Abstract
Introduction
Acute hepatitis C virus infection is associated with high rates of spontaneous clearance and variable rates of treatment-induced clearance. The benefit of early treatment versus awaiting spontaneous clearance is unknown, as is the optimal timing of treatment.
Methods
We performed a MEDLINE and EMBASE search for the time period 1950 to October 2008. All English language abstracts using the search terms acute hepatitis C, hepatitis C and acute and hepatitis C and acute disease or acute infection were reviewed. Bibliographies were reviewed.
Results
Twenty-two studies including 1075 patients met inclusion criteria. The sustained virologic response (SVR) rate for treated patients was 78%, significantly higher than 55.1% in untreated patients (OR =3.08, 95% CI 1.8-4.8 p value<0.0001). Mean time from diagnosis to spontaneous clearance was 9.7 weeks (SD 6.5). SVR rates varied inversely with time from acute HCV diagnosis. SVR rates for treatment within 12 weeks was 82.5% (95% CI 75.6-89.3), significantly better than the clearance rates in untreated patients (p<0.001). Response rates fell to 66.9% for treatment between 12 and 24 weeks, and decreased further to 62.5% for treatment beyond 24 weeks.
Conclusions
Rates of viral clearance in treated patients with acute hepatitis C virus infection were significantly higher than that in untreated patients. Treatment rates were highest when treatment was initiated within 12 weeks of diagnosis. Based on these findings, we would advocate a 12 week period of observation for spontaneous clearance before treatment initiation. If no clearance has occurred by 12 weeks, treatment should be initiated.
Keywords: acute hepatitis C, meta-analysis, spontaneous clearance, sustained virologic response
Chronic hepatitis C virus infection infects more than 170 million people worldwide and more than 4 million Americans.(1) The incidence of new hepatitis C virus infections in the United States is estimated to be 100-200 cases per 100,000 people each year, with 28,000 new cases globally.(2) Unfortunately, 75-80% of patients who acquire hepatitis C virus are asymptomatic, delaying diagnosis and preventing early treatment.(3) However, symptomatic patients who present with acute hepatitis C virus may have higher rates of spontaneous clearance, with some studies noting spontaneous clearance rates as high as 66%.(4-7) Treatment studies of patients with symptomatic acute HCV have yielded varying rates of treatment-induced SVR from as low as 21% to as high as 98%.(8-10) Trials have been limited by small patient numbers, the lack of randomized controls, and variability in enrollment criteria, including the definition of acute hepatitis C virus infection and of sustained virologic response.(11, 12) Additionally, in several of these studies, early treatment (12-35 days after diagnosis) was initiated.(13, 14) Early initiation of treatment most likely includes a proportion of patients who would have spontaneously cleared HCV which may overestimate the treatment response.
Recommendations for the timing of treatment initiation vary widely from 35 days after diagnosis to 120 days, with some studies suggesting that earlier treatment is associated with higher rates of SVR.(8, 14-18) With high rates of spontaneous clearance and varying rates of treatment-induced clearance in symptomatic patients with acute hepatitis C virus infection, it is unknown whether treatment in the acute phase is clearly superior to watchful waiting. Furthermore, if treatment is chosen, the optimal timing of initial therapy remains unknown. Premature initiation of treatment in the acute phase may subject patients who would otherwise spontaneously clear HCV to unnecessary and costly treatment. However, delay in treatment initiation may lower rates of treatment-induced clearance.
This meta-analysis sought to more definitively establish the rate of spontaneous and treatment-induced clearance in patients with acute hepatitis C virus infection, and further sought to evaluate whether treatment outcomes vary based on the timing of treatment initiation.
Materials and Methods
We performed a MEDLINE and EMBASE search for the period of 1950 to October 2008. All English language abstracts using the search terms “acute hepatitis C”, “hepatitis C and acute” and “hepatitis C and acute disease or acute infection” were reviewed. Bibliographies of retrieved papers were also reviewed for eligible articles.
