Abstract
Aim
To describe the pharmacokinetics and safety of indinavir boosted with ritonavir (IDV/r) during the second and third trimesters of pregnancy and in the post-partum period.
Methods
IMPAACT P1026s is an on-going, prospective, non-blinded study of antiretroviral pharmacokinetics (PK) in HIV-infected pregnant women with a Thai cohort receiving IDV/r 400/100 mg twice daily during pregnancy through to 6–12 weeks post-partum as part of clinical care. Steady-state PK profiles were performed during the second (optional) and third trimesters and at 6–12 weeks post-partum. PK targets were the estimated 10th percentile IDV AUC (12.9 μg ml−1 h) in non-pregnant historical Thai adults and a trough concentration of 0.1 μg ml−1, the suggested minimum target.
Results
Twenty-six pregnant women were enrolled; thirteen entered during the second trimester. Median (range) age was 29.8 (18.9–40.8) years and weight 60.5 (50.0–85.0) kg at the third trimester PK visit. The 90% confidence limits for the geometric mean ratio of the indinavir AUC(0,12 h) and Cmax during the second trimester and post-partum (ante : post ratios) were 0.58 (0.49, 0.68) and 0.73 (0.59, 0.91), respectively; third trimester/post-partum AUC(0,12 h) and Cmax ratios were 0.60 (0.53, 0.68) and 0.63 (0.55, 0.72), respectively. IDV/r was well tolerated and 21/26 women had a HIV-1 viral load < 40 copies ml−1 at delivery. All 26 infants were confirmed HIV negative.
Conclusion
Indinavir exposure during the second and third trimesters was significantly reduced compared with post-partum and ∼30% of women failed to achieve a target trough concentration. Increasing the dose of IDV/r during pregnancy to 600/100 mg twice daily may be preferable to ensure adequate drug concentrations.
Keywords: antiretrovirals, HIV, pregnancy, prevention of mother-to-child transmission
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Highly active antiretroviral therapy (HAART) is recommended during pregnancy for HIV/AIDS treatment and/or the prevention of mother-to-child transmission of HIV.
Physiological changes during pregnancy can reduce antirerotivral drug exposure.
Indinavir trough concentrations have been reported to be reduced during the third trimester of pregnancy following an indinavir/ritonavir dose of 400/100 mg twice daily. However, indinavir and ritonavir exposures have not been fully characterized during the second and third trimesters of pregnancy and in the early post-partum period.
WHAT THIS STUDY ADDS
Median indinavir exposures during the second and third trimesters were significantly reduced compared with post-partum.
Approximately 30% of women failed to achieve a target trough concentration during the third trimester.
Indinavir and ritonavir were tolerated well during pregnancy and an increased dose of indinavir during pregnancy may be preferable to ensure adequate exposure throughout pregnancy.
Introduction
Drug disposition can be altered by physiological changes during pregnancy. Physiological changes that manifest during pregnancy include longer gastrointestinal emptying/transit times, reduced gastric acid secretion, increased body water, plasma volume, fat stores and hepatic/renal blood flow, and temporal changes of hepatic metabolizing enzyme activities [1]. It is important to assess drugs susceptible to these changes during pregnancy to ensure optimal drug exposures are achieved.
Highly active antiretroviral therapy (HAART) is recommended during pregnancy for HIV/AIDS treatment and/or the prevention of mother to child transmission of HIV [2]. Several antiretroviral drugs metabolized via hepatic cytochrome P450 enzymes have reduced exposure during the third trimester of pregnancy, particularly ritonavir boosted protease inhibitors [3, 4]. Indinavir is a potent HIV protease inhibitor that has been successfully used as part of triple antiretroviral drug regimens for the treatment of HIV/AIDS [5]. Indinavir is primarily metabolized by the CYP3A4 isoenzyme, and similar to other protease inhibitors, co-administration with low dose ritonavir, a potent CYP3A4 inhibitor, enhances its pharmacokinetic profile allowing less frequent dosing and the removal of food restrictions [6]. An indinavir/ritonavir (IDV/r) dose of 400/100 mg twice daily has been shown to be safe and efficacious in non-pregnant adults [7, 8].
Pregnancy greatly reduces indinavir exposure in the absence of ritonavir [9]. The impact of pregnancy on indinavir boosted with ritonavir has not been fully characterized. Indinavir trough concentrations were reported to be lower during the third trimester of pregnancy in women receiving HAART-containing indinavir/ritonavir (400 mg/100 mg, twice daily) compared with HIV-infected men and non-pregnant women. However, HIV RNA levels remained well-controlled [10]. No data on the pharmacokinetic parameters of indinavir/ritonavir during the second and third trimesters of pregnancy and the post-partum period are available. Optimal antiviral exposure throughout pregnancy is critical to ensure durable viral load suppression to prevent mother-to-child transmission of HIV and the selection of resistance. Our aim was to investigate the pharmacokinetics of indinavir boosted with ritonavir during the second and third trimesters of pregnancy and in the early post-partum period.
