Abstract
Study Design
An experimental study to investigate the characterization of 3 chordoma cell lines.
Objective
To characterize chordoma cell lines and generate hypothesis for further chordoma studies.
Summary of Background Data
Three cultured human chordoma cell lines have been successfully generated; however, their characterization is incomplete. Complete characterization of chordoma cell lines is necessary for these reagents to be a useful preclinical model.
Methods
Three chordoma cell lines, CH 8, U-CH1, and GP 60, were cultured in different commercially available tissue culture media. They were also cultured in different environments, which included collagen substrate, various concentrations of glucose, and various levels of hypoxic conditions. The rate of cell proliferation was assessed by either MTT or numeration assay. A 3-dimensional (3D) cell culture model of these chordoma cell lines was also studied, and the expression of vimentin and cytokeratin was measured by immunofluorescence and Western blot. Additionally, the sensitivity of the 3 chordoma cell lines to 6 chemotherapeutic drugs was analyzed.
Results
CH 8, GP 60, and U-CH1 cells proliferate more actively in Iscove Modified Dulbecco Medium or Dulbecco modified Eagle Medium and less actively in RPMI medium. All 3 chordoma cell lines universally grow better in collagen substrate and survive in hypoxic conditions, whereas glucose concentration has no significant influence on their growth properties. Chordoma cell lines grew well in 3D culture systems and formed acini-like spheroids and retained the expression of vimentin and cytokeratin. MTT analysis indicates that all 3 chordoma cell lines are sensitive to doxorubicin, yondelis, zalypsis, and cisplatin.
Conclusion
We characterized 3 chordoma cell lines for differential growth properties in a variety of media and response to chemotherapeutic agents.
Keywords: chordoma, characteristics, 3D cell culture, chemotherapy
Chordoma is an uncommon neoplasm with the majority arising in the axial skeleton, most commonly the sacrum, followed by the skull base and the mobile spine.1–3 Morphologically, immunohistochemically, and ultrastructurally, chordomas are identical to the normal notochord, and it has been hypothesized that they arise from benign notochordal rests or so-called benign notochordal cell tumors.4
Chordomas are relatively resistant to radiation and refractory to chemotherapy.4–7 Therefore, surgery is the primary modality to achieve the best long-term control.3,8–11 However, as chordomas are typically large and locally invasive at the time of diagnosis, obtaining tumor-free margins around the spine are challenging and frequently impossible to achieve.9–12 A large number of patients with chordoma (~50%–70%) are not cured and ultimately die.8 The overall median survival for chordoma has been estimated to be ~6 years, with a survival rate of 70% at 5 years, falling to 40% at 10 years.8 To date, there is limited understanding of the biology of chordomas.
Cultured chordoma cell lines are invaluable tools for the study of these tumors and the identification of drugs that can affect their growth. To date, only 3 cultured human chordoma cell lines have been successfully created; however, their characterization is incomplete.13–15 Complete characterization of chordoma cell lines is necessary for them to be a useful preclinical model. In this study, we sought to further characterize chordoma cell lines by (a) measuring the growth rates of cell lines in different media, levels of oxygen, and collagen substrates; (b) studying cell lines in 3-dimensional (3D) culture; and (c) assessing each of the cell lines for sensitivity to a variety of chemotherapeutic agents.
Materials and Methods
Cell Culture
The human chordoma cell line GB 60 was provided by the Catholic University School of Medicine, Rome, Italy.13 It was cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (all obtained from Invitrogen, Carlsbad, CA). The human chordoma cell line U-CH1 was obtained from the University Hospitals of Ulm, Ulm, Germany.14 It was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (all obtained from Invitrogen, Carlsbad, CA) in flask or plates coated with rat tail collagen type I (BD Biosciences, Bedford, MA), according to the recommendation of the provider.
Establishment of Novel Chordoma Cell Line CH 8
We established the third human chordoma cell line CH 8 from a patient with chordoma at lumbar spine. The tumor was a locally recurrent chordoma 7 months after the initial surgery. The diagnosis of chordoma was confirmed pathologically. Immediately after the surgical removal, chordoma tissue samples were mechanically dissociated. Cells were cultured in DMEM supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin G and 100 mg/mL streptomycin) (all obtained from Invitrogen, Carlsbad, CA). After confluence, these cells were detached using trypsin/EDTA (0.25%/0.02%, Boehringer, Ingelheim, Germany) and subcultured. At present, CH 8 has been growing for more than 8 months in culture and has a doubling time of about 1 week. The CH 8, GB 60, and U-CH1 cells were also stained with hematoxylin-eosin and observed under Nikon Eclipse 50i microscope.
Chordoma Cells Cultured in Different Media
To evaluate whether different media could influence the proliferative activity of chordoma cell lines, cells were seeded at 4 × 103 cells per well in 96-well plates and cultured in different commercial available tissue culture media, including RPMI 1640, DMEM, F 12, McCoy 5A Medium, or Iscove Modified Dulbecco Medium (IMDM). After 7 days, cell proliferation was assessed by MTT assay as described previously.16 Ten microliters of MTT (5 mg/mL PBS) was added to each well and incubated for 4 hours. After dissolving the resulting formazan product with acid isopropanol, the absorbance was read on a BT 2000 Microkinetics Reader (Bio-Tek Instrument, Inc., Winooski, VT) at a wavelength of 490 nm. Five samples were measured by MTT assay in each chordoma cell cultured in different media to obtain the average values.
