of DR4 or DR5 (or mutations of these receptors), and the possibility that the scheduling of combinations with cytotoxic agents is suboptimal. Other possible reasons for failure include overexpression of decoy receptors, requirement of O-glycosylation for full activation, and alterations in the downstream pathway, such as high c-FLIP, IAP or Bcl-2 proteins, that block activation/amplification [7].
A number of future approaches for death receptor targeting are being pursued preclinically. These include fusion proteins of TRAIL, recombinant soluble TRAIL genetically linked to a receptor-selective antibody fragment, and designed PARAs with higher affinity binding [49].
Agents that target and inhibit anti-apoptotic Bcl-2 proteins
Many inhibitors of Bcl-2 family proteins have reached the clinic. An overview of the preclinical and clinical results for the lead clinical Bcl-2 inhibitors is discussed below, with a more detailed list of later phase trials shown in Table 2.
Table 2.
Clinical studies of Bcl-2 family inhibitors
| Clinical drug | Drug type | Target | Phase* | Treatment | Patients | Status† |
|---|---|---|---|---|---|---|
| Genasense | Antisense oligonucleotide | Bcl-2 | I | +Cytarabine and daunorubicin | AML | Closed |
| I | +Abraxane and temodar | Melanoma | Closed | |||
| II | +Carboplatin and paclitaxel | Melanoma | Closed | |||
| III | +Dacarbazine | Melanoma | Completed | |||
| III | +Cytarabine and daunorubicin | Untreated AML | Completed | |||
| III | +Dexamethasone | Relapsed or refractory MM | Completed | |||
| III | +Fludarabine and cyclophosphamide | Relapsed or refractory CLL | Completed | |||
| II/III | +Docetaxel | NSCLC | Unknown | |||
| II/III | Monotherapy | Advanced melanoma | Terminated | |||
| III | +Rituximab and fludarabine | Untreated CLL | Withdrawn | |||
| Gossypol/AT-101 | Polyphenolic aldehyde | Pan-Bcl-2, ER stress | II | +Docetaxel | Laryngeal cancer | Active |
| II | +Docetaxel | Squamous cell carcinoma of the head and neck | Active | |||
| Obatoclax (GX-15–070) | BH3 mimetic | Pan-Bcl-2, Kinases | I | +Bortezomib | Aggressive relapsed or recurrent NHL | Active |
| I | +Vincristine, doxorubicin and dexrazoxane | Relapsed or refractory solid tumours, lymphoma or leukaemia | Closed | |||
| I/II | +Topotecan | Relapsed or refractory SCLC or advanced tumours | Closed | |||
| I/II | +Bortezomib | Relapsed or refractory melanoma | Closed | |||
| III | +Carboplatin and etoposide | Chemotherapy-naïve SCLC | Withdrawn | |||
| Navitoclax (ABT-263) | BH3 mimetic | Bcl-2, Bcl-Xl | I | +Fludarabine/cyclophosphamide/rituximab or +bendamustine/rituximab | Relapsed or refractory CLL | Closed |
| I | +Rituximab | Lymphoid cancers | Closed | |||
| I | ±Erlotinib or irinotecan | Solid tumours | Closed | |||
| I/II | Monotherapy | Relapsed or refractory CLL | Closed | |||
| II | Monotherapy | Relapsed or refractory lymphoid malignancies | Closed | |||
| II | Monotherapy | CLL | Closed | |||
| ABT-199 | BH3 mimetic | Bcl-2 | I | Monotherapy | Relapsed or refractory CLL and NHL | Active |
| I | +Rituximab and bendamustine | Relapsed or refractory NHL | Active | |||
| I | +Rituximab and bendamustine | Relapsed or refractory CLL | Active | |||
| I | +Obinutuzumab | CLL | Suspended | |||
| I | +Rituximab and bendamustine | CLL | Suspended |
Abbreviations are as follows: AML, acute myeloid leukaemia; CLL, chronic lymphocytic leukaemia; MM, multiple myeloma; NHL, non-Hodgkin's lymphoma; NSCLC, nonsmall cell lung cancer; SCLC, small cell lung cancer.
Only active or late phase clinical trials are shown. A more comprehensive list of Bcl-2 drug targeting trials can be found at http://www.clinicaltrials.gov.
Status according to http://www.clinicaltrials.gov as of 3 January 2013: closed = active, not recruiting; and suspended = temporarily halted patient recruitment.
Oblimersen (Genasense)
Preclinical studies
The antisense oligonucleotide for Bcl-2, oblimersen (Genasense), targets the first six codons of Bcl-2 mRNA and is active in preclinical models of prostate cancer, melanoma, NHL, NSCLC, AML, B cell malignancies and several other malignancies [15, 50]. Preclinical studies of oblimersen showed enhanced antitumour activity of several chemotherapeutics, e.g. taxanes, vinca alkaloids, anthracyclines, alkylators, platinum-containing agents [6], endocrine therapy [51] and radiation [52].
Clinical studies
Studies with oblimersen (as a single agent or in combination) in many solid and haematological malignancies have unfortunately not confirmed the preclinical promise of this agent. An example of this is in melanoma. Early work controversially suggested that Bcl-2 was a viable target in melanoma [53]. Using the recommended phase II dose of oblimersen, 7 mg kg−1 day−1, as a continuous infusion for 5 days, followed by 1000 mg m−2 darcabazine (DTIC), a small phase II trial showed efficacy [54]. Therefore, a nonblinded, randomized trial in 775 patients comparing oblimersen + DTIC vs. DTIC alone was undertaken. Patients were prestratified by baseline lactate dehydrogenase (LDH). A continuous improvement in overall survival was observed in the patients receiving oblimersen as a function of baseline LDH. Patients with LDH ≤0.8 times the upper limit of normal showed the greatest benefit in overall survival (OS), and those with LDH ≥1.1 times the upper limit of normal showed no difference in OS [55]. On the basis of these results, a randomized, phase III trial of oblimersen + DTIC vs. DTIC alone was performed in 300 patients with LDH ≤0.8 times the upper limit of normal. This study revealed no difference in OS. Thus ended clinical trials of oblimersen, which had also failed in myeloma and whose development in chronic lymphocytic leukaemia (CLL) was halted [56]. Other bispecific antisense oligonucleotides targeting Bcl-2 and Bcl-Xl have been designed [57], but currently none has yet reached the clinic.