Methods
Study population
International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network Protocol 1026s is an ongoing, multicentre, non-blinded, prospective study to evaluate the pharmacokinetics (PK) of antiretrovirals among pregnant HIV-infected women [ClinicalTrials.gov Identifier: NCT00042289]. This report includes women receiving indinavir boosted ritonavir 400/100 mg twice daily that was open to study sites in Thailand.
The eligibility criterion for this indinavir boosted ritonavir arm of P1026s was initiating the standard dose of indinavir/ritonavir (400 mg/100 mg, twice daily) as part of clinical care before the beginning of the 35th week of gestation. The choice of additional antiretrovirals and duration of treatment (i.e. continuation of anti-retroviral treatment) was determined by the subject's physician, who prescribed all medications and remained responsible for her clinical management throughout the study. Exclusion criteria were: concurrent use of medications known to interfere with the absorption, metabolism or clearance of indinavir/ritonavir, multiple gestation pregnancy and clinical or laboratory toxicity that, in the opinion of the site investigator, would likely require a change in the medication regimen during the study. The study was approved in Thailand by the Ethics Committee Institute for the Development of Human Research Protections (IHRP), Ministry of Public Health (Sor Kor Mor 236/2552), and signed informed consent was obtained from all subjects prior to participation. Subjects continued to take their prescribed medications until post-partum blood sampling was completed. Maternal and infant safety follow-up continued until 24 weeks post-partum.
Women were enrolled during either the second or third trimester. Enrolment during the second trimester was optional with PK sampling between 20 and 26 weeks gestation. Women enrolled during the second or third trimester had PK sampling performed between 30 and 36 weeks gestation. All women had PK sampling scheduled between 6 and 12 weeks post-partum. Indinavir area under the concentration vs. time curve [AUC(0,12 h)] was calculated at each PK sampling visit and compared with indinavir AUC(0,12 h) in non-pregnant Thai adults receiving IDV/r 400/100 mg twice daily [11]. The 10th percentile for indinavir AUC(0,12 h) in non-pregnant Thai adults, 12.9 μg ml−1 h, was chosen as the minimum target for drug exposure. Each subject's physician was notified of the subject's indinavir AUC(0,12h) within 2 weeks of sampling. If the AUC(0,12 h) was below the target, the treating physician offered the option of discussing the results and possible dose modifications with the study team.
Clinical and laboratory monitoring
Maternal data accessed were: maternal age, ethnicity, weight, concomitant medications, CD4 cell count and plasma viral load assay results. Plasma viral load assays were performed locally at laboratories certified by the NIAID Virology Quality Assurance (VQA) programme. Maternal clinical and laboratory toxicities were assessed through clinical evaluations (history and physical examination) and laboratory assays (ALT, AST, creatinine, BUN, albumin, bilirubin, haemoglobin) on each pharmacokinetic sampling day and at delivery. Infant data included birth weight, gestational age at birth, and HIV infection status. Infants received physical examinations at 24–48 h, 4–21 days and 24 weeks after delivery. The study team reviewed toxicity reports on monthly conference calls, although the subject's physician was responsible for toxicity management. The Division of AIDS (DAIDS)/NIAID Toxicity Table for Grading Severity of Adult Adverse Experiences was used to report adverse events for study subjects August 1992. All toxicities were followed through to resolution.
Sample collection
Subjects were stable on their antiretroviral regimen for at least 2 weeks prior to pharmacokinetic sampling. Seven plasma samples were drawn at the second and/or third trimester, and at the post-partum pharmacokinetic evaluation visits, starting immediately before an oral indinavir/ritonavir dose and at 1, 2, 4, 6, 8, and 12 h post-dose. Indinavir/ritonavir was given as an observed dose without regard to food but the last food intake was documented. Other information collected included the time of the two prior doses, the two most recent meals and maternal height and weight. A single maternal plasma sample and an umbilical cord sample after the cord was clamped were collected at delivery.
Antiretroviral drug assays
Indinavir and ritonavir plasma samples were assayed at the PHPT-IRD laboratory at the Faculty of Associated Medical Sciences, Chiang Mai University. This laboratory participates in the AIDS Clinical Trials Group (ACTG), USA, Pharmacology Quality Control (Precision Testing) programme, which performs standardized inter-laboratory testing twice a year [13]. Plasma drug concentrations were measured following a published reversed phase high performance liquid chromatography (HPLC) method with ultraviolet detection at 215 nm [14]. This HPLC method was validated internally using the AIDS Clinical Trials Group (ACTG) method validation guidelines over the concentration range of 0.043–17.2 μg ml−1 for indinavir (free base) and 0.050–20.0 μg ml−1 for ritonavir. Plasma samples with drug concentrations above the upper limit of quantification were diluted and re-assayed. The average accuracy for indinavir was 99–107% and precision (inter- and intra-assay) was <9% of the coefficient of variation (CV) and for ritonavir the average accuracy was 99–109% and precision (inter- and intra-assay) was <4% of the CV. Overall extraction recovery from plasma was 107% for indinavir and 104% for ritonavir.