Chordoma Cells Cultured in Hypoxic Conditions and Under Different Glucose Concentrations
To evaluate the growth properties of chordoma cell lines under hypoxic conditions and to see whether the concentration of glucose could influence the proliferation of chordoma cells, all 3 cell lines were cultured in different glucose concentrations and incubated in different oxygen levels. Chordoma cells were seeded at 4 × 103 cells per well in 96-well plates and incubated at 2 different oxygen levels. Cells that were cultured in hypoxic condition were incubated in Hot Box System (Billups-Rothenberg, Inc., Del Mar, CA). The chamber was filled with gas, which contained 5% O2 (Airgas Inc.) Cells that were cultured in normoxic condition were incubated in conventional incubator which contained 20% O2. After culture for 7 days, MTT assay was performed in duplicate. For stimulation with glucose, 4 × 103 chordoma cells per well were seeded in 96-well plates. The culture medium was complete DMEM/F-12 containing increasing concentrations of glucose (2, 3, 4, 5, and 6 g/L). Chordoma cells were incubated in hypoxic or normoxic conditions for 7 days, then MTT assay was performed. The growth of chordoma cells in hypoxic conditions and under different glucose concentrations were also confirmed by Hoechst assay. The chordoma cells were incubated with PBS containing 1 μg/mL Hoechst 33342 (Invitrogen, Carlsbad, CA) for 1 minute and then visualized on a Nikon Eclipse Ti-U fluorescence microscope (Nikon Corp., Tokyo, Japan) equipped with a SPOT RT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
Chordoma Cells Cultured in Collagen Substrate
To evaluate whether the collagen as substrate could influence the proliferative activity of the chordoma cells, the chordoma cells were seeded at 5 × 103 cells per well in 24-well plates coated with rat tail collagen type I (BD Biosciences, Bedford, MA). After culture for 7 days, cell proliferative activity was assessed by cell numeration as described previously and compared with the proliferative activity of chordoma cells cultured in collagen free plates.16 The cell numbers were also confirmed by Hoechst assay.
3D Culture
At present, there is no report describing the 3D cultures of chordoma cell lines. We performed 3D cultures on a reconstituted basement membrane as described previously.17 The Growth Factor Reduced Matrigel was added to each well of the 8-well glass chamber slides and spread evenly in the well to form the basement membrane measuring ~1 to 2 mm in thickness. A single cell suspension was than seeded on the solidified layer. Cells were grown in an assay medium (DMEM/F12 containing 2% horse serum, 0.5 μg/mL hydrocortisone, 100 ng/mL cholera toxin, 10 μg/mL insulin, 100 U/mL penicillin G, and 100 mg/mL streptomycin) in which 5 ng/mL epidermal growth factor (EGF) and 2% Matrigel were added. The cells were grown in a 5% CO2 humidified incubator at 37°C. The cells were refed with assay medium containing 2% Matrigel and 5 ng/mL EGF every 4 days. After culture for 16 days, the chordoma cells were fixed with 2% paraformaldehyde to perform immunofluorescence to measure the expression of biomarkers.
Western Blot Analysis
Vimentin and cytokeratin are 2 biomarkers of chordomas. Western blot analysis was performed to confirm their expression in the chordoma cells. Protein lysates from cells were generated through lysis with 1 × RIPA lysis buffer (Upstate Biotechnology, Charlottesville, VA). The concentration of the protein was determined by Protein Assay Reagents (Bio-Rad, Hercules, CA) and spectrophotometer (Beckman DU-640, Beckman Instruments, Inc., Columbia, MD). Forty micrograms of total protein was processed on v-Page 4% to 12% Bis-Tris Gel (Invitrogen, Carlsbad, CA) and transferred to a pure nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Primary antibodies were incubated at 1:1000 dilution in Tris-buffered saline, pH 7.4, with 0.1% Tween 20 and overnight at 4°C. Signal was generated through incubation with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA) incubated in Tris-buffered saline, pH 7.4, with 5% nonfat milk and 0.1% Tween 20 at 1:2000 dilution for 1 hour at room temperature. Positive immunoreactions were detected by using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). Densitometric analysis of Western blot results was performed with Adobe Photoshop 7.0 (San Jose, CA) as described in the User Guide (http://www.lukemiller.org/journal/2007/08/quantifying-western-blots-without.html).