BH3 mimetics
Numerous BH3 mimetics have been developed to target the anti-apoptotic Bcl-2 proteins. These moieties are designed to bind the BH3 hydrophobic groove of these anti-apoptotic proteins, displacing pro-apoptotic proteins, which then trigger intrinsic apoptosis. Most of these BH3 mimetics are reported as pan-Bcl-2 inhibitors (i.e. bind Bcl-2, Bcl-Xl and Mcl-1). The problems with many of these designed mimetics (terphenyl-based structures, HA14-1, compound 6, antimycin A, S1, chelerythrine and stabilized BH3 peptides) include poor pharmacological properties, weak binding affinities and induction of Bax/Bak-independent cell death [58–62]. Thus, most do not appear to function as BH3 mimetics.
Gossypol and AT-101
Preclinical studies
Several natural compounds have been reported to bind the anti-apoptotic Bcl-2 proteins, including gossypol (cotton seeds), epigallecatechin gallate (green tea), theaflavins (black tea), chelerythrine (tropical plants) and antimycin A (bacterial metabolites), many of which are unstable in vivo and degrade to an inactive form, with the pan-Bcl-2 inhibitor gossypol showing the most promise as a potential therapeutic agent [15]. The measured binding affinities of gossypol for the anti-apoptotic proteins have varied due to its poor solubility [58]. AT-101 is the racemically purified version of gossypol that is being used for clinical trials due to increased potency in preclinical studies [63, 64].
There are reports of AT-101 activity as a single agent and in combination with rituximab in animal models of MM, B cell lymphoma and primary CLL (reviewed in [58]). Gossypol has been modified to apogossypol to make a more stable, effective form of the compound, but this compound has not yet reached clinical trials. Another gossypol derivative, apogossypolone, inhibited growth of follicular lymphoma cell lines and primary mantle cell lymphoma and CLL tumours. Xenograft models showed apogossypolone protection from tumour-cell bone marrow infiltration and extended survival times (review [58]). Whether gossypol is a BH3 mimetic has been questioned, because it does not appear to function as such in vitro [62, 65].
Clinical studies
Phase I studies of AT-101 undertaken in prostate cancer patients established the recommended monotherapy phase II dose as 30 mg day−1 for 21 days out of 28. However, during phase II studies, better tolerability was achieved with 20 mg day−1 for 21 out of 28 days. The major adverse effects were gastrointestinal disturbances, anorexia, fatigue and bone and back pain [66]. Other Phase I studies of combination treatments of 1–5 days have established a maximal tolerated dose of AT-101 of 40 mg twice daily. Pharmacokinetic studies revealed a half-life of 3–5 h, and food produced a 45% increase in AUC [67]. Phase II randomized studies of AT-101 plus docetaxel vs. docetaxel alone in chemonaïve prostate cancer patients (n = 221) did not show improved overall survival, although the study suggests that patients with high-risk disease may benefit [68].
Obatoclax (GX15-070)
Preclinical studies
The reported pan-Bcl-2 inhibitor obatoclax is a synthetic prodiginine derivative with single agent activity in multiple cancer cells lines, with IC50 values of 0.26–15 μm. Synergy with several anticancer molecules has also been reported; however, obatoclax can kill cells independently of Bax and Bak, causing S–G2 cell cycle arrest [58, 60, 69]. These studies suggest that obatoclax sensitizes cells independent of the intrinsic apoptosis family and has off-target effects.
Clinical studies
Infusion of obatoclax mesylate was studied in phase I and phase II studies in haematological cancers. Unfortunately, it was found that the concentrations needed for preclinical anticancer efficacy could not be sustained clinically because of dose-limiting neurotoxicity (ataxia, mood alteration, somnolence and cognitive dysfunction) [70–72].
Navitoclax and its congeners (e.g. ABT-199)
The BH3 mimetics, such as ABT-737, ABT-263 and ABT-199, directly bind the BH3 groove of anti-apoptotic proteins, leading to Bax/Bak activation and true intrinsic apoptosis [9]. ABT-737 and ABT-263 bind to Bcl-2, Bcl-Xl and Bcl-w, while ABT-199 binds more selectively to Bcl-2. Navitoclax (formerly ABT-263) is an analogue of ABT-737 that is orally bioavailable.
Preclinical studies
ABT-737 showed in vitro toxicity as a monotherapy in numerous malignant cell lines and primary leukaemia and lymphoma cells (reviewed in [73]). Navitoclax (ABT-263) showed synergy with etoposide and carboplatin that was most likely to be due to p53 activation and upregulation of pro-apoptotic proteins Puma, Noxa and Bim by the DNA-damaging agents. A mechanism of resistance against navitoclax and ABT-737 is the expression of Mcl-1. Rationalized targeting of Mcl-1, a short-lived protein, with cyclin-dependent kinase inhibitors that block global transcription in combination with ABT-737 can sensitize leukaemia cells [74]. When navitoclax was combined with rituximab, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or bortezomib in mouse models of large B cell lymphoma and mantle cell lymphoma, it demonstrated enhanced antitumour effects (reviewed in [58]). However, navitoclax absorption is limited by its dissolution, with a half-life of 4.6–8.4 h in the mouse, rat, dog and monkey (reviewed in [58]), leading to a daily dosing regimen in human clinical trials.
ABT-199, the most recent navitoclax congener, was effective alone or in combination with rituximab ± bendamustine in several leukaemia and lymphoma animal models. ABT-199 was more potent at killing peripheral blood CLL cells while sparing circulating platelets in comparison to navitoclax, suggesting that it may be more successful clinically 75.
Clinical studies
A phase I trial of oral navitoclax was conducted in 29 patients with relapsed or refractory CLL. Navitoclax was given daily for 14 days (10, 110, 200 or 250 mg day−1; n = 15) or 21 days (125, 200, 250 or 300 mg day−1; n = 14) of each 21 day cycle. Lymphocytosis was reduced by more than 50% in 19 of 21 patients with baseline lymphocytosis. Among 26 patients treated with navitoclax ≥110 mg day−1, nine (35%) achieved a partial response and seven maintained stable disease for >6 months. Dose-related thrombocytopenia was the major dose-limiting toxicity, with some milder gastrointestinal adverse effects. Navitoclax had a mean half-life of 17 h with first-order elimination, and could not be detected in urine, indicating negligible navitoclax renal clearance. A navitoclax dose of 150–250 mg day−1 in solid tumours or haematological malignancies in a continuous dosing schedule was defined as optimal for phase II studies [76–78]. Navitoclax has little activity as monotherapy for NSCLC [79]. However, several phase II studies with navitoclax monotherapy in lymphoid malignancies are nearing completion, as are combination studies with bendamustine and rituximab.
