Abstract
Monitoring inosine monophosphate dehydrogenase (IMPDH) activity as a biomarker of mycophenolic acid (MPA)–induced immunosuppression may serve as a novel approach in pharmacokinetics (PK)/pharmacodynamics (PD)–guided therapy. The authors prospectively studied MPA pharmacokinetics and IMPDH inhibition in 28 pediatric de novo kidney transplant recipients. Pretransplant IMPDH activity and full PK/PD profiles were obtained at 3 different occasions: 1 to 3 days, 4 to 9 days, and approximately 6 months after transplant. Large intra- and interpatient variability was noted in MPA pharmacokinetics and exposure and IMPDH inhibition. MPA exposure (AUC0-12 h) was low early posttransplant and increased over time and stabilized at months 3 to 6. Mean pretransplant IMPDH activity (6.4 ± 4.6 nmol/h/mg protein) was lower than previously reported in adults. In most of the patients, IMPDH enzyme activity decreased with increasing MPA plasma concentration, with maximum inhibition coinciding with maximum MPA concentration. The overall relationship between MPA concentration and IMPDH activity was described by a direct inhibitory Emax model (EC50 = 0.97 mg/L). This study suggests the importance of early PK/PD monitoring to improve drug exposure. Because IMPDH inhibition is well correlated to MPA concentration, pre-transplant IMPDH activity may serve as an early marker to guide the initial level of MPA exposure required in a pediatric population.
Keywords: pediatric patient, mycophenolic acid, pharmacodynamics, inosine monophosphate dehydrogenase (IMPDH), pharmacokinetics, kidney transplantation
There exists a clinical need to better understand intra-and interpatient variability inthe dose- concentration response to mycophenolate mofetil (MMF, CellCept) and in the susceptibility to adverse events related to this drug in pediatric kidney transplant recipients.1–3 MMF is a prodrug that is rapidly converted presystemically to its active immunosup-pressive moiety, mycophenolic acid (MPA). MPA exhibits its immunosuppressive effect through reversible noncompetitive inhibition of inosine monophosphate dehydrogenase (IMPDH), a key enzyme in the de novo synthesis of guanosine nucleotides in T and B lymphocytes.4,5 According to the Scientific Registry for Transplant Recipients (SRTR) in 2007, approximately 70% of pediatric renal transplant recipients with functioning grafts 30 days posttrans-plant were treated with some form of MPA.6 The recommended MMF dose in children is 600 to 1200 mg/m2 divided in 2 doses, depending on comedication.1,7,8 Earlier clinical studies in pediatric patients showed large intra- and interpatient variability in MPA pharmacokinetics (PK) and exposure, despite dosing normalization to body surface area. Accordingly, possible benefits of therapeutic drug monitoring of MMF therapy have been outlined by several groups.9–11 In addition, data from adult populations indicate that concentration-controlled dosing strategies with an exposure target of 30 to 60 mg·h/L are associated with improved clinical outcomes and reduced drug-related toxicity.12–15 However, MPA PK are quite complex both in adult and pediatric transplant recipients because of variable absorption profiles, enterohepatic recycling, and changing drug clearance over time.3,16,17 Drug exposure in plasma may also not be directly reflective of intracellular pharmacodynamics (PD) in the immune system. Therefore, plasma drug concentrations alone may not fully predict freedom from rejection or side effects or long-term graft function.18,19
Recently, assays for measuring the pharmacodynamic effect of MPA by evaluating the level of IMPDH inhibition in mononuclear cells during MPA therapy have been introduced,20–22 adding a potential new dimension to the optimization of individual MPA therapy. To date, only few data on the PK-IMPDH relationship are available in MPA-treated adult kidney transplant patients,23,24 and no data have been reported in children. In adults, IMPDH activity displays wide interpatient but relatively small intrapatient variability even after long-term administration of MPA.25 Targeting IMPDH activity as a surrogate pharmacodynamic marker of MPA-induced immunosuppression may thus allow increased precision when used in an integrated PK/PD fashion, providing a more accurate assessment of efficacy and aid in limiting toxicity.25,26 In adult transplant patients, an association between pretransplant IMPDH activity and outcome of therapy has in fact been documented,24 but the impact of MPA PK on IMPDH activity variations posttrans-plant and the relationship between the level of post-transplant IMPDH inhibition and clinical outcomes have not been well established. In addition, the advent of calcineurin inhibitor- and corticosteroid-sparing immunosuppressive regimens may necessitate more patient- and protocol-specific PK/PD targets.27 Assessment of posttransplant IMPDH inhibition may thus serve as a useful pharmacodynamic marker of MPA-induced immunosuppression when combined with current PK monitoring strategies.
The objective of this investigation was to study for the first time the PK and PD of MPA using IMPDH inhibition as a PD marker in pediatric renal transplant recipients early posttransplant and during maintenance treatment.