Immunofluorescence
To confirm whether chordoma cells retain their phenotype in 3D culture, their expression of cytokeratin and vimentin was evaluated by immunofluorescence. After culture in 3D for 16 days, chordoma cells were fixed in 2% paraformaldehyde for 20 minutes, followed by permeabilization with 0.1% Triton X-100 (PBST). Permeabilized cells were blocked with 1% albumin bovine serum. Cells were then incubated with primary antibodies at 1:1000 dilution in PBST at room temperature for 1 hour. Antibodies directed against cytokeratin and vimentin were obtained from Invitrogen (Invitrogen, Carlsbad, CA). The cells were washed with PBST and incubated with alexa conjugated secondary antibody (Invitrogen, Carlsbad, CA) at room temperature for 1 hour. To counterstain nuclei, the chordoma cells were incubated with PBST containing 1 μg/mL Hoechst 33342 (Invitrogen, Carlsbad, CA) for 1 minute. Chordoma cells were then visualized on a Nikon Eclipse Ti-U fluorescence microscope (Nikon Corp., Tokyo, Japan) equipped with a SPOT RT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
Chemotherapy Drug Sensitivity Assay
To evaluate the sensitivity of chordoma cells to various potential chemotherapeutic agents, in vitro cytotoxicity effects were performed by MTT assay as described previously.16 Four × 103 cells per well were seeded in 96-well plates and treated with doxorubicin, methotrexate, cisplatin, yondelis, zalypsis, and paclitaxel in various concentration. After culture for 7 days, MTT was performed to evaluate the sensitivity of all 3 chordoma cell lines to these drugs.
Data Analysis
Values from MTT assay are representative of duplicate determinations in 2 or more experiments. Treatment effects were evaluated using a 2-sided Student t test (GraphPad PRISM 4 software, GraphPad Software, San Diego, CA). Errors are standard deviation of averaged results, and P < 0.05 values were accepted as a significant difference between means.
Results
Effects of Different Cell Culture Media on Chordoma Cell Proliferation
Three chordoma cell lines, CH 8, GB 60, and U-CH1, all demonstrated typical morphology for chordomas containing cells with highly vacuolated appearance (so-called physaliphorous cells) (Figure 1). We incubated all 3 chordoma cell lines in different commercially available tissue culture media, and proliferation was measured using the MTT assay. We observed that the CH 8 and GB 60 cells proliferated more actively in IMDM and DMEM medium but less in RPMI 1640 (P < 0.05). U-CH1 cells proliferated more rapidly in DMEM and slower in RPMI 1640 medium (P < 0.05) (Figures 2A–C).
Figure 1.
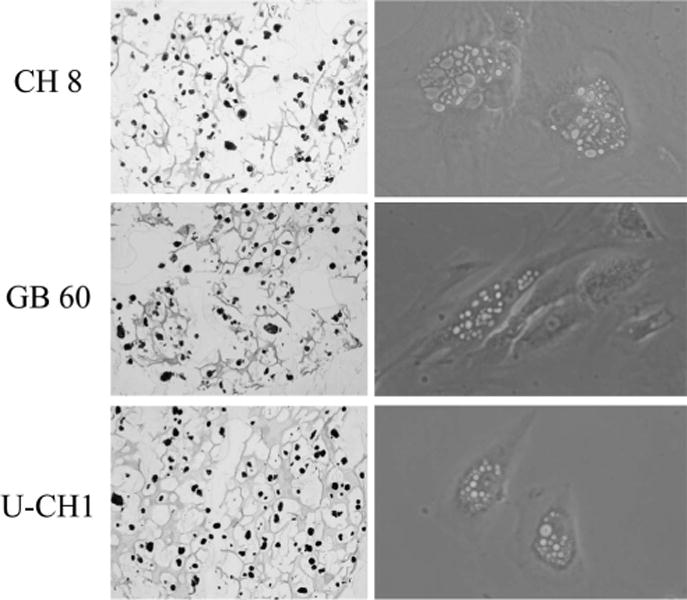
The morphology and appearance of the chordoma cells. The morphology and appearance of the chordoma cells were observed under the microscope, including live cells and cells after hematoxylin and eosin stain. All 3 chordoma cell lines demonstrated vacuolated cytoplasm (so called physaliphorous cells).
Figure 2.
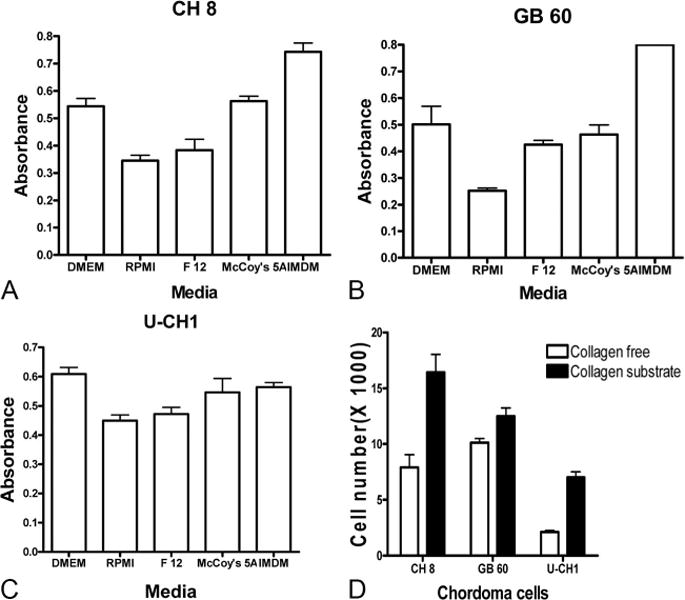
The chordoma cells were cultured in different commercial available tissue culture media and collagen substrate, and their proliferate activity was evaluated by MTT and numerication assay. A, MTT assay indicated that the CH 8 cells proliferated less actively in RPMI medium and more actively in DMEM and IMDM media. B, MTT assay indicated that the GB 60 cells proliferated less actively in RPMI medium and more actively in DMEM and IMDM media. C, MTT assay indicated that the U-CH1 cells proliferated less actively in RPMI medium and more actively in DMEM medium. D, Cell numeration assay indicated that all 3 chordoma cell lines universally grew better in collagen substrate than collagen free plate.