The newest drug in the BH3-mimetic pipeline, ABT-199, has already entered the clinic for patients with NHL. Data from an ongoing phase I study revealed that ABT-199 is tolerated at oral doses ranging from 50 to 600 mg daily, with a time to maximum concentration (Tmax) at 7 h and a mean half-life of approximately 15 h. Its AUC is increased threefold when ingested with food. The most common adverse effects were gastrointestinal disturbances (nausea, dyspepsia and diarrhoea) and fatigue, as well as grade 3 anaemia and grade 4 neutropenia. The maximal tolerated dose has not yet been determined. It caused little or no thrombocytopenia, with encouraging antitumour efficacy in eight of 15 patients and four of five with follicular lymphoma [80].
Agents that target the inhibitors of apoptosis proteins
The lead clinical IAP inhibitors are discussed below, and a detailed list of current clinical trials for all IAP inhibitors can be found in Table 3.
Table 3.
Clinical studies of inactivators of inhibitors of apoptosis (IAP) proteins
| Clinical drug | Drug type | Target | Phase | Treatment | Patients | Status* |
|---|---|---|---|---|---|---|
| YM155 | Small molecule | Survivin | I | Monotherapy | Advanced cancers | Completed |
| II | Monotherapy | HRPC | Completed | |||
| II | Monotherapy | NSCLC | Completed | |||
| II | Monotherapy | Melanoma | Completed | |||
| II | Monotherapy | Diffuse large B cell lymphoma | Terminated | |||
| II | +Docetaxel ± prednisone | HRPC, solid tumours | Completed | |||
| II | +Docetaxel | Refractory melanoma | Completed | |||
| II | +Docetaxel | Untreated Her2-negative breast cancer | Closed | |||
| II | +Rituximab | NHL | Closed | |||
| I/II | +Paclitaxel and carboplatin | Solid tumours, NSCLC | Closed | |||
| AEG35156 | Antisense oligonucleotide | XIAP | I | Monotherapy | Advanced cancers | Terminated |
| I | +Docetaxel | Solid tumours | Completed | |||
| II | +Cytarabine and idarubicin | AML | Terminated | |||
| I /II | Monotherapy | CLL, B cell lymphoma | Terminated | |||
| I/II | +Carboplatin and paclitaxel | Advanced NSCLC | Terminated | |||
| I/II | +Sorafenib | Advanced hepatocellular carcinoma | Completed | |||
| I/II | +Gemcitabine | Pancreatic cancer | Terminated | |||
| I/II | +Paclitaxel | Advanced breast cancer | Terminated | |||
| AT-406 | Small molecule | IAPs | I | Monotherapy | Solid tumours, lymphoma | Active |
| I | +Daunorubicin and cytarabine | AML | Active | |||
| LCL161 | SMAC mimetic | IAPs | I | Monotherapy | Advanced solid tumours | Completed |
| I | +Paclitaxel | Advanced solid tumours | Active | |||
| II | +Paclitaxel | Triple-negative breast cancer | Active | |||
| HGS1029 | Small molecule | IAPs | I | Monotherapy | Advanced solid tumours | Completed |
| I | Monotherapy | Lymphoid malignancies | Terminated | |||
| GDC-0917 | SMAC mimetic | IAPs | I | Monotherapy | Solid tumours | Completed |
| GDC-0152 | SMAC mimetic | IAPs | I | Monotherapy | Solid cancers | Completed |
| TL32711 | SMAC mimetic | IAPs | I | Monotherapy | Refractory solid tumours, lymphoma | Closed |
| I | +Gemcitabine | Advanced solid tumours | Active | |||
| I/II | Monotherapy | AML | Active | |||
| I/II | +Carboplatin and paclitaxel, +irinotecan, +docetaxel, +gemcitabine or +liposomal doxorubicin | Adult cancers | Closed | |||
| II | Monotherapy | Advanced ovarian, Fallopian tube and peritoneal cancer | Active |
Abbreviations are as follows: AML, acute myeloid leukaemia; CLL, chronic lymphocytic leukaemia; HRPC, hormone-refractory prostate cancer; NHL, non-Hodgkin's lymphoma; NSCLC, nonsmall cell lung cancer.
Status according to http://www.clinicaltrials.gov as of 3 January 2013: closed = active, not recruiting.
IAP antisense oligonucleotides
Preclinical studies
There are a number of antisense oligonucleotides in development that target IAPs. Of these, AEG35156, which targets XIAP, has made the most clinical progress. AEG35156 reduces XIAP mRNA and protein in the nanomolar range in cell lines, enhancing sensitivity to TRAIL in NSCLC and pancreatic cancer lines. In paediatric tumour cell lines, AEG35156 demonstrated XIAP downregulation and antitumour efficacy in combination with doxorubicin or etoposide [81]. Tumour growth delay was observed in colon and prostate cancer xenografts with AEG35156 used as a single agent, which was enhanced in combination with docetaxel [82].
Clinical studies
Early clinical studies with AEG35156 revealed several problematic pharmacological properties, including poor oral bioavailability, requiring continuous intravenous infusions over hours or days [21]. The drug caused chills and lethargy and accumulated in the liver, causing transaminitis and liver toxicities. Tumour lysis syndrome, hypophosphataemia and QTc prolongation (with one death) were all observed dose-limiting toxicities. Shorter infusions were studied in combination with chemotherapy but caused neuropathy, and altering the dosing schedule to make AEG35156 safer limited its effectiveness (reviewed in [21]). Even with these problems, XIAP targeting is considered a better approach than SMAC mimetics that target cIAP1 and cIAP2, even though the latter may be tumour suppressors in NHL, Hodgkin's disease, small lymphocytic leukaemia, ALL, CLL, MM or Waldenstrom's macroglobulinaemia [21]. AEG35156 development seems to be halted, but antisense oligonucleotides for IAPs are still attracting considerable interest as potential anticancer therapeutics.
Antisurvivin agents
Preclinical studies
The role of survivin as an IAP protein is controversial, because it contains only one BIR domain, leading to poor caspase binding. However, survivin association with other proteins (e.g. XIAP and hepatitis B X-interacting protein) enables caspase binding and inhibition. Furthermore, survivin has a critical role in cell division, making it an attractive target for cancer therapy [83].
The preclinical studies of survivin inhibitors EZN3042/SPC3042 showed reduced tumour weight when combined with paclitaxel in prostate cancer xenografts [84]. A survivin antisense oligonucleotide, LY2181308, showed inhibition of survivin protein in glioblastoma and melanoma xenografts with tumour growth delay as a single agent. LY2181308 combined with gemcitabine or paclitaxel increased caspase 3 activity and delayed tumour growth by 2–5 days [85].