Methods
Patients
This was a multicenter open-label, inpatient/outpatient PK/PD study of mycophenolate-mofetil in de novo pediatric kidney transplant recipients aged 2 to 18 years during the early posttransplant period and stable treatment after approximately 6 months. Patients were treated as per institutional protocols with a fixed MMF dose of 450 to 600 mg/m2 (as CellCept, 250-mg capsule or 500-mg tablet) twice a day up to a maximum dose of 2 g daily in combination with tacrolimus and corticosteroids. Intravenous administration and oral suspension (200 mg/mL) were allowed early posttransplant and documented in the case report form (CRF). Patients on concomitant cyclosporine were excluded. Basiliximab (Simulect) or rabbit antithymocyte globulin (Thymo-globulin, Genzyme, Inc) was used as induction therapy in all patients according to clinical protocols. MMF dose adjustments were at the discretion of the treating physician and were not based on drug concentration measurements.
Patients with any medical condition (active or chronic) or prior surgery that could interfere with the pharmacokinetic behavior of MMF (absorption, distribution, and elimination) were not eligible. Concurrent use of antacids, cholestyramine, and iron supplements was not allowed. The study was approved by the institutional review boards of the participating institutions, and parents/guardians and patients provided written consent and assent as required.
Cincinnati Children’s Hospital Medical Center (CCHMC) was the lead site with 2 additional sites from the National Institute of Child Health and Human Development (NICHD) Pediatric Pharmacology Research Units (PPRU) network participating: Dallas South Western and University of Utah. The goal was to enroll a total of 24 patients (72 PK/ PD profiles), with participants being stratified into 3 age categories: ≥2 and <6, ≥6 and <12, and ≥12 to ≤18 years of age.28
PK/PD Evaluation
Full PK/PD profiles were collected early posttrans-plant (days 1–3), at discharge from the hospital (days 5–9), and during stable MMF treatment (at least 3–6 months posttransplant). For PK sampling, the schedule included predose (trough), 20 minutes, 40 minutes, and 1, 1.5, 2, 3, 4, 6, and 9 hours after the morning MMF dose. An alternate sparse sampling schedule was offered for incidental patients coming from afar where a 9-hour profile posed a problem either by interfering with the clinical visit and/or travel arrangements: predose (trough), 20 minutes, and 1 and 3 hours postdose and a fifth sample at one additional later time point, randomly selected from those of the full sampling schedule according to the Limoges University Hospital schedule.29
For the PD sampling, IMPDH activity measurements were obtained pretransplant (baseline), pre-dose, and 1, 3, 4, 6, and 9 hours postdose on all sampling days. An abbreviated profile consisting of predose, 20 minutes, and 1, 3, and 4 hours on the sample day was offered as an alternative for select patients during the final visit. The times for the samples during each study day were allowed to vary by ±5 minutes for samples before the 1-hour sample and by ±10 minutes for all samples after 1 hour. Exact time points were recorded in the CRF. For the youngest patients, fewer samples (abbreviated sampling schedule) were collected because of restrictions in total blood volume allowed to draw.
A standard light meal was offered 1 and 4 hours postdose, and intake was recorded. Demographic data collected included age, gender, weight, height, race, ethnicity, and donor type. Concentrations of serum creatinine were obtained, and creatinine clearance (CrCL) was used as a predictor of renal function calculated using Schwartz’s formula.30
Measurement of MPA Concentrations
MPA and its metabolite MPA-glucuronide (MPAG) were analyzed in plasma using a validated high-performance liquid chromatography (HPLC) assay according to a modification of a previously described method.31,32 In short, plasma samples were extracted using solid-phase extraction (Oasis HLB 15 μm 2.1 × 20 mm; Waters, Milford, Massachusetts) and separated on a Synergi reversed-phase column (4 μm Hydro-RP 80 Å 250 × 3.0 mm; Phenomenex, Torrance, California) using an Agilent 1100 HPLC system with diode array detection at 215 nm with a semigradient elution (Agilent Technologies, Santa Clara, California). The method was selective and reproducible in the range of 0.25 to 25.0 mg/L for MPA and 1.0 to 250 mg/L for MPAG. Extraction efficiencies were between 91% and 100% over the working range for both analyses. The lower limit of quantification (LLOQ) was 0.25 mg/L for MPA and 1.0 mg/L for MPAG, respectively. The level of detection (LOD) was 0.03 mg/L for both analyses. Intra- and interday precision and accuracy for quality control (QC) samples was always <5%.
Measurement of IMPDH Activity
IMPDH activity in mononuclear cells (MNCs) was determined according to the method described by Glander et al.20 Whole blood samples (2.5 mL) were collected in 4-mL Li-heparin-containing tubes and stored at room temperature (12-hour maximum). MNCs were isolated using Ficoll-Hypaque gradient centrifugation and lysed after being counted. At remote sites, LeucoSep tubes (Greiner Bio-one, Monroe, North Carolina) were allowed for MNC isolation after validation of this method versus Ficoll workup.33 The lysate was incubated with inosine 5'-monophosphate (IMP), and the product xantho-sine 5'-monophosphate (XMP) and intracellular adenosine 5'-monophosphate (AMP) were measured by reversed-phase HPLC (Synergi column, 4 μm Hydro-RP 80 Å 250 × 3.0 mm; Phenomenex) with UV detection (1100 Series, Agilent Technologies). The flow rate was 0.7 mL/min at a column temperature of 45°C. Detection was at 254 nm.