Effect of Hypoxia and Level of Glucose on Chordoma Cell Proliferation
We investigated the condition of hypoxia to the growth of chordoma cells. After culture in hypoxic conditions, all 3 chordoma cell lines survived and grew unaffected by either hypoxic or normoxic conditions (P > 0.05). The effects of hypoxia on chordoma cell proliferation were also confirmed by using Hoechst assay (Suplemental Digital Content 1, Figure 1, available at: http://links.lww.com/BRS/A432). We also evaluated the influence of glucose concentration to the proliferation of chordoma cells because neoplastic transformation usually causes a marked increase in glucose uptake and catabolic conversion to lactate even under normoxic conditions. After culture in various glucose concentration, the MTT assay demonstrated that the concentration of glucose had no significant effect on the proliferative activity of all 3 chordoma cell lines in either hypoxic or normoxic conditions (P > 0.05). The effects of glucose on chordoma cell proliferation were also confirmed by using Hoechst assay (Supplemental Digital Content 2, Figure 2, available at: http://links.lww.com/BRS/A433; Supplemental Digital Content 3, Figure 3, available at: http://links.lww.com/BRS/A434).
Figure 3.
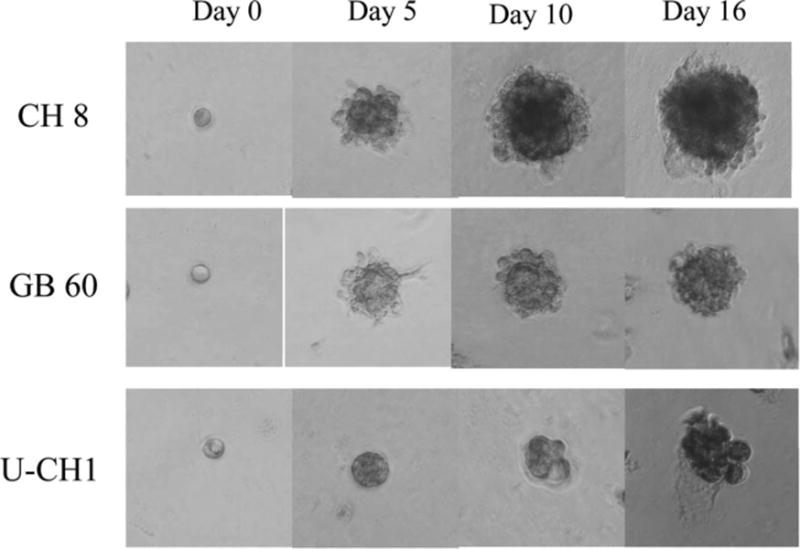
Three-dimensional culture model of chordoma cells. After seeded cells in 3-dimensional culture system, the chordoma cells became spheroid. They continued to proliferate and formed clusters after 5 to 6 days and subsequently formed acini-like spheroids.
Effect of Collagen Substrate on Chordoma Cell Proliferation
Because the presence of extracellular matrix components as substrates can drastically modulate the phenotype and gene expression of cultured cells, we compared the morphology and proliferation of chordoma cells cultured in plates with or without collagen substrate. The morphology of the chordoma cells did not change when cultured on collagen substrate, when compared with culture in conventional plates. The cell numeration assay indicated that all 3 chordoma cell lines universally grew better in collagen substrate than in collagen free plate (P < 0.05, Figure 2D). The effect of collagen substrate on the proliferation of chordoma cells was also confirmed by Hoechst assay (Supplemental Digital Content 4, Figure 4, available at: http://links.lww.com/BRS/A435).
Figure 4.