For inclusion into the meta-analysis the articles were required to meet the following criteria: 1) Acute hepatitis C virus infection, defined by seroconversion of anti-hepatitis C antibody, negative antibody and positive HCV RNA or positive antibody, positive HCV RNA, elevated ALT with a history of recent HCV exposure, 2)Ability to determine the patient’s management (treatment versus observation), 3) Ability to determine patient’s outcome (viral clearance versus the development of chronic disease), 4) Follow-up period of 6 months or more, 5)Absence of co-infection with hepatitis B or HIV, 6)Ability to determine the timing from acute HCV diagnosis to treatment, 7) Ability to determine the duration of HCV treatment, 8)Absence of end stage renal disease. Only trials prospective in nature were included.
The majority of studies were rejected because of the lack of a clear definition of acute hepatitis C virus infection, the inability to determine time to treatment, or the inability to assess patient outcomes.
Data were extracted using a pre-specified data extraction sheet by two independent reviewers. Variables extracted included study type, year, definition of acute hepatitis C virus infection, number of patients treated, number of patients with sustained virologic response, number of patients with spontaneous clearance, distribution of genotype. Differences between reviewers were resolved by consensus.
We fit a Bayesian model to take into account the between-study variability and estimated the treatment effect based on 22 studies. Specifically, we modeled the number of responses in study i
We used WinBUGS software to fit this model and draw inferences regarding the overall treatment rates as well as response rates among treated or control groups.
Categorical variables were analyzed using the chi-square test, while continuous variables were evaluated using the Student’s t-test. A two-sided p-value of < 0.05 is considered statistically significant.
Results
Study and Patient Characteristics
A total of 2262 abstracts were found and all were reviewed. Of these reviewed abstracts, 139 articles were retrieved and 22 met our inclusion criteria. (Table 1)
Table 1.
Studies Included in Meta-Analysis
| Study Number | Author | Source | Country of Origin | Study Type | Treatment |
|---|---|---|---|---|---|
| 1 | Hwang et al.(9) | Journal of Hepatology 1994 | China | RCT | Yes |
| 2 | Kamal et al.(4) | Gastroenterology 2006 | Egypt, USA, Germany |
Randomized trial* | Yes |
| 3 | Kamal et al.(22) | Hepatology 2004 | Egypt, Germany | Randomized trial* | Yes |
| 4 | Kamal et al.(15) | Hepatology 2006 | Egypt, USA, Germany |
Randomized trial* | Yes |
| 5 | Nomura et al.(23) | Hepatology 2004 | Japan | Randomized trial* | Yes |
| 6 | Broers et al.(24) | Journal of Hepatology 2005 | Switzerland | Prospective cohort** | Yes |
| 7 | Calleri et al.(25) | Journal of Viral Hepatitis 2007 |
Italy | Prospective cohort** | Yes |
| 8 | De Rosa et al.(13) | Journal of Antimicrobials & Chemotherapeutics 2006 |
Italy | Prospective cohort** | Yes |
| 9 | De Rosa et al.(13) | Clinical Infectious Disease 2007 |
Italy | Prospective cohort** | Yes |
| 10 | Delwaide et al.(18) | Alimentary Pharmacology and Therapeutics 2004 |
Belgium | Prospective cohort** | Yes |
| 11 | Gerlach et al.(19) | Gastroenterology 2003 | Germany | Prospective cohort** | Yes |
| 12 | Hofer et al.(14) | Hepatology 2003 | Austria | Prospective cohort** | Yes |
| 13 | Jaeckel et al.(8) | New England Journal of Medicine 2001 |
Germany | Prospective cohort** | Yes |
| 14 | Santantonio et al.(20) | Journal of Hepatology 2005 | Italy | Prospective cohort** | Yes |
| 15 | Wiegand et al. (26) | Hepatology 2006 | Germany | Prospective cohort** | Yes |
| 16 | Guobuzaite et al.(27) | Medicina 2008 | Lithuania | Prospective cohort** | No |
| 17 | Hwang et al.(10) | Journal of Medical Virology 2001 |
China | Prospective cohort** | No |
| 18 | Larghi et al.(6) | Hepatology 2002 | Italy, USA | Prospective, observational cohort*** |
No |
| 19 | Pekova et al.(7) | Journal of Hospital Infection 2007 |
Bulgaria | Prospective, observational cohort*** |
No |
| 20 | Santantonio et al.(21) | Digestive and Liver Diseases 2003 |
Italy, USA | Prospective cohort** | Yes |
| 21 | Spada et al.(10) | Gut 2004 | Italy | Prospective, observational cohort*** |
No |
| 22 | Wang et al.(28) | Journal of Infectious Diseases 2007 |
USA | Prospective, observational cohort*** |
No |
Randomized trials, compared to randomized controlled trials, lacked a randomized control group. In these trials patients who refused treatment were followed as controls.