The method was reproducible over the working range of 90 to 4800 pmol/sample. Extraction efficiencies were between 96% and 100% over the working range. The LLOQ was 90 pmol/sample for both XMP and AMP. The LOD was 45 pmol/sample for XMP and 11 pmol/sample for AMP. Intra- and interday precision and accuracy for XMP and AMP quality control samples was between 0.5% and 6.7% throughout. Cross-validation was achieved by exchanging QC samples with the Glander laboratory.22 Enzyme activity was expressed as produced XMP (nmol) per time unit (h) per mg protein (nmol/h/ mg protein). To date, this activity measure has been used most frequently and is therefore reported here. For quality assurance purposes, enzyme activity was also expressed as produced XMP per pmol of measured intracellular AMP (XMP/AMP activity ratio).
Pharmacokinetic Analysis
Individual and mean pharmacokinetic parameter estimates for MPA and MPAG were generated using noncompartmental analysis (WinNonlin, Version 5.2, Pharsight Corporation, Mountain View, California). Parameters obtained included (1) actual and dose-normalized plasma MPA concentrations at each sampling time; (2) time to peak concentration (tmax); (3) actual and dose-normalized peak (Cmax), minimum (Cmin), and predose (Ctrough) concentrations; (4) actual and dose-normalized AUC (AUC0-12 h); and (5) oral clearance (CL/F) and weight-normalized CL/F. The extrapolated AUC0-12 h was estimated by using the method described by Hale et al18,34 as described before. CL/F was calculated using an adjusted MPA dose calculated from the MMF dose according to MPA dose = 0.739 · MMF dose, where 0.739 is the fractional difference in molecular mass between MPA and MMF.
Pharmacokinetic-Pharmacodynamic Modeling
The relationship between MPA concentrations and IMPDH inhibition was explored graphically by plotting all MPA concentration data (linear and log scale) versus IMPDH activity data. The MPA concentration-IMPDH activity relationship was described according to
where E0 is IMPDH baseline activity (no inhibition), Emax is the maximum IMPDH inhibition, C is MPA concentration, and EC50 is the concentration C when 50% of maximum IMPDH inhibition is reached. Correlations between MPA and IMPDH activity parameters were explored visually with nonlinear regression models by using GraphPad Prism 4.03 (GraphPad Software, La Jolla, California). The model fit was evaluated graphically with consideration of standard error (SE) and 95% confidence intervals.
Clinical outcomes
Clinical outcomes were assessed by reviewing the clinical information collected in the case report forms of all participating patients. Rejection episodes and side effects (diarrhea and leucopenia) observed during the course of the study were recorded and compared to MPA exposure and pre- and posttrans-plant levels of IMPDH activity/inhibition.
Statistical Analysis
Descriptive statistics were used to characterize the demographics of the patients. Noncategorical variables are presented as mean with standard deviation. Categorical variables were reported using frequency and proportions. Statistical significance was defined as P < .05. A 1-way analysis of variance (ANOVA) and Kruskal-Wallis test were used to compare MMF doses and PK parameter estimates among different sampling days. Separate analyses by paired t test and Wilcoxon signed-rank test were conducted for subgroups where appropriate. Between-subject and between-occasion variability, relationships between parameters, and influence of covariates were described visually and with statistical methods. The descriptive analyses were performed with MINITAB statistic software (Release 15.1.1.0, MINITAB Inc., State College, Pennsylvania) and GraphPad Prism for Windows (Version 4.03, GraphPad Software).
As the main objective of the study was to collect preliminary PK/PD data in children aged 2 years or older, determination of the sample size was not solely based on statistical power considerations.
Results
Enrollment and Immunosuppressive Regimen
A total of 28 pediatric patients were enrolled in the study from February 2006 through December 2008. Patient demographic data are summarized in Table I. One patient with severe growth retardation was enrolled as her developmental age was considered much younger than her actual age (20 years). Two patients withdrew from the study after the first day of sampling, 1 due to acute graft rejection and 1 for personal reasons. The PK/PD profiles of these 2 patients were analyzed with the data for the first sampling day. One patient with focal segmental glomerulosclerosis was temporarily switched to cyclosporine and excluded when she was on it. Despite efforts to recruit patients in the 6- to 12-year age group, we were not able to enroll more than 2, with 6 patients being enrolled in the youngest 2- to 5-year age category.
Table I.
Patient Demographics (n = 28)
| Mean (Number of Patients) | Standard Deviation | Range | |
|---|---|---|---|
| Age, y | 12.5 (28) | 5.3 | 2.1–20.2 |
| Age group, y | |||
| ≥2 and <6 | 3.5 (6) | 1.5 | 2.1–5.3 |
| ≥6 and <12 | 9.5 (2) | - | 8.3, 10.6 |
| ≥12 to 18 | 15.5 (20) | 2.0 | 12.3–20.2a |
| Height, cm | 140.5 (28) | 30.5 | 81.2–175.8 |
| Weight, kg | 44.8 (28) | 26.2 | 10.3–106.4 |
| Pharmacokinetic/pharmacodynamic evaluation day (days after transplant) | |||
| Early posttransplant | 2.4 (27) | 1.3 | 1–5 |
| Prior to discharge | 7.0 (25) | 3.0 | 3–17 |
| Stable period | 193 (19) | 138 | 81–611 |
| Creatinine clearance (mL/min per 1.73 m3) | |||
| Early posttransplant | 102 (27) | 67 | 14–243 |
| Prior to discharge | 116 (25) | 48 | 21–228 |
| Stable period | 100 (19) | 36 | 63–223 |
| Number of Patients | |||
| Gender (female/male) | 11/17 | ||
| Race (African American/ white) | 4/24 | ||
| Ethnicity (Hispanic/ non-Hispanic) | 2/26 | ||
| Donor type (living/cadaver) | 17/11 | ||
Parameter values are reported as means with standard deviations (SD) and ranges. Creatinine clearance (CrCL) was estimated with the Schwartz equation.30
One patient >18 years with developmental delay, both physically and mentally, was accepted for enrollment.