EZN-3042 is a locked nucleic acid oligonucleotide that effectively targets survivin in several ALL cell lines. Apoptosis initiated by EZN-3042 was enhanced by several individual chemotherapeutic drugs, with ALL xenografts showing reduced blast counts [86].
YM155 (now known as sepantropium bromide) is an imidazolium-based small molecule that exclusively downregulates survivin according to a promoter activity assay. Preclinical studies show that YM155 reduces survivin protein in hormone-refractory prostate cancer PC-3 cells, accompanied by increased caspase 3 activity and decreased cell viability. Furthermore, several human tumour xenograft models (hormone-refractory prostate cancer, NSCLC, breast, bladder and melanoma) showed reduced survivin protein in the tumour and tumour regression in response to YM155 [87, 88].
Clinical studies
LY2181308 (antisense oligonucleotide against survivin)
LY2181308 has been studied as single agent therapy as a 3 h intravenous infusion of 750 mg for 3 days consecutively, then once weekly thereafter in refractory solid tumours in Japanese patients (n = 12) and in relapsed/refractory AML in US patients (n = 16). LY2181308 was reasonably well tolerated, with reversible and low-grade toxicities of flu-like syndrome, prolonged prothrombin time, thrombocytopenia and fatigue. A dose-limiting toxicity of reversible grade 3 elevation of aspartate or alanine aminotransferase/gamma-glutamyl transpeptidase was reported in one patient. The systemic disposition LY2181308 revealed a long terminal elimination half-life of 21 days with a large apparent volume of distribution, indicating tissue accumulation. There was reduced survivin expression in tumours of patients with high survivin expression. One of the 12 Japanese patients in this study was observed to have stable disease [89]. The US study initially treated a patient cohort (n = 8) with LY2181308 as monotherapy (dosed as described above). In a subsequent cohort (n = 16), LY2181308 was combined with cytarabine (1.5 g m−2 4 h intravenous infusion) and idarubicin (12 mg m−2, 30 min intravenous infusion) on days 3, 4 and 5 and then again on days 1, 2 and 3 of subsequent 28 day cycles. The combination produced four of 16 complete responses, one of 16 incomplete response, and four of 16 AML patients showed cytoreduction. Six of eight patients who received LY2181308 monotherapy died, and three of 16 who received combination therapy died [90].
YM155 (sepantropium bromide)
YM155 has been studied as single agent therapy given as a continuous intravenous infusion for 168 h every 21 days in two phase I studies in patients with advanced solid tumours. The maximal tolerated dose in US patients (n = 41) was 4.8 mg m−2 day−1, while in Japanese patients (n = 33) using a hydration regimen it was 8 mg m−2 day−1. The dose-limiting toxicity in both studies was unexpected reversible acute tubular necrosis. Other milder toxicities included fever, arthralgia, nausea and diarrhoea. YM155 pharmacokinetics revealed an elimination half-life of 20.7 h. In the US study, responses were observed in refractory NHL (three of five; one complete) and in refractory prostate cancer (two of nine; both partial responses) and a minor response in one of two NSCLC patients. In the Japanese patients, a pretreatment hydration and hydration regimen supplemented the 168 h infusion of YM155. The dose-limiting toxicity was nephrotoxicity; additional lesser toxicities were microalbuminuria (suggesting glomerular effects), fever, anaemia, lymphopenia and infusion-site reactions. Pharmacokinetics in the Japanese study were similar to those found in the US study, with dose linearity and a similar terminal elimination half-life. The fractional dose excreted in the urine varied from 25 to 42% and was not related to dose. Stable disease in nine of 33 patients was observed on tumour burden assessment [91, 92]. YM155 pharmacokinetics were further studied, and a 30% increase in YM155 exposure (AUC) in patients with moderate (but not mild) renal dysfunction was observed, suggesting that dose modification would be beneficial in such patients [93].
Further clinical development of YM155 included phase II monotherapy studies in hormone-refractory prostate cancer, diffuse B cell lymphoma and NSCLC. Clinical data from these studies showed limited single agent YM155 activity [94–97]. However, strong preclinical data suggested beneficial effects of YM155 when combined with approved cytotoxic agents; thus, several phase II studies of combinations of YM155 are ongoing (see Table 3).
IAP antagonists
Preclinical studies
An alternative means to target IAP proteins is by using mimetics of their natural antagonists (e.g. SMAC). Many small molecule IAP antagonists have been designed, including AT-406, LCL161, HGS1029, GDC-0917, GDC-0152 and TL32711. AT-406 is an orally active antagonist of multiple IAP proteins and has antitumour activity as single agent and in combination with carboplatin in ovarian cancer cells and in xenografts [98]. This activity may rely on its ability to downregulate XIAP. LCL161 has shown limited in vitro and in vivo activity as a single agent against paediatric cancer preclinical models and is under investigation as combination therapy [99]. GDC-0152 demonstrated Ki values of 17–43 nm for XIAP, cIAP1 and cIAP2 and showed remarkable tumour growth inhibition in a breast cancer xenograft model [100]. TL32711 is reported as a SMAC mimetic, whose preclinical data suggests that in combination with a DR4/5 agonist it may be effective against follicular and germinal cell lymphomas [101].
Clinical studies
IAP antagonists currently reported in phase I trials include AT-406, LCL161, HGS1029, GDC-0917 and GDC-0152, with TL32711 in phase II trials [102] (see Table 3). Early clinical reports of HGS1029 suggest that it is well tolerated, with dose-limiting toxicities including severe fatigue, elevated amylase and lipase levels. Tumour regression was seen in one colon cancer patient and stable disease in two patients (NSCLC and adrenocortical carcinoma) in response to HGS1029 treatment [103]. TL32711 is currently under investigation under the name of birinapant, with early clinical results reporting that this SMAC mimetic is well tolerated and results in rapid and sustained cIAP1 suppression in tumour biopsies [101, 104].
Rationale for combining pro-apoptotic agents
The extrinsic and intrinsic apoptotic pathways can be stimulated independently of one another, although crosstalk can occur via caspase 8 activation of Bid. For example, FADD−/− and caspase 8−/− fibroblasts are resistant to death receptor stimulation but sensitive to cytotoxic drugs (activation of the intrinsic pathway), and caspase 9−/− embryonic stem cells and Apaf1−/− thymocytes are sensitive to death receptor agonists but resistant to cytotoxic drugs (activation of the extrinsic pathway) [6].