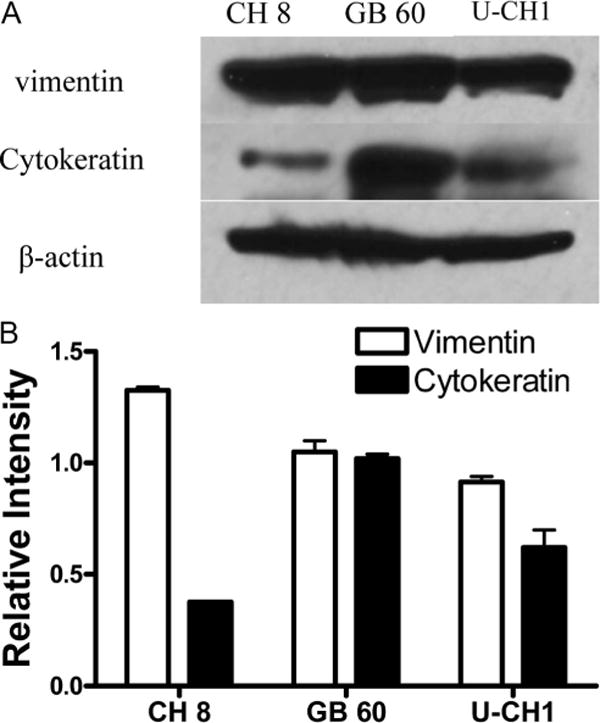
Western blot analysis of biomarkers of chordoma cells. A, It was confirmed that cytokeratin and vimentin were expressed in all chordoma cell lines. B, Densitometric analysis of Western blot results.
Chordoma Cell Lines Grew Well in 3D Culture System
In addition to growth parameters, we established a 3D culture model for chordoma cells. The morphology of the chordoma cells in 3D culture was also observed microscopically. The shape of the chordoma cells first became spheroid after being seeded in 3D culture. They then formed clusters after 5 to 6 days and subsequently formed acini-like spheroids. In this setting, the CH 8 cells grew faster than GB 60 cells and U-CH1 cells (Figure 3).
To further characterize these cell lines under 3D culture, we also measured the expression of biomarkers in chordoma cells, which were grown in 3D culture to see whether the chordoma cells retained their phenotype. Western blot analysis confirmed the expression of cytokeratin and vimentin in all chordoma cell lines (Figure 4). The immunofluorescence staining confirmed that the expression of cytokeratin and vimentin was retained in all chordoma cell lines after culture in 3D culture system (Figures 5 and 6).
Figure 5.
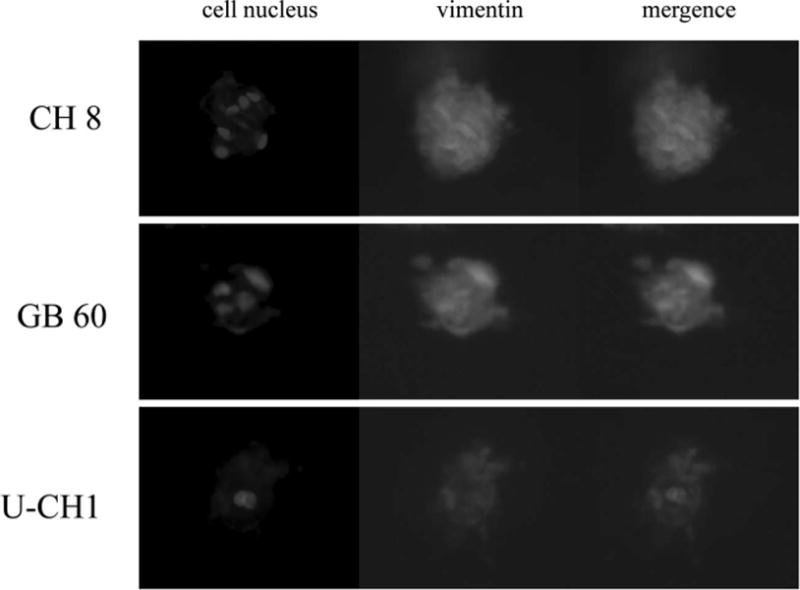
The immunofluorescence staining of vimentin for chordoma cells in 3-dimensional culture. The immunofluorescence staining confirmed that the expression of vimentin was retained in all chordoma cell lines after culture in 3-dimensional culture system.
Figure 6.
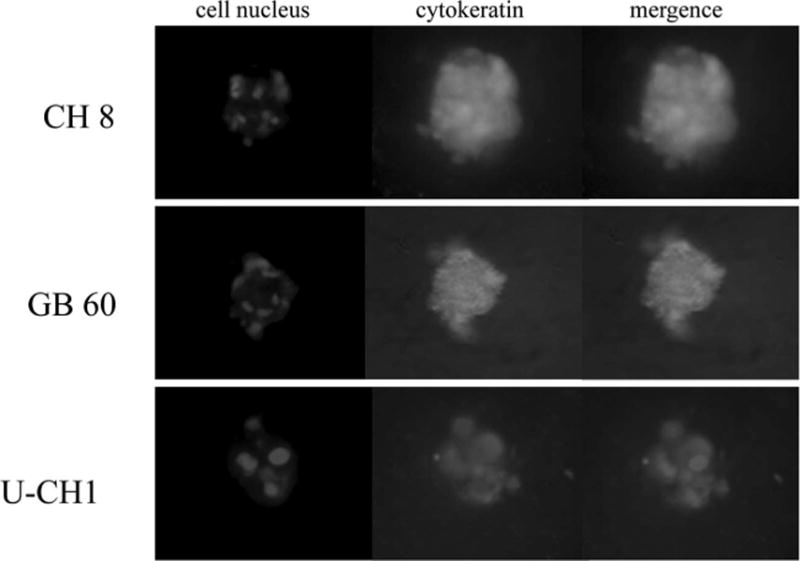
The immunofluorescence staining of cytokeratin for chordoma cells in 3-dimensional culture. The immunofluorescence staining confirmed that the expression of cytokeratin was retained in all chordoma cell lines after culture in 3-dimensional culture system.