Prospective cohorts: open label, unrandomized trials offering all subjects treatment but not randomizing patients, untreated patients were also followed
Prospective observational cohort: no treatment offered
RCT: Randomized controlled trial
Sixteen studies were treatment studies of acute hepatitis C virus infection; five studies were randomized trials, with or without control groups and 11 studies were prospective, open label treatment trials. Six studies were observational in nature only and offered no acute hepatitis C virus infection treatment.
These studies included 1075 patients with acute hepatitis C virus infection. The mean age of the patients was 36.4 years. Fifty-four percent of subjects were men. Genotype 1 predominated with 46.9% of subjects, 12.6% with genotype 2, 12% with genotype 3, 17.3% genotype 4 and 11.2% unknown. (Table 2) The mean ALT at time of diagnosis was 471 IU/L. Risk factors included intravenous drug abuse (IVDA) in 26.8%, occupational exposure in 29.3%, medical procedures in 15%, transfusion in 10%, sexual contact in 8.3% and unknown in 9.7%.
Table 2.
Characteristics of Studies Included in Meta-Analysis
| Study Number |
Total patients |
Mean Age | Male/Female | Genotype 1 / 2 / 3 / 4 |
|---|---|---|---|---|
| 1 | 33 | 54 | 24/9 | 0 / 17 /5 / 4 |
| 2 | 168 | 36.7 | 79/89 | 57 / 17 /20 / 71 |
| 3 | 54 | 34.4 | 31/23 | 20 /0 /0/ 34 |
| 4 | 161 | 29.5 | 68/93 | 68 / 15/14 / 64 |
| 5 | 34 | 39 | 19/11 # | 25 / 0 /0/ 0 |
| 6 | 27 | 31.7 | 20/7 | 9 / 1 /11/ 0 |
| 7 | 46 | 32.8 | 26/20 | 22 / 10/ 9 / 4 |
| 8 | 16 | 29 | 12/4 | 9 / 1 / 6 / 0 |
| 9 | 23 | 26.6 | 15/8 | 12 / 2 / 6 / 3 |
| 10 | 44 | 34.6 | 20/24 | 17 / - / - / 2 |
| 11 | 60 | 35.5 | 25/35 | 36 / 3/ 11/ 2 |
| 12 | 12 | 39.5 | 3/9 | 7 / - / 3 / 0 |
| 13 | 44 | 36 | 19/25 | 27 / -/ - / 0 |
| 14 | 28 | 35 | 23/5 | 6 / 7/ 3 / 0 |
| 15 | 89 | 34 | 56/33 | 59 / 3 / 17 / 1 |
| 16 | 8 | 33 | 4/4 | 4 / 1 / 3 / 0 |
| 17 | 67 | 42 | 47/20 | 42 / 22 / 0 / 0 |
| 18 | 14 | 31.2 | 3/11 | 0 / 14 / 0 / 0 |
| 19 | 6 | 65.8 | 6/0 | 6 / 0 / 0 / 0 |
| 20 | 40 | 40 | 23/17 | 21 / 11/ 3 / 2 |
| 21 | 34 | 30 | 27/7 | 16 / 3 / 12 / 0 |
| 22 | 67 | 31 | 35/32 | 42 / 9 / 7 / 0 |
| Total | 1075 | 36.4 +/−8.7 | 585/486 | 505/ 136/ 130 /187 |
NA= Not available. # = Gender unknown in four patients
Clearance Rates Higher in Treated Patients
Four hundred and seventy-three patients (44%) received no treatment for acute hepatitis C virus infection. These patients deferred treatment, had contraindications to therapy, or underwent viral clearance prior treatment initiation. The overall clearance rate for untreated patients was 55.1% (Figure 1), indicating that more than half of patients diagnosed with acute hepatitis C virus infection achieved viral clearance without treatment. Additionally, the mean time from symptom onset and diagnosis of acute hepatitis C virus infection to spontaneous clearance was 9.7 weeks (SD 3.2) and the mean time from exposure to spontaneous clearance was 13 weeks (SD 8.1), indicating that the majority of clearance occurred within 3 months of diagnosis and 4 months of presumed exposure.