Malignancies are notorious for having defects in one or both apoptotic pathways and cannot be successfully targeted by single agent therapies. Thus, combination therapies are more likely to be successful. Multistep targeting of the apoptotic cascade by combining rhTRAIL with Bcl-2 antagonists or IAP-blocking mimetics enhances apoptosis rates in malignant cells in vitro [105–108]. Certain chemotherapeutic drugs cause endogenous expression and/or translocation of DR4/5 to the extracellular membrane, which could enhance their combined use with other pro-apoptotic agents. Furthermore, the expression of anti-apoptotic and pro-apoptotic proteins can be transcriptionally regulated by nuclear factor-κB and p53, respectively, in response to chemotherapeutic drugs. It should be emphasized that combining targeted pro-apoptotic agents with classical cytotoxic chemotherapy has an indirect effect on enhancing the pro-apoptotic equilibrium in cells compared with the use of the targeted pro-apoptotic agent alone.
Conclusions and future directions
Targeting apoptosis is an exciting paradigm in cancer drug development. Antibodies and recombinant TRAIL that target the death receptors are in phase II clinical trials against a range of solid tumours, but the maturing clinical data on their antitumour efficacy are disappointing. Bcl-2 family and IAP antagonists with preclinical efficacy are showing promising antitumour efficacy in early clinical trials against certain tumours, especially haematological malignancies. Preclinical data will be important in identifying the genetic bases for the development of tumour resistance to this class of compounds by comparing the DNA, RNA and proteins in resistant and sensitive cancers. Tissue can then be collected in subsequent clinical trials to address these hypotheses in cancer patients. One aspect of further clinical trials with these agents should be an improved understanding of the systemic drug exposure (i.e. drug dose and schedule) relationship to drug molecular target tumour pharmacodynamics, emphasizing the importance of obtaining tumour tissue or developing a validated biomarker for the antitumour effects of such targeted drugs. Effective treatment for some tumours will probably require that pro-apoptotic agents are administered with other established anticancer drugs in scientifically guided combinations.
Competing Interests
D.J.P.B. has no competing interests to declare. L.D.L. has received research grants from Abbott Pharmaceuticals and Astellas for early clinical development studies of navitoclax (ABT-263) and sepantronium bromide (YM155), respectively.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre N. Hippocrates of Cos and apoptosis. Lancet. 2003;361:1306. doi: 10.1016/S0140-6736(03)13017-6. [DOI] [PubMed] [Google Scholar]
- 5.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 6.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Lostao L, Marzo I, Anel A, Naval J. Targeting the Apo2L/TRAIL system for the therapy of autoimmune diseases and cancer. Biochem Pharmacol. 2012;83:1475–1483. doi: 10.1016/j.bcp.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Walensky LD. BCL-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ. 2006;13:1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- 9.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 12.Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campana D, Coustan-Smith E, Manabe A, Buschle M, Raimondi SC, Behm FG, Ashmun R, Arico M, Biondi A, Pui CH. Prolonged survival of B-lineage acute lymphoblastic leukemia cells is accompanied by overexpression of bcl-2 protein. Blood. 1993;81:1025–1031. [PubMed] [Google Scholar]
- 14.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, Archimbaud E, Magaud JP, Guyotat D. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–3096. [PubMed] [Google Scholar]
- 15.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3:388–398. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- 16.Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645–655. doi: 10.1038/sj.emboj.7600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stennicke HR, Ryan CA, Salvesen GS. Reprieval from execution: the molecular basis of caspase inhibition. Trends Biochem Sci. 2002;27:94–101. doi: 10.1016/s0968-0004(01)02045-x. [DOI] [PubMed] [Google Scholar]
- 18.Simons M, Beinroth S, Gleichmann M, Liston P, Korneluk RG, MacKenzie AE, Bahr M, Klockgether T, Robertson GS, Weller M, Schulz JB. Adenovirus-mediated gene transfer of inhibitors of apoptosis protein delays apoptosis in cerebellar granule neurons. J Neurochem. 1999;72:292–301. doi: 10.1046/j.1471-4159.1999.0720292.x. [DOI] [PubMed] [Google Scholar]
- 19.Wright ME, Han DK, Hockenbery DM. Caspase-3 and inhibitor of apoptosis protein(s) interactions in Saccharomyces cerevisiae and mammalian cells. FEBS Lett. 2000;481:13–18. doi: 10.1016/s0014-5793(00)01962-1. [DOI] [PubMed] [Google Scholar]
- 20.Ravi R, Fuchs EJ, Jain A, Pham V, Yoshimura K, Prouser T, Jalla S, Zhou X, Garrett-Mayer E, Kaufmann SH, Schulick RD, Pardoll DM, Bedi A. Resistance of cancers to immunologic cytotoxicity and adoptive immunotherapy via X-linked inhibitor of apoptosis protein expression and coexisting defects in mitochondrial death signaling. Cancer Res. 2006;66:1730–1739. doi: 10.1158/0008-5472.CAN-05-3377. [DOI] [PubMed] [Google Scholar]
- 21.Lacasse EC. Pulling the plug on a cancer cell by eliminating XIAP with AEG35156. Cancer Lett. 2012;332:215–224. doi: 10.1016/j.canlet.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Small S, Keerthivasan G, Huang Z, Gurbuxani S, Crispino JD. Overexpression of survivin initiates hematologic malignancies in vivo. Leukemia. 2010;24:1920–1926. doi: 10.1038/leu.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacasse EC, Kandimalla ER, Winocour P, Sullivan T, Agrawal S, Gillard JW, Durkin J. Application of XIAP antisense to cancer and other proliferative disorders: development of AEG35156/ GEM640. Ann N Y Acad Sci. 2005;1058:215–234. doi: 10.1196/annals.1359.032. [DOI] [PubMed] [Google Scholar]