Pharmacokinetic analyses
The maximum plasma concentration (Cmax), corresponding time (tmax), minimum plasma concentration (Cmin), and 12 h post-dose concentration (C12) were determined by direct inspection. AUC(0,12 h) during the dose interval (from time 0 to 12 h post-dose) for indinavir and ritonavir was calculated using the trapezoidal rule. Apparent clearance (CL/F) from plasma was calculated as dose divided by AUC(0,12 h).
Statistical analyses
Target enrolment was at least 25 women with evaluable third trimester indinavir pharmacokinetics. The statistical rationale for this sample size has been previously described [4]. The number of subjects with an AUC below 12.9 μg ml−1 h and a trough concentration below 0.1 μg ml−1, the suggested minimum target trough concentration [15, 16], was determined during pregnancy and post-partum. Indinavir and ritonavir pharmacokinetic parameters during pregnancy and post-partum were compared at the within-subject level using 90% confidence limits for the geometric mean ratio of AUC(0,12 h), Cmax and C12. When the true geometric mean of the ratio (the antilog of the true mean of the log ratios) of the pharmacokinetic parameters for pregnant and non-pregnant conditions has a value of 1, this indicates equal geometric mean pharmacokinetic parameters for the pregnant and non-pregnant conditions. If the 90% confidence intervals are entirely outside the limits (0.8 and 1.25), the pharmacokinetic parameters for the pregnant and non-pregnant conditions are considered different. If, on the other hand, the 90% confidence intervals are entirely within the limits (0.8, 1.25), the parameters are considered equivalent. If the 90% confidence interval overlaps with (0.8, 1.25), these data alone do not support any conclusions. Wilcoxon signed-rank test was used to assess the difference between second trimester, third trimester and post-partum pharmacokinetic parameters. Descriptive statistics were calculated for pharmacokinetic parameters of interest during each study period.
Results
Subject characteristics
Twenty-six HIV-infected pregnant women were enrolled in the study, with thirteen women entering the study during the second trimester. The clinical characteristics of the study population at the second and third trimesters, delivery and 6–12 weeks post-partum visits are presented in Table 1. There were 26 live born infants and the median (range) birth weight was 2830 (2200–3548) g.
Table 1.
Characteristics of Thai women during the second and third trimesters of pregnancy, at delivery and at 6–12 weeks post-partum, as well as infant characteristics at delivery
| Median (range) or # of subjects (%) | |
|---|---|
| Second trimester (n = 13) | |
| Age (years) | 32.5 (18.8–35.7) |
| Weight (kg) | 59.0 (48.0–84.0) |
| Gestational age (weeks) | 25.1 (21.0–27.0) |
| CD4 + cell count (cells μl−1) | 352 (110–581) |
| HIV-1 RNA (copies ml−1) | 144 (<40–68 920) |
| Duration of indinavir/r use (weeks) | 4.9 (2.4–8.0) |
| Third trimester (n = 25)* | |
| Age (years) | 29.8 (18.9–40.8) |
| Weight (kg) | 60.5 (50.0–85.0) |
| Gestational age (weeks) | 31.1 (29.3–37.0) |
| Duration of indinavir/r use (weeks) | 9.0 (2.1–18.0) |
| CD4 + cell count (cells μl−1) | 393 (129–758) |
| HIV-1 RNA (copies ml−1) | <40 (<40–903) |
| HIV1 RNA ≤ 40 cps ml−1 | 14/25 (56%) |
| Other ARVs: ZDV/ d4T/3TC/TDF | 21/4/26/1 |
| Delivery (n = 26) | |
| Weight (kg) | 62.5 (50.0–87.0) |
| Gestational age (weeks) | 38.4 (35.3–41.9) |
| HIV-1 RNA (copies ml−1) | <40 (<40–1309) |
| HIV-1 RNA ≤ 400 cps ml−1 | 25/26 (96%) |
| HIV-1 RNA ≤ 40 cps ml−1 | 21/26 (81%) |
| Post-partum (n = 26) | |
| Weight (kg) | 55.0 (44.0–81.0) |
| Timing of PK sampling (weeks) | 8.6 (2.6–10.3) |
| Infants (n = 26) | |
| Infant weight at birth (g) | 2 830 (2 200–3 548) |
Includes those women enrolled during the second trimester.
Indinavir/ritonavir pharmacokinetics during pregnancy and post-partum
Antepartum pharmacokinetic assessments during the second trimester were performed at a median (range) gestational age of 25.1 (21–27) weeks. Ten of 13 women (77%) had an AUC above the target during the second trimester. Two of the three women with an indinavir AUC below target maintained a C12 h above 0.1 μg ml−1 and following consultation with the study team did not have a dose adjustment. The woman with an AUC and C12 h below the predefined targets had a dose increase to 600/100 mg twice daily and did not have PK sampling performed during the third trimester pregnancy.