Figure 1.
Acute Hepatitis C Virus Infection Response Rates for Untreated Patients. Only studies which reported response rates in the specific treatment period are included.
Six hundred and two patients (56%) received treatment for acute hepatitis C virus. The majority of patients (77%) received peginterferon monotherapy. Sixteen percent of patients received interferon monotherapy, 4% received peginterferon and ribavirin and 3% of patients received interferon and ribavirin. The average duration of treatment was 19.7 weeks (+/− 12.5 weeks). (Table 3)
Table 3.
Treatment Characteristics of Studies Included in Meta-Analysis
| Study Number |
Mean Treatment Duration (weeks) |
Treatment Regimen |
Mean Time from Diagnosis to Treatment |
Mean Time from Exposure to Treatment |
|---|---|---|---|---|
| 1 | 12 | IFNα-2b 3M TIW | 8.8 ± 1.0 weeks | 16 weeks |
| 2 | 12 | PEGIFN α-2b 1.5μg/kg Qweek | 8 or 12 or 20 weeks | 67 days, 95 days or 151 days |
| 3 | 24 | PEGIFN α 2a 180 mcg Q week or PEG-IFN α 2a 180 mcg Qweek +RBV 800 mg qd |
12 weeks | 19.8 weeks |
| 4 | 8, 12, or 24 | PEGIFN α-2b 1.5μg/kg Qweek | 8 or 12 or 24 weeks | NA |
| 5 | 4 or 24 | IFN α 6M qd or IFN α 6M TIW |
8 or 52 weeks | 15.6 ± 2.7 weeks |
| 6 | 24 | PEGIFN α-2b 1.5μg/kk Q week | 9.7 weeks | NA |
| 7 | 12 | PEGIFN α-2b 1.5μg/kg Qweek | 15 days (1-90)* | NA |
| 8 | 12 | PEGIFN α-2b 1.0-1.6μg/kg Qweek | 31 days (0 -116)* | NA |
| 9 | 12 | PEGIFN α-2b 1.0-1.6μg/kg Qweek | 13.5 days (0 – 166)* | NA |
| 10 | 8 | IFN 5MU qd | 55 ± 41 days | 110 ± 44 days |
| 11 | 38.5 | IFN α 5 or 3MU TIW, IFN + RBV 1000- 1200mg, PEG-FNα 2a 180 mcg, PEGIFNα + RBV 1000-1200 mg qd |
6.1 months | NA |
| 12 | 24 | PEGIFN α-2b 1.5μg/kg Qweek | 6 weeks | NA |
| 13 | 24 | IFN 5MU qd × 4 w + IFN 5MU TIW × 20 w | 89 days (30 – 112) | NA |
| 14 | 24 | PEGIFN α-2b 1.5μg/kg qweek | NA | NA |
| 15 | 24 | PEGIFN α-2b 1.5μg/kg qweek | 27 days (5 – 131)* | 76 days (14 – 150)* |
| 20 | 52 | IFN α 5MU TIW × 6 Mo + IFN 3 MU TIW x 6Mo or IFN α 5MU TIW × 6 Mo + IFN 3 MU TIW × 6Mo + RBV 1000-1200mg qd |
24 weeks | NA |
NA = Information Not available, TIW = three times per week, IFN = interferon, PEG-IFN – peginterferon, RBV = ribavirin, qd = daily
These studies reported time as a median value with a range.