Chordoma Cells are Sensitive to Doxorubicin, Yondelis, Zalypsis, and Cisplatin
Chordoma cells were treated with doxorubicin, methotrexate, cisplatin, yondelis, zalypsis, and paclitaxel. The doses of the chemotherapeutic drugs were those conventionally used clinically. MTT assay indicated that all 3 chordoma cell lines were more sensitive to doxorubicin, yondelis, zalypsis, and cisplatin than to methotrexate and paclitaxel (Figure 7).
Figure 7.
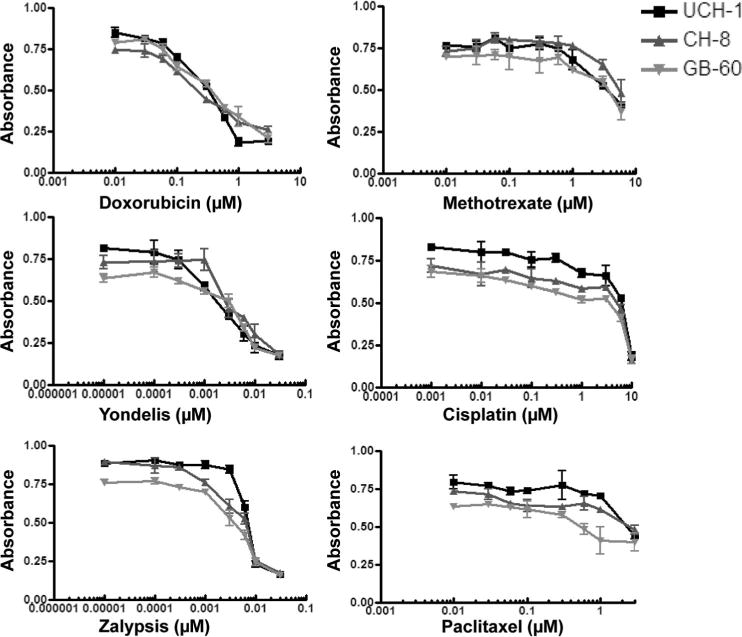
Chemotherapy drug sensitivity assay. All 3 chordoma cell lines are more sensitive to doxorubicin, yondelis, zalypsis, and cisplatin and relatively resistant to methotrexate and paclitaxel.
Discussion
Chordoma cell lines can play an important role in improving the understanding of the mechanisms of chordoma carcinogenesis and contribute to the search for new molecular targets in the treatment for chordoma. Because chordoma cells are difficult to maintain in long-term cultures, before this study, the characteristics of chordoma cells was incompletely understood, although 3 permanent human chordoma cell lines have been reported to date.13–15 Establishing novel chordoma cell lines and further characterizing existing chordoma cell lines would benefit chordoma research and treatment. We have recently established a chordoma cell line, CH 8. This cell line showed a typical morphology for chordomas containing physaliphorous cells (Figure 1). In addition, Affymetrix gene array analysis showed CH-8 gene transcripts clustered more close to chordoma tissues and chordoma cell lines than to any other types of human cancer (unpublished data).
The chordoma cells usually grow slowly in vitro, and these cells have been cultured in different media in different reports. The U-CH1 cells were cultured in RPMI medium, whereas the GB 60 cells were cultured in DMEM.13,14 Another chordoma cell line reported by Ostroumov and Hunter15 was cultured in DMEM/F-12. We compared the proliferation of 3 chordoma cell lines in 5 different commercially available tissue culture media. All 3 chordoma cell lines showed the same proliferation levels in 5 different media, although they all were found to proliferate slower in RPMI medium and faster in IMDM or DMEM. These differences may be due to the biochemical make up of the media, because we found that the concentrations of most amino acids in IMDM and DMEM were higher than the concentration of amino acids in RPMI, F 12, and McCoy 5A. We hypothesize that the chordoma cells proliferate more actively in media containing high concentrations of amino acids. This suggests that either IMDM or DMEM would be the preferred medium in the research of chordoma cells.
Although hypoxia is toxic to both cancer cells and normal cells, cancer cells can undergo genetic and adaptive changes that allow them to survive and even proliferate in a low oxygen environment.18–21 These processes contribute to their malignant phenotype and aggressive behavior.22,23 Our study demonstrated that the chordoma cells could flourish equally in hypoxic and normoxic conditions. This is in contrast to the report by Ostroumov and Hunter15 who suggested that chordoma cells are metabolically more active when incubated under low oxygen conditions.
In tumor tissue, even under normoxic conditions, >50% of the cellular energy is produced by glycolysis with the remainder being generated by the mitochondria.24 Reliance on glycolysis for energy production causes tumor cells to consume glucose inefficiently to generate ATP.23 It has been suggested that chordoma cells are metabolically more active when incubated in high-glucose media.15 However, our study did not demonstrate that the glucose concentration had a significant effect on the proliferative activity on any of the 3 chordoma cell lines.