Twenty-five women receiving IDV/r 400/100 mg twice daily during the third trimester had pharmacokinetic sampling performed at a median gestational age of 31.1 (29 to 37) weeks. Seventeen women (68%) had an AUC above the target during the third trimester. The indinavir/ritonavir dose was increased to 600/100 mg twice daily in three of eight women with low exposure during the third trimester. Indinavir/ritonavir PKs were repeated in one women receiving 600/100 mg and her indinavir AUC increased above the 10th percentile target.
All 26 women completed post-partum PK sampling at a median duration of 8.6 (2.6–10.3) weeks after delivery. All women had an indinavir AUC and C12 h above their respective targets post-partum. Median (±interquartile range; IQR) indinavir concentration vs. time curves during the second and third trimesters and post-partum are shown in Figure 1.
Figure 1.
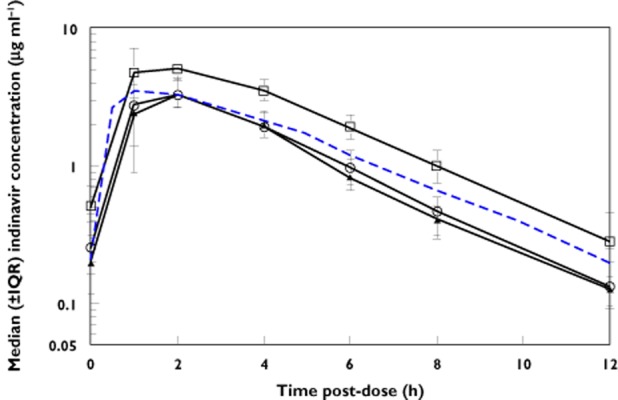
Median (± interquartile range) indinavir concentration vs. time curves for HIV-infected pregnant women using indinavir/ritonavir 400/100 mg twice daily during the second and third trimesters of pregnancy and post-partum. Dashed line represents the typical 50th percentile concentrations in non-pregnant historical Thai adults.  , second trimester;
, second trimester;  , third trimester;
, third trimester;  , post-partum;
, post-partum;  , non-pregnant
, non-pregnant
Indinavir and ritonavir pharmacokinetic parameters during the second and third trimesters and post-partum are presented in Table 2. The indinavir AUC(0,12 h) was significantly lower during the second and third trimesters when compared with post-partum (Table 2). Indinavir oral clearance (CL/F) was significantly higher during the second and third trimesters compared with post-partum. Indinavir C12 was also significantly reduced during the second and third trimesters compared with post-partum. Individual indinavir AUC and C12 h during the second trimester, third trimester and post-partum are presented in Figure 2. The 90% CIs for the geometric mean ratio of the indinavir AUC and Cmax during the second trimester and post-partum (ante : post ratios) were 0.58 (0.49, 0.68) and 0.73 (0.59, 0.91), respectively. Similarly, the third trimester/post-partum AUC and Cmax ratios were 0.60 (0.53, 0.68) and 0.63 (0.55, 0.72), respectively. Lower ritonavir exposure during pregnancy was also observed when compared with the post-partum period (Table 2). The change in indinavir pharmacokinetic parameters during pregnancy was less apparent when comparing with historical data in non-pregnant Thai adults (Table 2) although median AUC and C12 h remained lower.
Table 2.
Indinavir/ritonavir pharmacokinetic parameters during the second and third trimesters of pregnancy and post-partum
| Second trimester (n = 13) | Third trimester (n = 25) | Post-partum (n = 26) | Historical data Non-pregnant adults [8] | |
|---|---|---|---|---|
| Indinavir † | ||||
| AUC(0,12h) (μg ml−1 h) | 14.9 (10.4–38.7)* | 16.10 (7.50–39.9)* | 27.1 (18.6–44.7) | 18.3 (11.1–33.0) |