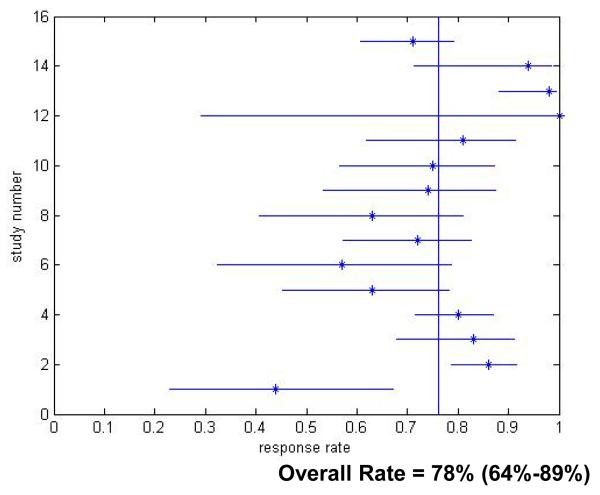
The overall SVR rate for all patients receiving treatment for acute hepatitis C virus infection was 78% (Figure 2). This is significantly higher than the clearance rate (55.1%) in untreated patients (OR =3.08, 95% CI 1.8-4.8 p value<0.0001).
Figure 2.
Acute Hepatitis C Virus Infection Treatment Response Rates for Treated Patients. Only studies which reported response rates in the specific treatment period are included.
Early Treatment Associated with Increased SVR Rates
Next, we sought to determine whether the timing of treatment initiation influenced SVR rates. Patients were divided into three subgroups, those treated at or within 12 weeks of acute hepatitis C virus infection diagnosis, patients treated between 12 and 24 weeks after diagnosis and patients treated 24 weeks post-diagnosis. Four hundred and seventeen patients initiated treatment within 12 weeks of diagnosis (12 Week Group). One hundred and ten patients initiated treatment between 12 and 24 weeks of diagnosis (12-24 Week Group) and forty-six patients received treatment 24 or more weeks after diagnosis (24 Week Group).
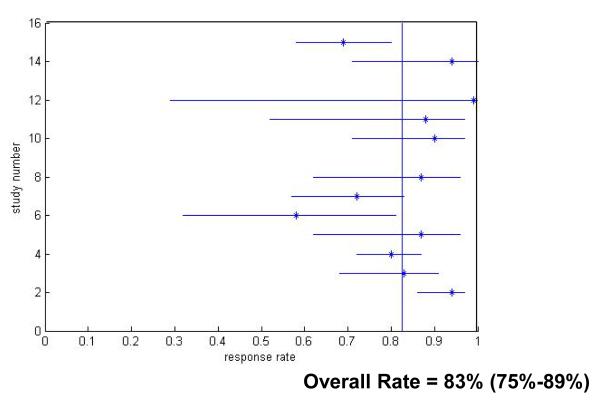
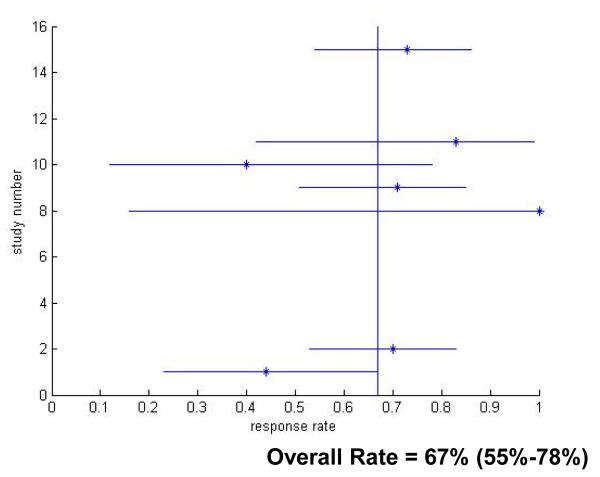
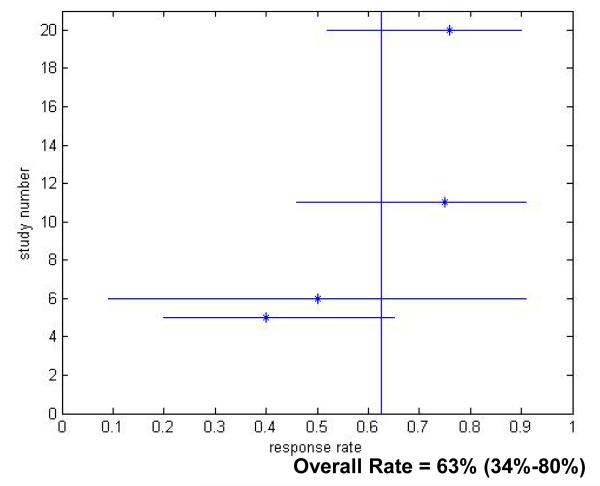
The rate of SVR varied inversely with time from acute HCV diagnosis. The sustained virologic response rates for patients with acute hepatitis C virus infection treated in the 12 Week Group was 82.5% (95% CI 75.6-89.3) (Figure 3), which was significantly better than the clearance rates in untreated patients (p<0.001). Response rates fell to 66.9% (p=0.24 compared to untreated) in the 12-24 Week Group (Figure 4) and decreased further to 62.5% for patients in the 24 Week Group (p=0.68 compared to untreated) (Figure 5).