It is generally acknowledged that the extracellular matrix does not function as a mere passive scaffold for connective tissue within organ architecture, but rather it plays a more active and “informational” role through a network of interactions between cells and signal molecules, which is of primary importance in the control of cellular proliferation, differentiation, and motility.25–29 When different collagens are used as culture substrates, they can promote different degrees of tumor cell growth, motility, and invasion.25,28,30 By light microscopy, chordoma cells are embedded in abundant extracellular myxoid matrix.31 Our study demonstrates that when the chordoma cells are cultured in vitro, collagen can be used as culture substrate to promote the proliferation of chordoma cells.
It is known that cells grown in 3D culture have different cell surface receptor expression and proliferation, cell density, metabolic functions, and extracellular matrix synthesis than cells grown in 2-dimensional (2-D) monolayer.32–36 Many important signals, key regulators, and tissue phenotypes are lost when cells are cultured on 2-D substrates such as culture plates.37 Because chordoma cell lines typically have a very slow rate of growth, they are notoriously challenging to study in vitro — particularly when trying to use cell lines as preclinical models for anticancer drug development. It has been suggested that drug efficacy may be significantly lower in 3D models in other cancer cell lines than in 2-D monolayer as cellular architecture may have a significant effect on drug uptake, distribution, and efficacy.38 We have successfully established the first 3D model of chordoma cells, which can be used to analyze the spatial and temporal aspects of key biologic processes (e.g., proliferation and apoptosis) and signal transduction events during morphogenesis of chordoma. Such a model can be used to develop effective drugs by improving the understanding of the role of chemical, biologic, and physical parameters in the process of drug diffusion through the tumor mass, drug retention, and therapeutic outcome. We noticed that the CH 8 cell line grow faster than GB 60 and U-CH1 cell lines in 3D culture. These results may reflect that the CH 8 cell line is more adaptive to Matrigel or EGF supplements in 3D culture condition than other 2 chordoma cell lines.
The sensitivity of the chordoma cells to chemotherapeutic drugs has not been described earlier. To assess the drug sensitivity, we analyzed the sensitivity of all 3 chordoma cell lines to the conventional chemotherapeutic drugs (doxorubicin, cisplatin, methotrexate, and paclitaxel) and some novel drugs (yondelis and zalypsis) and found that the chordoma cells were relatively sensitive to doxorubicin, yondelis, zalypsis, and cisplatin. Therefore, these drugs may be useful in the treatment of chordomas as adjuvant drugs given alone or in combination with other forms of therapy.
There are some limitations of this study. The most characterizations of the chordoma cell lines described in our study were in 2-dimensional culture. Because 3D culture of cancer cell lines has long been advocated as a better model of the malignant phenotype that is most closely related to tumorigenicity in vivo, further study is needed to describe the characterization of chordoma cell lines in 3D culture.
In conclusion, we have characterized in detail 3 chordoma cell lines CH 8, GB 60, and U-CH1. We demonstrate that various culture media and different environments can affect the proliferation of chordoma cells. The chordoma cells can grow in 3D culture system and form acini-like spheroids, and the chordoma cells seem to be relatively sensitive to doxorubicin, yondelis, zalypsis, and cisplatin. These information will have important implications for further chordoma studies.
Supplementary Material
Key Points.
The characterization of current chordoma cell lines is incomplete.
The proliferation of chordoma cells can be affected by various culture media and different environments.
The chordoma cells can grow in 3-dimensional culture system and form acini-like spheroids.
The chordoma cells seem to be relatively sensitive to doxorubicin, yondelis, zalypsis, and cisplatin.
Acknowledgments
Foundation funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Footnotes
The manuscript submitted does not contain information about medical device (s)/drug (s).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.spinejournal.com).