MMF dosage at the different sampling days is summarized in Table II. Two patients received intravenous MMF on the first day of PK sampling (on days 1 and 3 after their transplantation, respectively). The youngest patients (age <6 years; n = 8) received MMF suspension throughout the study. Three patients received MMF suspension via a nasogastric tube while in the hospital. In all other instances, MMF was administered orally as MMF (CellCept) capsules or tablets in combination with tacrolimus and steroids. Five patients were switched from tacrolimus to sirolimus (Rapamune) once stable.
Table II.
Mean Values, Ranges, and Standard Deviation for MPA Parameters
| Early Posttransplant
(1–3 Days) (n = 27)a
|
Prior to Discharge
(5–9 Days) (n = 25)a,b
|
Stable Period (Approximately
6 Months) (n = 19) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range |
Range |
Range |
||||||||||
| Mean | Min | Max | SD | Mean | Min | Max | SD | Mean | Min | Max | SD | |
| Age, y, at transplant | 12.3 | 2.1 | 20.2 | 5.3 | 12.3 | 2.1 | 20.2 | 5.5 | 13.0 | 2.1 | 18.7 | 5.1 |
| MMF dose, mg | 527 | 200 | 1000 | 267 | 542 | 200 | 1000 | 244 | 592 | 100 | 1500 | 316 |
| MPA | ||||||||||||
| Cmax, mg/L | 5.4 | 0.7 | 13.4 | 3.7 | 8.1 | 1.8 | 20.3 | 4.7 | 11.0 | 3.5 | 30.2 | 6.8 |
| Cmin, mg/L | 0.6 | 0.0 | 1.4 | 0.4 | 0.7 | 0.1 | 2.4 | 0.5 | 1.5 | 0.3 | 4.3 | 1.0 |
| Ctrough, mg/L | 1.0 | 0.1 | 3.0 | 0.8 | 1.2 | 0.2 | 5.0 | 1.0 | 2.3 | 0.3 | 8.0 | 2.0 |
| tmax, h | 2.8 | 0.03 | 12.0 | 3.2 | 1.6 | 0.4 | 4.0 | 1.2 | 1.5 | 0.2 | 6.0 | 1.5 |
| AUC0-12 h, h·mg/L | 20.7 | 4.0 | 48.1 | 12.1 | 25.2 | 10.2 | 44.5 | 8.9 | 41.6 | 11.8 | 91.8 | 21.8 |
| CL/F, L/h | 26.7 | 6.4 | 140 | 26.6 | 16.4 | 6.5 | 28.5 | 5.8 | 13.4 | 3.5 | 62.8 | 13.2 |
| MPAG | ||||||||||||
| Ctrough, mg/L | 29 | 4 | 82 | 21 | 24 | 2 | 72 | 17 | 26 | 5 | 51 | 13 |
| Cmax, mg/L | 49 | 9 | 106 | 27 | 56 | 19 | 114 | 26 | 52 | 24 | 86 | 19 |
| tmax, h | 3.4 | 0.12 | 9.1 | 2.8 | 2.5 | 0.62 | 6.0 | 1.3 | 2.3 | 1.0 | 6.0 | 1.6 |
Parameter values are reported as means with standard deviations (SD) and ranges. MPA, mycophenolic acid; MMF, mycophenolate mofetil; MPAG, MPA-glucuronide; Cmax (Cmin), maximum (minimum) concentration of MPA or MPAG observed after the MMF dose; Ctrough, predose concentration of MPA or MPAG; tmax, time after dose when Cmax is reached; AUC0-12 h, calculated with the trapezoidal method (missing data points acquired with the method according to Hale et al18); CL/F, calculated as AUC0-12 h/adjusted MPA dose.
One patient was excluded because of taking cyclosporine at the moment of sampling (for first 2 sampling days).
Two patients were dropped from this study because of rejection and personal reasons after the first sampling day.
Pharmacokinetic Data
Table II summarizes the number of PK/PD profiles obtained across the different sampling days. A total of 620 blood samples were available for PK analysis. For the final evaluation, 71 PK (MPA and MPAG) and IMPDH activity (PD) profiles obtained in 28 patients were available: 27 PK/PD profiles early posttransplant (1–3 days posttransplant), 25 at discharge from the hospital, and 19 in the maintenance phase (approximately 6 months posttransplant).