- 24.Fulda S, Debatin KM. Targeting inhibitor of apoptosis proteins (IAPs) for diagnosis and treatment of human diseases. Recent Pat Anticancer Drug Discov. 2006;1:81–89. doi: 10.2174/157489206775246539. [DOI] [PubMed] [Google Scholar]
- 25.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 26.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O'Connell M, Kelley RF, Ashkenazi A, de Vos AM. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4:563–571. doi: 10.1016/s1097-2765(00)80207-5. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 29.Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–38. [PubMed] [Google Scholar]
- 30.Jin H, Yang R, Fong S, Totpal K, Lawrence D, Zheng Z, Ross J, Koeppen H, Schwall R, Ashkenazi A. Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res. 2004;64:4900–4905. doi: 10.1158/0008-5472.CAN-04-0408. [DOI] [PubMed] [Google Scholar]
- 31.Hylander BL, Pitoniak R, Penetrante RB, Gibbs JF, Oktay D, Cheng J, Repasky EA. The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med. 2005;3:22. doi: 10.1186/1479-5876-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman R, Hideshima T, Anderson KC. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood. 2001;98:795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- 33.Daniel D, Yang B, Lawrence DA, Totpal K, Balter I, Lee WP, Gogineni A, Cole MJ, Yee SF, Ross S, Ashkenazi A. Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood. 2007;110:4037–4046. doi: 10.1182/blood-2007-02-076075. [DOI] [PubMed] [Google Scholar]
- 34.Pollack IF, Erff M, Ashkenazi A. Direct stimulation of apoptotic signaling by soluble Apo2l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res. 2001;7:1362–1369. [PubMed] [Google Scholar]
- 35.Roth W, Isenmann S, Naumann U, Kugler S, Bahr M, Dichgans J, Ashkenazi A, Weller M. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265:479–483. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- 36.Brooks AD, Ramirez T, Toh U, Onksen J, Elliott PJ, Murphy WJ, Sayers TJ. The proteasome inhibitor bortezomib (Velcade) sensitizes some human tumor cells to Apo2L/TRAIL-mediated apoptosis. Ann N Y Acad Sci. 2005;1059:160–167. doi: 10.1196/annals.1339.042. [DOI] [PubMed] [Google Scholar]
- 37.Johnson TR, Stone K, Nikrad M, Yeh T, Zong WX, Thompson CB, Nesterov A, Kraft AS. The proteasome inhibitor PS-341 overcomes TRAIL resistance in Bax and caspase 9-negative or Bcl-xL overexpressing cells. Oncogene. 2003;22:4953–4963. doi: 10.1038/sj.onc.1206656. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Guo W, Zhang L, Wu S, Teraishi F, Davis JJ, Dong F, Fang B. Proteasome inhibitors-mediated TRAIL resensitization and Bik accumulation. Cancer Biol Ther. 2005;4:781–786. doi: 10.4161/cbt.4.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene. 2004;23:6261–6271. doi: 10.1038/sj.onc.1207830. [DOI] [PubMed] [Google Scholar]
- 40.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 41.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O'Dwyer PJ, Gordon MS, Novotny W, Goldwasser MA, Tohnya TM, Lum BL, Ashkenazi A, Jubb AM, Mendelson DS. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 42.Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M, Mahoney A, Carrell J, Boyd E, Yao XT, Zhang L, Zhong L, von Kerczek A, Shepard L, Vaughan T, Edwards B, Dobson C, Salcedo T, Albert V. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92:1430–1441. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marini P, Denzinger S, Schiller D, Kauder S, Welz S, Humphreys R, Daniel PT, Jendrossek V, Budach W, Belka C. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene. 2006;25:5145–5154. doi: 10.1038/sj.onc.1209516. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Zhang X, Barrisford GW, Olumi AF. Lexatumumab (TRAIL-receptor 2 mAb) induces expression of DR5 and promotes apoptosis in primary and metastatic renal cell carcinoma in a mouse orthotopic model. Cancer Lett. 2007;251:146–157. doi: 10.1016/j.canlet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, Moriguchi J, Coxon A, Huard JN, Xu R, Peach ML, Juan G, Kaufman S, Chen Q, Bianchi A, Kordich JJ, Ma M, Foltz IN, Gliniak BC. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol Ther. 2010;9:618–631. doi: 10.4161/cbt.9.8.11264. [DOI] [PubMed] [Google Scholar]
- 46.Yada A, Yazawa M, Ishida S, Yoshida H, Ichikawa K, Kurakata S, Fujiwara K. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–1067. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- 47.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, Corey A, Cohen RB. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 48.Von Pawel J, Harvey JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, Kumm E, Gallant G, Fox N, Camidge DR. A randomized phase II trial of mapatumumab, a TRAIL-R1 agonist monocolonal antibody, in combination with carboplatin with paclitaxel in patients with advanced NSCLC. J Clin Oncol. 2010;28(Suppl):abstr LBA7501. doi: 10.1016/j.cllc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 49.den Hollander MW, Gietema JA, de Jong S, Walenkamp AM, Reyners AK, Oldenhuis CN, de Vries EG. Translating TRAIL-receptor targeting agents to the clinic. Cancer Lett. 2013;332:194–201. doi: 10.1016/j.canlet.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12:193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 51.Kim R, Tanabe K, Emi M, Uchida Y, Toge T. Modulation of tamoxifen sensitivity by antisense Bcl-2 and trastuzumab in breast carcinoma cells. Cancer. 2005;103:2199–2207. doi: 10.1002/cncr.21029. [DOI] [PubMed] [Google Scholar]
- 52.Mu Z, Hachem P, Pollack A. Antisense Bcl-2 sensitizes prostate cancer cells to radiation. Prostate. 2005;65:331–340. doi: 10.1002/pros.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benimetskaya L, Lai JC, Khvorova A, Wu S, Hua E, Miller P, Zhang LM, Stein CA. Relative Bcl-2 independence of drug-induced cytotoxicity and resistance in 518A2 melanoma cells. Clin Cancer Res. 2004;10:8371–8379. doi: 10.1158/1078-0432.CCR-04-1294. [DOI] [PubMed] [Google Scholar]