References
- 1.Sciubba DM, Chi JH, Rhines LD, et al. Chordoma of the spinal column. Neurosurg Clin N Am. 2008;19:5–15. doi: 10.1016/j.nec.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Cheng EY, Ozerdemoglu RA, Transfeldt EE, et al. Lumbosacral chordoma. Prognostic factors and treatment. Spine. 1999;24:1639–45. doi: 10.1097/00007632-199908150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]
- 4.Chugh R, Tawbi H, Lucas DR, et al. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–50. doi: 10.1634/theoncologist.12-11-1344. [DOI] [PubMed] [Google Scholar]
- 5.Forsyth PA, Cascino TL, Shaw EG, et al. Intracranial chordomas: a clinicopathological and prognostic study of 51 cases. J Neurosurg. 1993;78:741–7. doi: 10.3171/jns.1993.78.5.0741. [DOI] [PubMed] [Google Scholar]
- 6.Catton C, O’Sullivan B, Bell R, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67–72. doi: 10.1016/s0167-8140(96)91805-8. [DOI] [PubMed] [Google Scholar]
- 7.Cummings BJ, Hodson DI, Bush RS. Chordoma: the results of megavoltage radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:633–42. doi: 10.1016/0360-3016(83)90228-6. [DOI] [PubMed] [Google Scholar]
- 8.Casali PG, Stacchiotti S, Sangalli C, et al. Chordoma. Curr Opin Oncol. 2007;19:367–70. doi: 10.1097/CCO.0b013e3281214448. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs B, Dickey ID, Yaszemski MJ, et al. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–6. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 10.Osaka S, Kodoh O, Sugita H, et al. Clinical significance of a wide excision policy for sacrococcygeal chordoma. J Cancer Res Clin Oncol. 2006;132:213–8. doi: 10.1007/s00432-005-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzortzidis F, Elahi F, Wright D, et al. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery. 2006;59:230–7. doi: 10.1227/01.NEU.0000223441.51012.9D. discussion 230–7. [DOI] [PubMed] [Google Scholar]
- 12.Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–34. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, Pierconti F, Falchetti ML, et al. Establishing tumor cell lines from aggressive telomerase-positive chordomas of the skull base. Technical note. J Neurosurg. 2006;105:482–4. doi: 10.3171/jns.2006.105.3.482. [DOI] [PubMed] [Google Scholar]
- 14.Scheil S, Bruderlein S, Liehr T, et al. Genome-wide analysis of sixteen chordomas by comparative genomic hybridization and cytogenetics of the first human chordoma cell line, U-CH1. Genes Chromosomes Cancer. 2001;32:203–11. doi: 10.1002/gcc.1184. [DOI] [PubMed] [Google Scholar]
- 15.Ostroumov E, Hunter CJ. The role of extracellular factors in human metastatic chordoma cell growth in vitro. Spine. 2007;32:2957–64. doi: 10.1097/BRS.0b013e31815cde91. [DOI] [PubMed] [Google Scholar]
- 16.Duan Z, Duan Y, Lamendola DE, et al. Overexpression of MAGE/GAGE genes in paclitaxel/doxorubicin-resistant human cancer cell lines. Clin Cancer Res. 2003;9:2778–85. [PubMed] [Google Scholar]
- 17.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basichelix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 20.Tanimoto K, Makino Y, Pereira T, et al. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 23.Denko NC. Hypoxia, HIF1 and glucose metabolisminthe solid tumour. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 24.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 25.Sirchia R, Ciacciofera V, Luparello C. Tumor cell-collagen interactions: identification and semi-quantitative evaluation of selectively-expressed genes by combination of differential display- and multiplex-PCR. Biol Proced Online. 2003;5:222–7. doi: 10.1251/bpo65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pucci Minafra I, Luparello C, Sciarrino S, et al. Quantitative determination of collagen types present in the ductal infiltrating carcinoma of human mammary gland. Cell Biol Int Rep. 1985;9:291–6. doi: 10.1016/0309-1651(85)90047-5. [DOI] [PubMed] [Google Scholar]
- 27.Schillaci R, Luparello C, Minafra S. Type I and I-trimer collagens as substrates for breast carcinoma cells in culture. Effect on growth rate, morphological appearance and actin organization. Eur J Cell Biol. 1989;48:135–41. [PubMed] [Google Scholar]
- 28.Luparello C, Sheterline P, Pucci-Minafra I, et al. A comparison of spreading and motility behaviour of 8701-BC breast carcinoma cells on type I, I-trimer and type V collagen substrata. Evidence for a permissive effect of type I-trimer collagen on cell locomotion. J Cell Sci. 1991;100(pt 1):179–85. doi: 10.1242/jcs.100.1.179. [DOI] [PubMed] [Google Scholar]
- 29.Luparello C. Adhesion to type V collagen and cloning efficiency in agar of 8701-BC breast cancer cells. Eur J Cancer. 1994;30A:1400–1. doi: 10.1016/0959-8049(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 30.Minafra S, Giambelluca C, Andriolo M, et al. Cell-cell and cell-collagen interactions influence gelatinase production by human breast-carcinoma cell line 8701-BC. Int J Cancer. 1995;62:777–83. doi: 10.1002/ijc.2910620622. [DOI] [PubMed] [Google Scholar]
- 31.Romeo S, Hogendoorn PC. Brachyury and chordoma: the chondroid-chordoid dilemma resolved? J Pathol. 2006;209:143–6. doi: 10.1002/path.1987. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Weaver VM, Petersen OW, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–6. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders M, Hansen R, Ding RX, et al. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci USA. 2003;100:1943–8. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng KW, Leong DT, Hutmacher DW. The challenge to measure cell proliferation in two and three dimensions. Tissue Eng. 2005;11:182–91. doi: 10.1089/ten.2005.11.182. [DOI] [PubMed] [Google Scholar]
- 35.Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci USA. 2004;101:18024–9. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes NP, Srivastava JK, Smith RF, et al. Metabolic and histological analysis of mesenchymal stem cells grown in 3-D hyaluronan-based scaffolds. J Mater Sci Mater Med. 2004;15:391–5. doi: 10.1023/b:jmsm.0000021108.74004.7e. [DOI] [PubMed] [Google Scholar]
- 37.Lee GY, Kenny PA, Lee EH, et al. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhiman HK, Ray AR, Panda AK. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials. 2005;26:979–86. doi: 10.1016/j.biomaterials.2004.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