| Cmax (μg ml−1) | 3.89 (2.38–10.25) | 3.62 (1.33–7.42)* | 5.37 (3.76–9.41) | 3.8 (2.2–7.8) |
| tmax (median, h) | 2.00 (1.00–2.00) | 2.00 (1.00–4.00) | 2.00 (1.00–4.00) | 1.9 (0.96–3.8) |
| C12 h (μg ml−1) | 0.13 (0.07–0.23)* | 0.13 (0.07–0.60)* | 0.28 (0.14–0.71) | 0.17 (0.10–0.39) |
| Cmin (μg ml−1) | 0.12 (0.07–0.23)* | 0.12 (0.07–0.60)* | 0.28 (0.03–0.71) | 0.17 (0.10–0.39) |
| CL/F (l h−1) | 26.85 (10.34–38.46)* | 24.84 (10.03–53.33)* | 14.79 (8.95–21.51) | 21.9 (12.1–35.8) |
| Met indinavir AUC(0,12 h) target‡ | 10/13 (77%) | 17/25 (68%) | 26/26 (100%) | 9/11 (82%) |
| Met indinavir C12 h target‡ | 10/13 (77%) | 19/25 (76%) | 26/26 (100%) | 11/11 (100%) |
| Ritonavir # | ||||
| AUC(0,12 h) (μg ml−1 h)) | 5.80 (2.82–19.78)* | 5.95 (1.79–21.71)* | 14.6 (5.18–25.37) | 9.2 (3.9–24.8) |
| Cmax (μg ml−1) | 0.92 (0.34–3.68)* | 0.80 (0.26–3.25)* | 2.50 (0.68–3.89) | 1.5 (0.53–3.4) |
| tmax (median, h) | 2.00 (1.00–4.00) | 2.00 (1.00–6.00) | 2.00 (1.00–6.00) | 2.9 (1.4–5.0) |
| C12 h (μg ml−1) | 0.14 (0.09–0.30)* | 0.16 (0.07–0.56)* | 0.32 (0.15–1.14) | 0.24 (0.11–0.94) |
| CL/F (l h−1) | 17.25 (5.06–35.52)* | 16.80 (4.61–55.73)* | 6.87 (3.94–19.31) | 10.9 (4.0–25.6) |
†Values: Median (range), AUC: Area under the concentration−time curve; CL/F: apparent oral clearance (CL/F = Dose[12 h]/AUC[12 h]); ‡AUC(0,12 h) target is 12.9 μg ml−1: estimated 10th percentile for non-pregnant adults; C12 h Target is 0.10 μg ml−1 h: recommended minimum threshold; *P < 0.05, second trimester compared with post-partum and third trimester compared with post-partum, Wilcoxon signed rank test.
Figure 2.
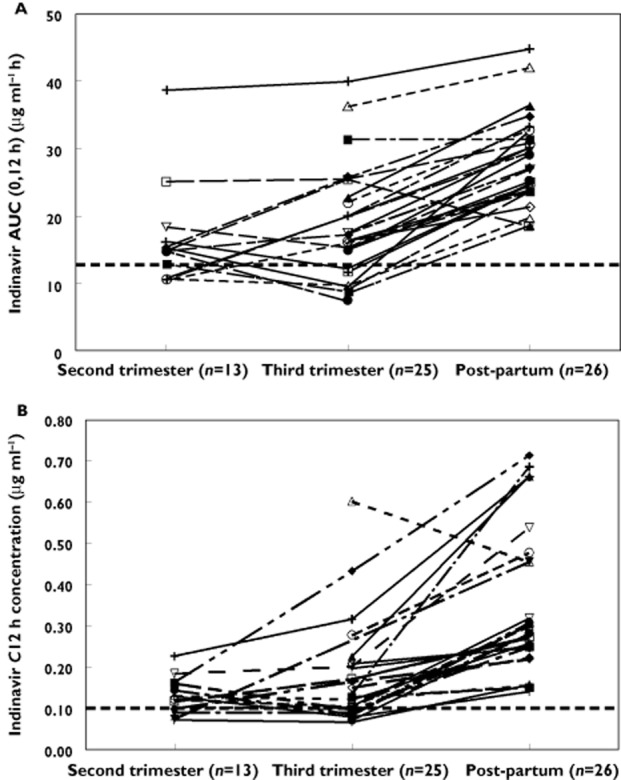
Individual indinavir (A) AUC(0,12 h) and (B) C12 h for HIV-infected pregnant women using 400/100 mg twice daily during the second and third trimesters of pregnancy and post-partum. Dashed line represents (A) the 10th percentile AUC (12.9 μg ml−1 h) in non-pregnant historical patients and (B) suggested minimum target trough concentration (0.1 μg ml−1)
Twenty-six pairs of maternal delivery and cord blood samples were collected. Indinavir concentrations were detectable in 25 maternal delivery samples. The median time interval between the last dose of indinavir/ritonavir and delivery was 3.7 (0.2 to 13.7) h. Seven cord blood samples had indinavir concentrations below the lower limit of detection of the drug assay (0.043 μg ml−1). Maternal plasma indinavir concentrations were 0.96 (<0.043–5.99) μg ml−1 at delivery and 0.12 (<0.043–0.75) μg ml−1 in the cord blood. The median ratio of cord blood : maternal delivery indinavir concentration was 0.12 (0.05–0.23, n = 19). Maternal delivery and cord blood indinavir concentrations and their ratios are plotted as a function of the time interval between maternal dosing and delivery in Figure 3. At delivery, 25 of 26 (96%) women had a HIV-1 RNA viral load less than 400 copies ml−1 and 21 of 26 women (81%) had a viral load below 40 copies ml−1 (five subjects had 51, 85, 86, 201, and 1309 copies ml−1 at delivery).
Figure 3.