Figure 3.
Treatment Response Rates for Treatment Within 12 Weeks of Hepatitis C Virus Infection Diagnosis. Only studies which reported response rates in the specific treatment period are included.
Figure 4.
Treatment Response Rates for Treatment Between 12-24 Weeks After Hepatitis C Virus Infection Diagnosis. Only studies which reported response rates in the specific treatment period are included.
Figure 5.
Treatment Response Rates for Treatment After 24 Weeks Following Hepatitis C Virus Infection Diagnosis. Only studies which reported response rates in the specific treatment period are included.
There was no statistically significant difference in the mean duration of treatment for responders and non-responders within the 12 Week Group (17.8 vs. 15.9 weeks, p=0.68), within the 12-24 Week group (16.7 vs. 16.4 weeks, p=0.98) or within the 24 Week Group (29.9 vs. 32.4 weeks, p=0.84).
There was no statistically significant difference between treatment durations when comparing responders in the 12 Week Group to responders in the 12-24 Week Group, responders within the 12 Week Group to the 24 Week Group or between responders in the 12-24 Week Group and 24 Week Group. (p=0.82, p=0.15, p=0.21, respectively). Non-responders in the 24 Week Group underwent a longer duration of treatment (32.5 weeks) than non-responders in the 12 Week Group or 12-24 Week Group (15.9 weeks, p=0.033, 16.4 weeks, p=0.057 respectively). Despite this significant increase in treatment duration, response rates remained lower in this group. (Table 4)
Table 4.
Characteristics of Treated Patients Based on Timing of Treatment Initiation
| Treatment Within 12 Weeks |
Treatment Between 12-24 Weeks |
Treatment After 24 Weeks |
|
|---|---|---|---|
| Number | 417 | 110 | 46 |
|
Mean Treatment
Duration in Responders (+/−SD) |
17.8 +/−9.1 | 16.7 +/−9.4 | 29.9 +/−21.3 |
|
Mean Treatment
Duration in Non- responders (+/−SD) |
15.9 +/−10.5 | 16.4+/−8.0 | 32.5 +/−13.3 |
Discussion
Our study answers several persistent questions regarding management of acute hepatitis C virus infection. First, we found a significant rate of spontaneous clearance in over half of untreated patients (55.1%) clearing HCV without treatment, indicating that more than half of patients diagnosed with acute hepatitis C virus infection do not require treatment. Importantly, the mean time from diagnosis to clearance was 9.7 weeks and mean time from exposure to clearance was 13 weeks. These findings are consistent with other data suggesting that spontaneous clearance of acute HCV typically occurs within 12 weeks of diagnosis.(19-21) Gerlach et al. found that the majority of patients who achieved spontaneous clearance cleared virus within 12 weeks of diagnosis and none cleared after 17 weeks following diagnosis.(19) Thus, our findings bolster the recommendation that a 12 week observation period is adequate to allow for spontaneous clearance.
While our study found that there was a high rate of spontaneous clearance we also confirmed that treatment was significantly better than no treatment with an odds ratio of 3.08 (95% CI 1.8-4.8). The superiority of treatment-induced SVR was driven by treatment initiated by the 12 week mark, with SVR rates higher the earlier treatment was initiated. Treatment initiated beyond 12 weeks is comparable to SVR rates for chronic hepatitis C virus infection.