Large interpatient PK variability was observed early posttransplant, with concentrations throughout the dosing interval and total exposure remaining rather low (mean AUC 20.7 mg·h/L; range, 4.0–48.1; Figure 1A and Table II). Over time, MPA exposure gradually increased (mean AUC 25.2 and 41.6 mg·h/L, respectively; Figure 1B,C and Table II). The MPA concentration-time profiles in this pediatric population exhibited both single peak and multiple peak profiles with early or later secondary peaks likely due to enterohepatic recycling. Substantial intrapatient variability was noted in the individual PK profiles (eg, changes and delay in absorption and/or appearance of secondary peaks) early post-transplant that stabilized over time.
Figure 1.
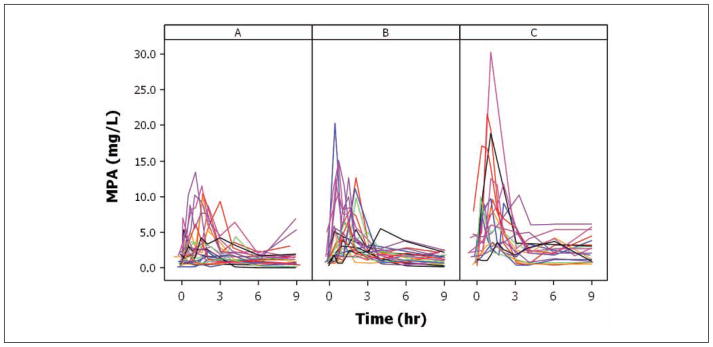
Individual mycophenolic acid (MPA) concentration-time profiles at the 3 sampling days following oral administration are shown in the separate panels: (A) early posttransplant (n = 27), (B) prior to discharge (n = 25), and (C) at stable mycophenolate mofetil (MMF) treatment (n = 19). Individual MPA concentration-time profiles at the 3 sampling days following oral administration are shown in the separate panels A, B, and C. A tendency of reduced complexity and increased concentrations is observed over time after transplantation.
Pharmacokinetic parameters such as Cmax, Cmin, Ctrough, tmax, AUC0-12 h, and CL/F are summarized in Table II. In most of our patients, MPA and MPAG concentration-time profiles were in parallel with a short delay in the occurrence of the MPAG peak concentration. In several patients, low MPA as well as low MPAG concentrations were observed suggestive of low bioavailability.
IMPDH Activity
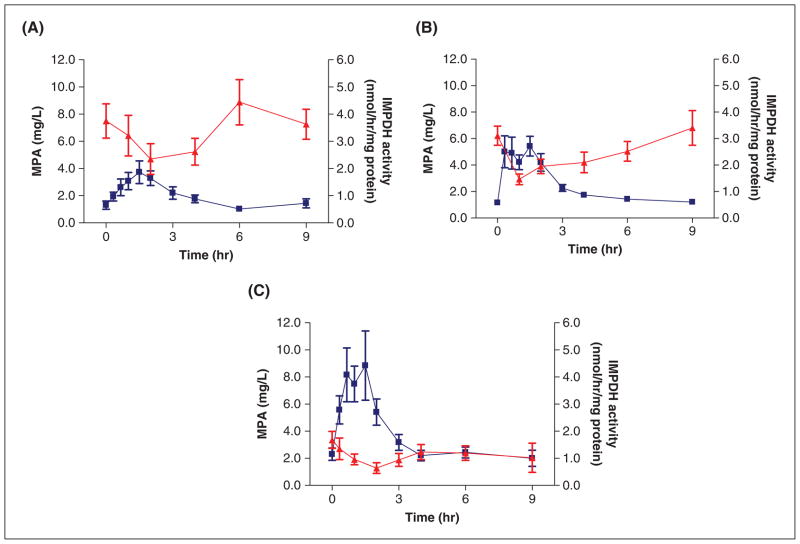
Out of a total of 377 PD samples from 28 patients, 285 IMPDH activity measurements were available for analysis. Twenty-one individual PD profiles were available from 1 to 3 days posttransplant, 19 from 5 to 9 days posttransplant, and 13 from approximately 6 months posttransplant. Reasons for having to exclude samples were low white blood cell count (<1.0 × 106/mL) due to thymoglobulin induction and, in several cases, stability issues during transport that interfered with reliable IMPDH activity measurement. In the 3- to 6-month evaluation group, 4 patients were lost for final follow-up. IMPDH activity profiles expressed as XMP/AMP activity ratio or as nmol/h/mg protein were very similar as judged from individual graphs and mean values. Mean IMPDH activity and MPA concentration profiles at each consecutive sampling day are shown in Figure 2. IMPDH activity was inversely related to MPA plasma concentration, with the highest level of inhibition associated with the highest concentration (Cmax), after which activity returned to near predose values. Over time, the overall IMPDH inhibition curve shifted downward as a result of increasing MPA exposure. Table III summarizes IMPDH activity, including baseline (pre-transplant), predose, and nadir values. In our pediatric patients, mean baseline IMPDH activity was 6.4 nmol/h/mg protein and revealed large interpatient variability (range, 1.0–18.4 nmol/h/mg protein). Pretransplant (baseline) IMPDH activity measurements could not be obtained in all patients as several were started on MMF at home 1 or more days prior to transplantation. As judged by the IMPDH inhibition profiles (Figure 2) and by the mean percentage inhibition (Table III), pharmacodynamic effects were not as pronounced very early after transplantation as compared to predischarge and several months post-transplant. Mean IMPDH inhibition ranged from 53% early posttransplant to 65% and 62%, at discharge and steady treatment, respectively, and revealed large interpatient differences.