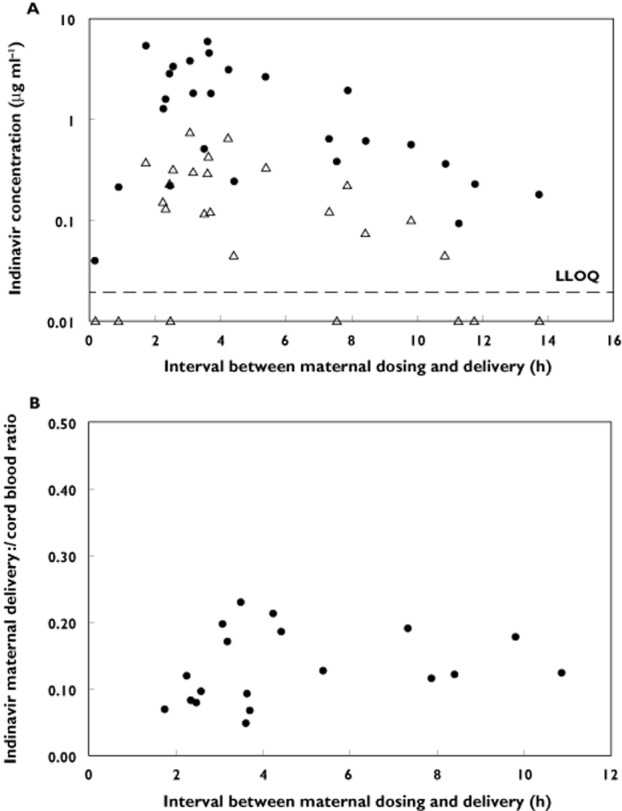
(A) Maternal delivery and cord blood indinavir concentrations plotted against the interval between maternal dosing and delivery. • maternal plasma indinavir concentration at delivery and ▵ cord blood indinavir concentrations; LLOQ, lower limit of assay quantification. (B) Maternal/cord blood indinavir concentrations ratio plotted against the interval between maternal dosing and delivery
Infant HIV status and safety
At 6 months of age, all 26 infants were confirmed HIV negative. Indinavir and ritonavir were tolerated well. Six women experienced adverse events grade ≥ 3. These included anaemia and neutropenia, dizziness, diarrhoea (shigella), cervical high-grade squamous intra-epithelial lesion (on pap smear), fever (5 days), and all events were deemed not attributed to indinavir/ritonavir treatment. One woman had preterm labour and preterm delivery (32 weeks) and this was deemed possibly related to indinavir/ritonavir treatment.
Discussion
Ensuring that adequate antiretroviral exposures are maintained throughout pregnancy is vital to provide maximal and durable viral load suppression to prevent mother to child transmission of HIV and the selection of viral drug resistance. We found that blood concentrations of indinavir boosted with ritonavir at a dose of 400/100 mg twice daily during the second and third trimester of pregnancy were significantly reduced compared with the early post-partum period. Median indinavir AUC during the second and third trimesters was reduced by ∼40% compared with post-partum and ∼30% of pregnant women failed to achieve the trough concentration target of 0.1 μg ml−1.
Ghosn et al. assessed steady-state indinavir trough concentration in 32 HIV-infected pregnant women receiving indinavir/ritonavir 400/100 mg twice daily during the third trimester [10]. The median trough concentration was 0.16 (range < 0.005−4.9) μg ml−1 and five women had an indinavir concentration below 0.005 μg ml−1 (assay limit of detection). These results are comparable with the trough concentrations of 0.13 μg ml−1 observed in the present study during the second and third trimesters (Table 2). Post-partum trough concentrations were also determined by Ghosn et al. in a subset of women and a two-fold increase in the median indinavir concentration after delivery was reported. A similar observation was found in the present study with the indinavir median trough concentration increasing from 0.13 μg ml−1 during the third trimester to 0.28 μg ml−1 post-partum.
Interestingly, post-partum indinavir exposures were greater than those observed in the historical non-pregnant control population of non-pregnant Thai adults receiving the 400/100 mg dose [11, 17]. Median indinavir AUC and trough concentrations were higher in these non-pregnant adults than were observed during pregnancy in the present study but considerably lower than during the post-partum period. In non-pregnant adults the trough concentrations of indinavir reported in the intensive pharmacokinetic studies were near the boundaries of the therapeutic window and the interpatient variability observed suggested that a small proportion of patients may not achieve adequate levels within the wider population. In a population pharmacokinetic analysis body weight was demonstrated to influence indinavir/ritonavir oral clearance but the majority of non-pregnant adults (>98%) weighing up to 80 kg were still expected to achieve target trough concentrations [18]. We expected that pregnancy-induced changes would have resolved by 6–12 weeks after delivery but it is unclear if the lower indinavir clearance during the post-partum period is related to continued physiological and/or metabolic issues associated with pregnancy. This apparent increase in post-partum drug exposure has been previously observed for lopinavir/ritonavir [19].