Our study has several limitations. First, we were unable to garner data from the individual studies including the hepatitis C virus infection genotypes for individual patients and their individual virologic outcomes (only overall genotypes for the study) and exact treatment regimen and duration for individual responders versus non-responders for each trial which are significant factors that may influence treatment outcomes. We did find that majority of our patients (77%) were treated with pegylated interferon monotherapy and our overall treatment response rate was 78%, suggesting that monotherapy may be adequate. However, we are unable to directly compare outcomes between pegylated interferon monotherapy and other regimens. We did find that mean treatment durations in the 12 Week Group were not significantly different between responders and non-responders (15.9 vs. 17.8 weeks). However, the wide standard deviations of 9.1 and 10.5 weeks reflect the variability in treatment durations, so we are thus unable to recommend an optimal duration to maximize treatment response while limiting treatment-induced adverse effects. Thus, while our study can provide treatment outcomes based on timing of treatment initiation, we cannot provide recommendations on the optimal duration of treatment or treatment regimen.
In conclusion, our meta-analysis found high rates of spontaneous clearance in patients with acute hepatitis C virus infection. In addition, we found superior rates of viral clearance in patients with acute hepatitis C virus infection who underwent early treatment when compared to patients who did not receive treatment. Treatment rates were highest when treatment was initiated within 12 weeks of diagnosis and the mean time to spontaneous clearance was 11.5 weeks. Thus, we would advocate for a 12 week period of watchful waiting before treatment initiation to allow for spontaneous clearance. This 12 week mark in untreated acute hepatitis C virus infection patients is analogous to the early virologic response (EVR) time point of treatment in chronic patients. We have therefore adopted the term “acute EVR” to denote spontaneous clearance of acute infection by 12 weeks. A lack of viral clearance at the 12 week mark in acute untreated patients, as in treated chronic patients, reflects an inadequate response and warrants a change in management. Just as failure to achieve EVR would justify cessation of treatment in chronic patients, failure to achieve acute EVR in acute patients would warrant initiation of treatment. If at the 12 week mark patients have not achieved an acute EVR, remaining viremic, we would recommend prompt initiation of treatment to ensure patients the greatest chance for treatment-induced clearance. Further studies are necessary to clarify the optimal duration of therapy and the optimal treatment regimen.
Acknowledgements and Disclosures
Statement of interest: KEC has received research support from Bristol-Myer-Squibb Virology Fellowship.
RTC has received research support from Roche Laboratories and Schering-Plough. RTC is supported by NIH AI69939 and DK78772.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999 Aug 19;341(8):556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000 Mar;31(3):777–82. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 3.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000 Feb 15;132(4):296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Al Tawil A, Khalifa KE, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology. 2006 Mar;130(3):632–8. doi: 10.1053/j.gastro.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Corey KE, Ross AS, Wurcel A, Schulze Zur, Wiesch J, Kim AY, Lauer GM, et al. Outcomes and treatment of acute hepatitis C virus infection in a United States population. Clin Gastroenterol Hepatol. 2006 Oct;4(10):1278–82. doi: 10.1016/j.cgh.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larghi A, Zuin M, Crosignani A, Ribero ML, Pipia C, Battezzati PM, et al. Outcome of an outbreak of acute hepatitis C among healthy volunteers participating in pharmacokinetics studies. Hepatology. 2002 Oct;36(4 Pt 1):993–1000. doi: 10.1053/jhep.2002.36129. [DOI] [PubMed] [Google Scholar]