Figure 2.
Mean mycophenolic acid (MPA) concentration versus inosine monophosphate dehydrogenase (IMPDH) activity on the 3 sampling days (A) early posttransplant (n = 27), (B) prior to discharge (n = 25), and (C) at stable mycophenolate mofetil (MMF) treatment (n = 19). MPA concentration is shown as squares and IMPDH activity as triangles. Bars indicate standard error of the mean (SEM).
Table III.
Pre- and Posttransplant IMPDH Activity Parameters Obtained, Expressed as nmol/h/mg Protein
| Pretransplant (Baseline
Before Dosing) |
Early Posttransplant
(1–3 Days) |
Prior to Discharge
(5–9 Days) |
Stable Period (Approximately
6 Months) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 19) | (n = 21) | (n = 19) | (n = 13) | |||||||||||||
| Range |
Range |
Range |
Range |
|||||||||||||
| Mean | Min | Max | SD | Mean | Min | Max | SD | Mean | Min | Max | SD | Mean | Min | Max | SD | |
| Age at transplant, y | 12.4 | 2.1 | 20.2 | 5.3 | 13.5 | 4.1 | 20.2 | 4.5 | 13.6 | 4.1 | 20.2 | 4.5 | 15.1 | 10.6 | 18.7 | 2.1 |
| Age group: ≥2 and <6/≥6 and <12/≥12 to 18 years | 4:2:13 | 3:1:17 | 3:0:16 | 0:1:12 | ||||||||||||
| Predose IMPDH activity, nmol/h/mg protein | 6.4 | 1.0 | 18.4 | 4.6 | 4.1 | 0.3 | 13.1 | 2.8 | 3.1 | 0.8 | 7.0 | 1.6 | 1.7 | 0.1 | 3.4 | 1.1 |
| Nadir IMPDH activity, nmol/h/mg proteina | 1.9 | 0.1 | 5.4 | 1.7 | 1.1 | 0.1 | 3.1 | 0.8 | 0.5 | 0.1 | 1.3 | 0.4 | ||||
| % Inhibition/ predose | 53 | 0 | 82 | 28 | 65 | 19 | 91 | 18 | 62 | 0 | 90 | 27 | ||||
| (n = 13) | (n = 12) | (n = 9) | ||||||||||||||
| % Inhibition/ baseline | 65 | –34 | 93 | 40 | 84 | 64 | 97 | 11 | 87 | 46 | 99 | 17 | ||||
Parameter values are reported as means with standard deviations (SD) and ranges. IMPDH, inosine monophosphate dehydrogenase.
Minimum IMPDH activity is defined as the lowest IMPDH activity observed after administration of a dose of mycophenolate mofetil.
Pharmacokinetic-Pharmacodynamic Data and Modeling
As illustrated by Figure 3, early posttransplant, 23 of 27 patients had AUCs below the lower consensus target for optimal effect of 30 mg·h/L. Increased AUC and trough levels, as a result of decreasing CL/F, were observed over time after transplantation, whereas between-subject variation in exposure (AUC) also decreased over time.
Figure 3.
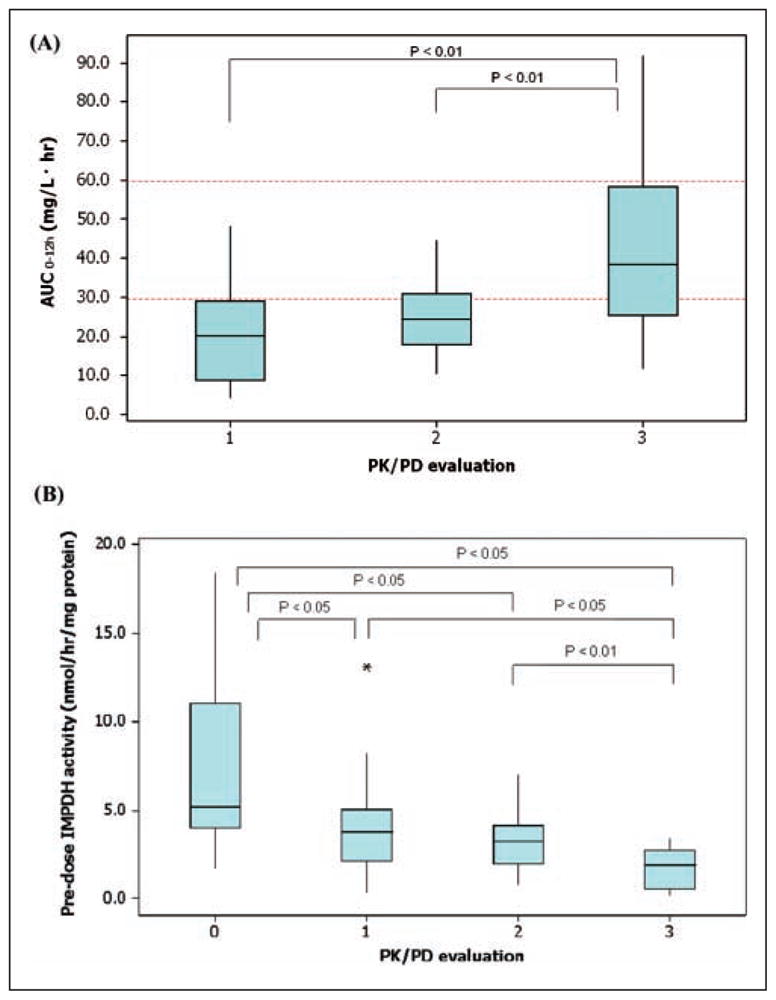
Box plots of mycophenolic acid (MPA) exposure as measured by the AUC with the current target range (dotted lines)12–15 (A) and the effect as measured by inosine monophosphate dehydrogenase (IMPDH) activity and inhibition (B) over time: (0) pretransplant, (1) early posttransplant, (2) prior to discharge, and (3) at stable mycophenolate mofetil (MMF) treatment. The bottom and top of the box represent the 25th and 75th percentiles, respectively. The 50th percentile (median) is represented by the band near the middle of the box. The ends of the whiskers represent the minimum and maximum data points. Data points represented by a star are considered outliers, defined as outside 1.5 interquartile range of the highest value of all values except the outlier.