- 54.Jansen B, Wacheck V, Heere-Ress E, Schlagbauer-Wadl H, Hoeller C, Lucas T, Hoermann M, Hollenstein U, Wolff K, Pehamberger H. Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet. 2000;356:1728–1733. doi: 10.1016/S0140-6736(00)03207-4. [DOI] [PubMed] [Google Scholar]
- 55.Agarwala SS, Keilholz U, Gilles E, Bedikian AY, Wu J, Kay R, Stein CA, Itri LM, Suciu S, Eggermont AM. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951) Eur J Cancer. 2009;45:1807–1814. doi: 10.1016/j.ejca.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 56.Chanan-Khan AA, Niesvizky R, Hohl RJ, Zimmerman TM, Christiansen NP, Schiller GJ, Callander N, Lister J, Oken M, Jagannath S. Phase III randomised study of dexamethasone with or without oblimersen sodium for patients with advanced multiple myeloma. Leuk Lymphoma. 2009;50:559–565. doi: 10.1080/10428190902748971. [DOI] [PubMed] [Google Scholar]
- 57.Zangemeister-Wittke U, Leech SH, Olie RA, Simoes-Wust AP, Gautschi O, Luedke GH, Natt F, Haner R, Martin P, Hall J, Nalin CM, Stahel RA. A novel bispecific antisense oligonucleotide inhibiting both bcl-2 and bcl-xL expression efficiently induces apoptosis in tumor cells. Clin Cancer Res. 2000;6:2547–2555. [PubMed] [Google Scholar]
- 58.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 59.Yin H, Lee GI, Sedey KA, Kutzki O, Park HS, Orner BP, Ernst JT, Wang HG, Sebti SM, Hamilton AD. Terphenyl-Based Bak BH3 alpha-helical proteomimetics as low-molecular-weight antagonists of Bcl-xL. J Am Chem Soc. 2005;127:10191–10196. doi: 10.1021/ja050122x. [DOI] [PubMed] [Google Scholar]
- 60.Albershardt TC, Salerni BL, Soderquist RS, Bates DJ, Pletnev AA, Kisselev AF, Eastman A. Multiple BH3 mimetics antagonize anti-apoptotic MCL1 by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem. 2011;286:24882–24895. doi: 10.1074/jbc.M111.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, Cohen GM. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 63.Varol U, Karaca B, Tunali D, Degirmenci M, Cirak Y, Purcu DU, Uzunoglu S, Sezgin C, Karabulut B, Sanli UA, Uslu R. The effect of racemic gossypol and at-101 on angiogenic profile of ovcar-3 cells: a preliminary molecular framework for gossypol enantiomers. Exp Oncol. 2009;31:220–225. [PubMed] [Google Scholar]
- 64.Liu S, Kulp SK, Sugimoto Y, Jiang J, Chang HL, Dowd MK, Wan P, Lin YC. The (-)-enantiomer of gossypol possesses higher anticancer potency than racemic gossypol in human breast cancer. Anticancer Res. 2002;22:33–38. [PubMed] [Google Scholar]
- 65.Albershardt TC, Salerni BL, Soderquist RS, Bates DJ, Pletnev AA, Kisselev AF, Eastman A. Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem. 2011;286:24882–24895. doi: 10.1074/jbc.M111.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009;15:3172–3176. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitot HC, Mould D, Maleski L, Leopold L. Analysis of a phase I pharmacokinetic (PK)/food effect study of AT-101 in patients with advanced solid tumors. J Clin Oncol. 2009;27(Suppl):abstr 2557. [Google Scholar]
- 68.Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, Galsky MD, Berry WR, Karlov P, Holmlund JT, Wood BA, Brookes M, Leopold L. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23:1803–1808. doi: 10.1093/annonc/mdr555. [DOI] [PubMed] [Google Scholar]
- 69.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, Bornmann W, Kantarjian H, Viallet J, Samudio I, Andreeff M. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang JJ, Kuruvilla J, Mendelson D, Pishvaian MJ, Deeken JF, Siu LL, Berger MS, Viallet J, Marshall JL. Phase I dose finding studies of obatoclax (GX15-070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin Cancer Res. 2010;16:4038–4045. doi: 10.1158/1078-0432.CCR-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schimmer AD, O'Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, Yee K, Ravandi F, Giles F, Schuh A, Gupta V, Andreeff M, Koller C, Chang H, Kamel-Reid S, Berger M, Viallet J, Borthakur G. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 72.Parikh SA, Kantarjian H, Schimmer A, Walsh W, Asatiani E, El-Shami K, Winton E, Verstovsek S. Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clin Lymphoma Myeloma Leuk. 2010;10:285–289. doi: 10.3816/CLML.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 73.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(Suppl. 1):S149–157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bates DJ, Salerni BL, Lowrey CH, Eastman A. Vinblastine sensitizes leukemia cells to cyclin-dependent kinase inhibitors, inducing acute, cell cycle phase-independent apoptosis. Cancer Biol Ther. 2011;12:314–325. doi: 10.4161/cbt.12.4.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 76.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, Cui Y, Busman TA, McKeegan EM, Krivoshik AP, Enschede SH, Humerickhouse R. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong H, Chiu YL, Cui Y, Busman T, Elmore SW, Rosenberg SH, Krivoshik AP, Enschede SH, Humerickhouse RA. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, Dive C, Enschede SH, Nolan C, Chiu YL, Busman T, Xiong H, Krivoshik AP, Humerickhouse R, Shapiro GI, Rudin CM. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, Chu Q, Giaccone G, Khaira D, Ramalingam SS, Ranson MR, Dive C, McKeegan EM, Chyla BJ, Dowell BL, Chakravartty A, Nolan CE, Rudersdorf N, Busman TA, Mabry MH, Krivoshik AP, Humerickhouse RA, Shapiro GI, Gandhi L. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davids MS, Roberts AW, Anderson M, Pagel JM, Kahl BS, Gerecitano JF, Darden DE, Nolan CE, Gressick LA, Yang J, Chyla BJ, Busman TA, Graham AM, Cerri E, Enschede SH, Humerickhouse RA, Seymour JF. The BCL-2-specific BH3-mimetic ABT-199 (GDC-0199) is active and well-tolerated in patients with relapsed non-hodgkin lymphoma: interim results of a phase I study ASH Annu Meeting Abstract 2012; 304.