- 7.Pekova LM, Teocharov P, Sakarev A. Clinical course and outcome of a nosocomial outbreak of hepatitis C in a urology ward. J Hosp Infect. 2007 Sep;67(1):86–91. doi: 10.1016/j.jhin.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001 Nov 15;345(20):1452–7. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SJ, Lee SD, Chan CY, Lu RH, Lo KJ. A randomized controlled trial of recombinant interferon alpha-2b in the treatment of Chinese patients with acute post-transfusion hepatitis C. J Hepatol. 1994 Nov;21(5):831–6. doi: 10.1016/s0168-8278(94)80246-7. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SJ, Lee SD, Lu RH, Chu CW, Wu JC, Lai ST, et al. Hepatitis C viral genotype influences the clinical outcome of patients with acute posttransfusion hepatitis C. J Med Virol. 2001 Nov;65(3):505–9. [PubMed] [Google Scholar]
- 11.Omata M, Yokosuka O, Takano S, Kato N, Hosoda K, Imazeki F, et al. Resolution of acute hepatitis C after therapy with natural beta interferon. Lancet. 1991 Oct 12;338(8772):914–5. doi: 10.1016/0140-6736(91)91774-o. [DOI] [PubMed] [Google Scholar]
- 12.Griveas I, Germanidis G, Visvardis G, Morice Y, Perelson AS, Pawlotsky JM, et al. Acute hepatitis C in patients receiving hemodialysis. Ren Fail. 2007;29(6):731–6. doi: 10.1080/08860220701460160. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa FG, Bargiacchi O, Audagnotto S, Garazzino S, Cariti G, Calleri G, et al. Twelve-week treatment of acute hepatitis C virus with pegylated interferon- alpha -2b in injection drug users. Clin Infect Dis. 2007 Sep 1;45(5):583–8. doi: 10.1086/520660. [DOI] [PubMed] [Google Scholar]
- 14.Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, Steindl-Munda P, et al. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology. 2003 Jan;37(1):60–4. doi: 10.1053/jhep.2003.50019. [DOI] [PubMed] [Google Scholar]
- 15.Kamal SM, Moustafa KN, Chen J, Fehr J, Abdel Moneim A, Khalifa KE, et al. Duration of peginterferon therapy in acute hepatitis C: a randomized trial. Hepatology. 2006 May;43(5):923–31. doi: 10.1002/hep.21197. [DOI] [PubMed] [Google Scholar]
- 16.Chung RT. Acute hepatitis C virus infection. Clin Infect Dis. 2005 Jul 1;41(Suppl 1):S14–7. doi: 10.1086/429490. [DOI] [PubMed] [Google Scholar]
- 17.Wiegand J, Deterding K, Cornberg M, Wedemeyer H. Treatment of acute hepatitis C: the success of monotherapy with (pegylated) interferon alpha. J Antimicrob Chemother. 2008 Nov;62(5):860–5. doi: 10.1093/jac/dkn346. [DOI] [PubMed] [Google Scholar]
- 18.Delwaide J, Bourgeois N, Gerard C, De Maeght S, Mokaddem F, Wain E, et al. Treatment of acute hepatitis C with interferon alpha-2b: early initiation of treatment is the most effective predictive factor of sustained viral response. Aliment Pharmacol Ther. 2004 Jul 1;20(1):15–22. doi: 10.1111/j.1365-2036.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003 Jul;125(1):80–8. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 20.Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005 Mar;42(3):329–33. doi: 10.1016/j.jhep.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Santantonio T, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, Gentile A, et al. Natural course of acute hepatitis C: a long-term prospective study. Dig Liver Dis. 2003 Feb;35(2):104–13. doi: 10.1016/s1590-8658(03)00007-0. [DOI] [PubMed] [Google Scholar]
- 22.Kamal SM, Ismail A, Graham CS, He Q, Rasenack JW, Peters T, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology. 2004 Jun;39(6):1721–31. doi: 10.1002/hep.20266. [DOI] [PubMed] [Google Scholar]
- 23.Nomura H, Sou S, Tanimoto H, Nagahama T, Kimura Y, Hayashi J, et al. Short-term interferon-alfa therapy for acute hepatitis C: a randomized controlled trial. Hepatology. 2004 May;39(5):1213–9. doi: 10.1002/hep.20196. [DOI] [PubMed] [Google Scholar]
- 24.Broers B, Helbling B, Francois A, Schmid P, Chuard C, Hadengue A, et al. Barriers to interferon-alpha therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005 Mar;42(3):323–8. doi: 10.1016/j.jhep.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Calleri G, Cariti G, Gaiottino F, De Rosa FG, Bargiacchi O, Audagnotto S, et al. A short course of pegylated interferon-alpha in acute HCV hepatitis. J Viral Hepat. 2007 Feb;14(2):116–21. doi: 10.1111/j.1365-2893.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006 Feb;43(2):250–6. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]
- 27.Guobuzaite A, Chokshi S, Balciuniene L, Voinic A, Stikleryte A, Zagminas K, et al. Viral clearance or persistence after acute hepatitis C infection: interim results from a prospective study. Medicina (Kaunas) 2008;44(7):510–20. [PubMed] [Google Scholar]
- 28.Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007 Nov 15;196(10):1474–82. doi: 10.1086/522608. [DOI] [PubMed] [Google Scholar]