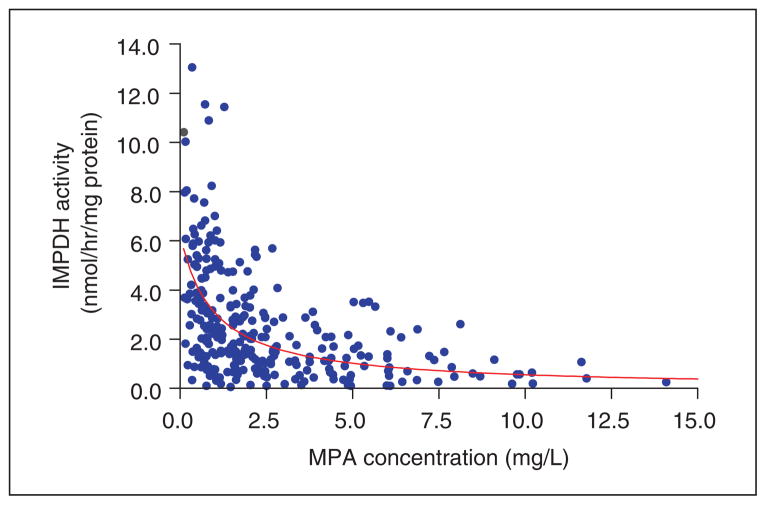
IMPDH enzyme activity decreased with increasing MPA plasma concentration, with maximum inhibition coinciding with maximum MPA concentration indicating a direct effect relationship (Figure 3). When analyzing all 285 PK/PD data points, the IMPDH-MPA concentration effect relationship could be well described by an inhibitory direct effect Emax model (Figure 4). This relationship was stable over time as judged by comparing EC50 estimates at each separate study day. The final model-based EC50 estimate was 0.97 mg/mL (SE 0.22; 95% confidence interval [CI]: 0.55–1.41 mg/L).
Figure 4.
The concentration-response relationship could be described by an inhibitory direct effect Emax model. The E0 and EC50 estimates were 6.2 nmol/h/mg protein (SE 0.65; 95% confidence interval [CI]: 4.9–7.5) and 0.97 mg/mL (SE 0.22; 95% CI: 0.55–1.41 mg/L), respectively, when Emax was fixed (value of 0) and is based here on 285 appropriately matched mycophenolic acid–inosine monophosphate dehydrogenase (MPA-IMPDH) data pairs out of 377 measurements. The mean observed baseline IMPDH activity of 6.4 nmol/h/mg protein (Table III) was close to the model-estimated E0.
Clinical outcomes
Although our study was not specifically designed to track outcomes, acute rejection and adverse event data were collected throughout. One patient who had received a deceased donor kidney lost his transplant 5 days posttransplant associated with histo-logical evidence of acute rejection and in the context of substantial MPA underexposure: immediately posttransplant, he had high IMPDH activity (~6.0–10.8 nmol/h/mg protein) and a low MPA AUC (<5.0 mg·h/L) despite a daily MMF dose of 1200 mg/m2. He also received induction therapy (basiliximab 20 mg) and a combination with tacrolimus 5 mg bid (trough level 8.0 ng/mL) and prednisone (60 mg/d). Another patient experienced an acute rejection after discharge before the last sampling day due to nonadherence. MPA therapy was stopped in 1 patient due to severe diarrhea, whereas in 6 patients, dose reduction was necessary due to leucopenia. No clear relation to PK/PD data could be detected in these cases.