Indinavir is primarily metabolized by CYP3A4 and increases in CYP3A4 activity occurs throughout pregnancy [20]. This increase in enzyme activity may be responsible for the lower indinavir exposure during pregnancy. Host genetic polymorphisms in drug metabolizing enzymes and drug transporters have been associated with antiretroviral drug concentrations and toxicity but limited data on indinavir boosted with ritonavir are available. One study reported that the CYP3A4*1B*1B genotype reduced indinavir absorption while ABCB1 (p-glycoprotein) and CYP3A5*3 and *6 polymorphisms were not found to influence significantly indinavir pharmacokinetics [21]. No data during pregnancy are available. We also found reduced ritonavir exposure during pregnancy and it is possible that the lower indinavir exposure is due to an indirect effect of lower ritonavir concentrations influencing indinavir clearance. Similar to other protease inhibitors indinavir is poorly distributed to the foetal compartment with a low cord : maternal blood ratio of 0.12 (0.05–0.23). However, therapeutic indinavir concentrations were detected in the cord blood in some infants.
Indinavir is now regarded as an older protease inhibitor with ‘second generation’ protease inhibitors with once daily dosing and improved safety profiles being preferred in many settings. Until recently, indinavir/ritonavir was one of the cheapest protease inhibitors available and was widely used within second-line HAART regimens in many resource limited settings. Today, following reductions in drug prices there is a shift towards using lopinavir/ritonavir or atazanavir/ritonavir PI-based regimens. However, in our clinical experience, many patients initiated indinavir/ritonavir and who have no tolerability problems prefer to remain on the same regimen.
In conclusion, indinavir exposure was reduced during pregnancy and led to a number of women with trough concentrations below the target efficacy threshold. An increased dose of indinavir during pregnancy may be preferable to ensure adequate exposure throughout pregnancy.
Acknowledgments
The authors wish to thank the women who participated in the protocol and the staff of the participating IMPAACT centres. Team/Site Investigators: Study Team: Elizabeth Hawkins, D. Heather Watts, Sandra K. Burchett, Francesca Aweeka, Steve Rossi, Michael Basar, Kathleen Kaiser, Emily Barr, Kenneth D. Braun, Jr, Jennifer Bryant, Kathleen A. Medvik, and Amy Jennings. Chiang Rai Prachanukroh Hospital, Chiang Rai, Thailand (#8352): Patcharee Kantipong, Jullapong Achalapong, Kannikar Saisawat, Chulapong Chanta, Kanchana Preedisripipat, Supaporn Utsaha, Chaniporn Yodsuwan, Pollawat Thongsuk and Yupawan Thaweesombat. Chonburi Hospital, Chonburi, Thailand (#8356): Chureeratana Bowonwatanuwong, Nantasak Chotivanich, Suchat Hongsiriwon, Ladda Argadamnuy, Donyapattra Ekkomonrat, Prakit Yothipitak, Duangporn Wiwattanasorn, Somrat Matchua, Suluck Soontaros and Kessarin Chaisiri. Bhumibol Adulyadej Hospital, Bangkok, Thailand (#8355): Sinart Prommas, Prapaisri Layangool, Jutarat Mekmallika, Sommai Tratong, Ladda Ruluk, Titima Taweewattanapan and Marina Thitathan. Prapokklao Hospital (#8354): Prapap Yuthavisuthi, Chaiwat Ngampiyaskul, Ubon Chanasit, Wanna Chamjamrat, Pathanee Teirsonsern, Nuttupassasorn Tungtongcha and Pisut Greetanukroh. Phayao Hospital, Phayao, Thailand, (#8353): Guttiga Halue, Wirawan Rasri, Pornnapa Suriyachai, Pornchai Techakunakorn, Kunlaya Jansook, Chutima Ruklao, Khanungnit Thungkham, Borwornluck Changlor and Wanpen Mooninta. PHPT-IRD174, Chiang Mai (#8351): Marc Lallemant, Gonzague Jourdain, Nicole Ngo-Giang-Huong, Pra-ornsuda Sukrakanchana, Kanchana Than-in-at, Nusara Krapunpongsakul, Renoo Wongsrisai, Patcharaporn Krueduangkam, Janjira Thonglo, Ruethai Wongchai, Tiwacha Thimakam, Purivis Chart, Yardpiroon Taworn, Pimpinun Punyati, Worathip Sripaoraya and Suriyan Tanasri. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Competing Interests
None of the authors have competing interests within the submitted manuscript. ‘All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.’