- 81.Holt SV, Brookes KE, Dive C, Makin GW. Down-regulation of XIAP by AEG35156 in paediatric tumour cells induces apoptosis and sensitises cells to cytotoxic agents. Oncol Rep. 2011;25:1177–1181. doi: 10.3892/or.2011.1167. [DOI] [PubMed] [Google Scholar]
- 82.LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, Wang H, Wang W, Zhang R, Agrawal S, Gillard JW, Durkin JP. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006;12:5231–5241. doi: 10.1158/1078-0432.CCR-06-0608. [DOI] [PubMed] [Google Scholar]
- 83.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 84.Hansen JB, Fisker N, Westergaard M, Kjaerulff LS, Hansen HF, Thrue CA, Rosenbohm C, Wissenbach M, Orum H, Koch T. SPC3042: a proapoptotic survivin inhibitor. Mol Cancer Ther. 2008;7:2736–2745. doi: 10.1158/1535-7163.MCT-08-0161. [DOI] [PubMed] [Google Scholar]
- 85.Carrasco RA, Stamm NB, Marcusson E, Sandusky G, Iversen P, Patel BK. Antisense inhibition of survivin expression as a cancer therapeutic. Mol Cancer Ther. 2011;10:221–232. doi: 10.1158/1535-7163.MCT-10-0756. [DOI] [PubMed] [Google Scholar]
- 86.Morrison DJ, Hogan LE, Condos G, Bhatla T, Germino N, Moskowitz NP, Lee L, Bhojwani D, Horton TM, Belitskaya-Levy I, Greenberger LM, Horak ID, Grupp SA, Teachey DT, Raetz EA, Carroll WL. Endogenous knockdown of survivin improves chemotherapeutic response in ALL models. Leukemia. 2012;26:271–279. doi: 10.1038/leu.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakahara T, Takeuchi M, Kinoyama I, Minematsu T, Shirasuna K, Matsuhisa A, Kita A, Tominaga F, Yamanaka K, Kudoh M, Sasamata M. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 88.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Kinoyama I, Matsuhisa A, Kudou M, Sasamata M. Broad spectrum and potent antitumor activities of YM155, a novel small-molecule survivin suppressant, in a wide variety of human cancer cell lines and xenograft models. Cancer Sci. 2011;102:614–621. doi: 10.1111/j.1349-7006.2010.01834.x. [DOI] [PubMed] [Google Scholar]
- 89.Tanioka M, Nokihara H, Yamamoto N, Yamada Y, Yamada K, Goto Y, Fujimoto T, Sekiguchi R, Uenaka K, Callies S, Tamura T. Phase I study of LY2181308, an antisense oligonucleotide against survivin, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:505–511. doi: 10.1007/s00280-010-1506-7. [DOI] [PubMed] [Google Scholar]
- 90.Erba HP, Sayar H, Juckett M, Lahn M, Andre V, Callies S, Schmidt S, Kadam S, Brandt JT, Van Bockstaele D, Andreeff M. Safety and pharmacokinetics of the antisense oligonucleotide (ASO) LY2181308 as a single-agent or in combination with idarubicin and cytarabine in patients with refractory or relapsed acute myeloid leukemia (AML) Invest New Drugs. 2013 doi: 10.1007/s10637-013-9935-x. doi: 10.1007/s10637-013-9935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P, Keating A, Antonia S. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y, Terashima M, Ueda S, Fukuoka M, Ariyoshi Y, Saito T, Masuda N, Watanabe H, Taguchi T, Kakihara T, Aoyama Y, Hashimoto Y, Nakagawa K. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–3880. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- 93.Aoyama Y, Nishimura T, Sawamoto T, Satoh T, Katashima M, Nakagawa K. Pharmacokinetics of sepantronium bromide (YM155), a small-molecule suppressor of survivin, in Japanese patients with advanced solid tumors: dose proportionality and influence of renal impairment. Cancer Chemother Pharmacol. 2012;70:373–380. doi: 10.1007/s00280-012-1913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tolcher AW, Quinn DI, Ferrari A, Ahmann F, Giaccone G, Drake T, Keating A, de Bono JS. A phase II study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancer. Ann Oncol. 2012;23:968–973. doi: 10.1093/annonc/mdr353. [DOI] [PubMed] [Google Scholar]
- 95.Cheson BD, Bartlett NL, Vose JM, Lopez-Hernandez A, Seiz AL, Keating AT, Shamsili S, Papadopoulos KP. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer. 2012;118:3128–3134. doi: 10.1002/cncr.26510. [DOI] [PubMed] [Google Scholar]
- 96.Lewis KD, Samlowski W, Ward J, Catlett J, Cranmer L, Kirkwood J, Lawson D, Whitman E, Gonzalez R. A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest New Drugs. 2010;29:161–166. doi: 10.1007/s10637-009-9333-6. [DOI] [PubMed] [Google Scholar]
- 97.Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, van Klaveren RJ, Chaudhary S, Gunther A, Shamsili S. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–4486. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 98.Brunckhorst MK, Lerner D, Wang S, Yu Q. AT-406, an orally active antagonist of multiple inhibitor of apoptosis proteins, inhibits progression of human ovarian cancer. Cancer Biol Ther. 2012;13:804–811. doi: 10.4161/cbt.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Houghton PJ, Kang MH, Reynolds CP, Morton CL, Kolb EA, Gorlick R, Keir ST, Carol H, Lock R, Maris JM, Billups CA, Smith MA. Initial testing (stage 1) of LCL161, a SMAC mimetic, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2011;58:636–639. doi: 10.1002/pbc.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flygare JA, Beresini M, Budha N, Chan H, Chan IT, Cheeti S, Cohen F, Deshayes K, Doerner K, Eckhardt SG, Elliott LO, Feng B, Franklin MC, Reisner SF, Gazzard L, Halladay J, Hymowitz SG, La H, LoRusso P, Maurer B, Murray L, Plise E, Quan C, Stephan JP, Young SG, Tom J, Tsui V, Um J, Varfolomeev E, Vucic D, Wagner AJ, Wallweber HJ, Wang L, Ware J, Wen Z, Wong H, Wong JM, Wong M, Wong S, Yu R, Zobel K, Fairbrother WJ. Discovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152) J Med Chem. 2012;55:4101–4113. doi: 10.1021/jm300060k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Graham MA, Mitsuuchi Y, Burns J, Chunduru S, Benetatos C, McKinlay M, Weng D, Wick MJ, Tolcher AW, Papadopoulo K, Amaravadi R, Schilder RJ, Adjei A, LoRusso P. Phase 1 PK/PD analysis of the Smac-mimetic TL32711 demonstrates potent and sustained cIAP1 suppression in patient PBMCs and tumor biopsies. Mol Cancer Ther. 2011;10:Abstract 25A. [Google Scholar]
- 102.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sikic BI, Eckhardt SG, Gallant G, Burris HA, Camidge DR, Colevas AD, Jones SF, Messersmith WA, Wakelee HA, Kaminker PG, Morris S, Infante JR. Safety, pharmacokinetics (PK), and pharmacodynamics (PD) of HGS1029, an inhibitor of apoptosis protein (IAP) inhibitor, in patients (Pts) with advanced solid tumors: results of a phase I study. J Clin Oncol. 2011;29(Suppl):abstr 3008. [Google Scholar]
- 104.Amaravadi RK, Schilder RJ, Dy GK, Ma WW, Fetterly GJ, Weng DE, Graham AM, Burns JM, Chunduru SK, Condon SM, McKinlay MA, Adjei AA. Phase 1 study of the Smac mimetic TL32711 in adult subjects with advanced solid tumors and lymphoma to evaluate safety, pharmacokinetics, pharmacodynamics, and antitumor activity. Cancer Res. 2011;71:Abstract LB-406. [Google Scholar]
- 105.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 106.Ray S, Bucur O, Almasan A. Sensitization of prostate carcinoma cells to Apo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis. 2005;10:1411–1418. doi: 10.1007/s10495-005-2490-y. [DOI] [PubMed] [Google Scholar]
- 107.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 108.Guo F, Nimmanapalli R, Paranawithana S, Wittman S, Griffin D, Bali P, O'Bryan E, Fumero C, Wang HG, Bhalla K. Ectopic overexpression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative-(BMS 247550) and Apo-2L/TRAIL-induced apoptosis. Blood. 2002;99:3419–3426. doi: 10.1182/blood.v99.9.3419. [DOI] [PubMed] [Google Scholar]