References
- 1.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. September 14, 2011; pp 1–207. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf (last accessed 5 June 2012)
- 3.Mirochnick M, Best BM, Stek AM, Capparelli EV, Hu C, Burchett SK, Rossi SS, Hawkins E, Basar M, Smith E, Read JS. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56:412–419. doi: 10.1097/QAI.0b013e31820fd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, Elgie C, Holland DT, Smith E, Tuomala R, Cotter A, Read JS. Reduced lopinavir exposure during pregnancy. Aids. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 5.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Jr, Feinberg JE, Balfour HH, Jr, Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 6.Hsu A, Granneman GR, Cao G, Carothers L, Japour A, El-Shourbagy T, Dennis S, Berg J, Erdman K, Leonard JM, Sun E. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob Agents Chemother. 1998;42:2784–2791. doi: 10.1128/aac.42.11.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosn J, Lamotte C, Ait-Mohand H, Wirden M, Agher R, Schneider L, Bricaire F, Duvivier C, Calvez V, Peytavin G, Katlama C. Efficacy of a twice-daily antiretroviral regimen containing 100 mg ritonavir/400 mg indinavir in HIV-infected patients. Aids. 2003;17:209–214. doi: 10.1097/00002030-200301240-00011. [DOI] [PubMed] [Google Scholar]
- 8.Cressey TR, Leenasirimakul P, Jourdain G, Tod M, Sukrakanchana PO, Kunkeaw S, Puttimit C, Lallemant M. Low doses of indinavir boosted with ritonavir in HIV-infected Thai patients: pharmacokinetics, efficacy and tolerability. J Antimicrob Chemother. 2005;55:1041–1044. doi: 10.1093/jac/dki143. [DOI] [PubMed] [Google Scholar]
- 9.Unadkat JD, Wara DW, Hughes MD, Mathias AA, Holland DT, Paul ME, Connor J, Huang S, Nguyen BY, Watts DH, Mofenson LM, Smith E, Deutsch P, Kaiser KA, Tuomala RE. Pharmacokinetics and safety of indinavir in HIV-infected pregnant women. Antimicrob Agents Chemother. 2007;51:783–786. doi: 10.1128/AAC.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosn J, De Montgolfier I, Cornelie C, Dominguez S, Perot C, Peytavin G, Marcelin AG, Pauchard M, Ouagari Z, Bonmarchand M, Agher R, Calvez V, Bricaire F, Dommergues M, Katlama C, Tubiana R. Antiretroviral therapy with a twice daily regimen containing 400 milligrams of indinavir and 100 milligrams of ritonavir in human immunodeficiency virus type 1-infected women during pregnancy. Antimicrob Agents Chemother. 2008;52:1542–1544. doi: 10.1128/AAC.01301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cressey TR, Leenasirimakul P, Sukrakanchana P, Kunkeaw S, Puttimit C, Tod M, Jourdain G, Lallemant M. A pharmacokinetic study comparing 600/100 IDV/RTV vs. 400/100 IDV/RTV as part of highly active antiretroviral therapy in HIV-infected Thai patients. In: XV International AIDS Conference, 11–16 July, Bangkok Thailand, 2004.
- 12.The Division of AIDS (DAIDS) Standardized Toxicity Table for Grading Severity of Adult Adverse Experiences. August 1992. Available at http://rcc.tech-res-intl.com (last accessed 3 August 2011)
- 13.Holland DT, DiFrancesco R, Stone J, Hamzeh F, Connor JD, Morse GD. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob Agents Chemother. 2004;48:824–831. doi: 10.1128/AAC.48.3.824-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Droste JA, Verweij-Van Wissen CP, Burger DM. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther Drug Monit. 2003;25:393–399. doi: 10.1097/00007691-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council. October 14, 2011. AIDSinfo Web site. Available at http://aidsinfo.nih.gov (last accessed 29 November 2011) [PubMed]
- 16.la Porte C, Back D, Blaschke T, Boucher CAB, Fletcher C, Flexner C, Gerber JG, Kashuba AD, Schapiro JM, Burger DM. Updated Guideline to Perform Therapeutic Drug Monitoring for Antiretroviral Agents Reviews. Antivir Ther. 2006;3:4–14. [Google Scholar]
- 17.Boyd M, Mootsikapun P, Burger D, Chuenyam T, Ubolyam S, Mahanontharit A, Sangkote J, Bunyaprawit P, Horsakulchai M, Lange J, Cooper D, Phanuphak P, Ruxrungtham K. Pharmacokinetics of reduced-dose indinavir/ritonavir 400/100 mg twice daily in HIV-1-infected Thai patients. Antivir Ther. 2005;10:301–307. [PubMed] [Google Scholar]
- 18.Cressey TR, Urien S, Hirt D, Halue G, Techapornroong M, Bowonwatanuwong C, Leenasirimakul P, Treluyer JM, Jourdain G, Lallemant M. Influence of body weight on achieving indinavir concentrations within its therapeutic window in HIV-infected Thai patients receiving indinavir boosted with ritonavir. Ther Drug Monit. 2011;33:25–31. doi: 10.1097/FTD.0b013e3182057f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best BM, Stek AM, Mirochnick M, Hu C, Li H, Burchett SK, Rossi SS, Smith E, Read JS, Capparelli EV. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54:381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand J, Treluyer JM, Panhard X, Tran A, Auleley S, Rey E, Salmon-Ceron D, Duval X, Mentre F. Influence of pharmacogenetics on indinavir disposition and short-term response in HIV patients initiating HAART. Eur J Clin Pharmacol. 2009;65:667–678. doi: 10.1007/s00228-009-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