Discussion
This study is the first to document the pharmacokinetics of MPA in relation to inhibition of the pharmacological target enzyme IMPDH in pediatric kidney transplant recipients. IMPDH activity assessment proved feasible and provided an attractive pharmacodynamic marker of MPA-induced immunosuppression at the lymphocyte level. As reported by other investigators, we also observed large intra- and interpatient variability in MPA PK as a function of time after transplantation.3,35,36 Variability was especially pronounced in the first week after transplantation and, as expected, not preventable by body surface area–based dosing.37,38 This might point toward a special need for early monitoring. As illustrated in Figures 1 to 3 and indicated in Table II, the complexity in MPA concentration-time profiles (eg, delayed Cmax or existence of pronounced secondary peaks) decreases and MPA exposure increases over time after transplantation. This gradual increase in AUC0-12 h can be explained by a transient decrease in MPA clearance, as has been reported in adult patients.39 The reason for this gradual decrease in clearance is not fully understood, but improving graft function, a rise in albumin concentrations, and tapering of the corticosteroid dose are all considered contributing factors.17,40 Of clinical importance is the observation that current dosing recommendations (1200 mg/m2/d of MMF in a cyclosporine-based regimen) lead to MPA underexposure early posttransplant in many patients.3 This finding is in agreement with recent observations by Filler et al,41 who have recommended higher starting doses of 1800 mg/m2/d when combined with cyclosporine and 1200 mg/m2/d with tacrolimus in older children that are now being implemented at our institution.
The within- and between-patient differences in the rate and extent of absorption and occurrence of reabsorption and secondary peaks after the initial absorption phase resulted in large variability in MPA exposure. Capturing this variability using therapeutic drug monitoring represents a major challenge in the clinical management of these patients because several blood samples drawn over a number of hours would be required to determine accurate AUCs.
Moreover, no true mechanism-based approaches that would accurately predict the absorption and likelihood of enterohepatic recycling (EHC) are currently available. The double-peak phenomenon can in part be explained by the degree of EHC. EHC is most likely influenced by the interplay between UGT drug-metabolizing enzymes, transporters such as MRP2 and P-glycoprotein, and bile physiology.42 Because these proteins play a key role in the handling of MPA, a physiological modeling approach may provide insight into these important contributing factors.43,44 In addition, study of underlying developmental changes and pharmacogenetic differences resulting in different phenotypes will further aid in these efforts. Several single nucleotide polymorphisms have been associated with differences in MPA clearance45–47 and can be used in prospective dosing.
Similar to PK variability, the present study revealed large interpatient variability in PD response (Figures 2–4). Mean predose IMPDH activity changed from 4.1 to 3.1 and 1.6 nmol/h/mg protein, respectively, at the different sampling occasions. IMPDH inhibition paralleled MPA plasma concentration and exposure. Accordingly, a direct effect Emax model was applied to describe the concentration-effect relationship. The EC50 value of 1.0 mg/L is well aligned with the clinically established predose (trough) concentration target range of 1.0 to 3.5 mg/L.15,48 The EC50 is also in line with a recently reported value of 1.6 mg/L using a similar modeling approach.26 This relationship was constant over time, with EC50 values ranging from 1.0 to 1.5 mg/L at the different evaluation days. Interestingly, the IMPDH activity data revealed what might be considered clinically relevant differences in baseline activity predictive of susceptibility and response. Our data showed a fairly wide range in baseline IMPDH activity in our population (range, 1.0–18.4). Of note, IMPDH activity in our pediatric population appears significantly lower than baseline IMPDH activity in 79 adult kidney transplant recipients (9.6 ± 4.4 nmol/h/mg protein) measured with an almost identical IMPDH assay method.24 Inspection of individual MPA-IMPDH profiles indicated that in patients with lower IMPDH activity, less MPA exposure would suffice for equivalent IMPDH inhibition as compared to patients who exhibited higher initial IMPDH activity. The observed mean percentage IMPDH inhibition was 53% directly after transplant and increased to 67% and 62% at discharge and steady treatment, respectively. These values are in concordance with the mean maximum inhibition of 67% as observed in 21 adult kidney transplant recipients.49
During the course of the study, we encountered 1 patient experiencing graft rejection 1 week post-transplant who had high IMPDH activity and low MPA exposure. Previously, it has been suggested that baseline IMPDH activity could be predictive of clinical outcome, with low and high initial IMPDH activity predisposing for toxicity and graft rejection, respectively.24 Recently, it was also demonstrated that higher IMPDH activity over 12 hours on day 6 posttransplant could be a risk factor for more acute rejection together with the observation of low IMPDH messenger RNA expressions predose on day 6 and high IMPDH mRNA pretransplant in patients with acute rejection.50 Based on these initial results, it is tempting to postulate that baseline IMPDH activity and/or mRNA in children may serve as an initial marker to guide the level and aggressiveness of required MPA exposure.
Conclusion
This study provides preliminary data exploring the potential utility of combining PK and IMPDH biomarker data to optimize MMF therapy. Our results suggest the importance of early monitoring of MPA PK and IMPDH inhibition to improve drug exposure and outcomes.
Baseline IMPDH activity in children may serve as a potential clinical marker to guide the level of required MPA exposure. Given the complexities of PK-assisted dosing, a combined PK/PD target strategy may provide an attractive new approach for tailoring MMF therapy to individual needs in pediatric renal transplant recipients that warrants further study.
Acknowledgments
This work was supported by NIH grants 5U10HD037249 (TF, DM, AAV) and 5K24HD050387 (AAV) and an investigator-initiated research grant from Roche Laboratories. We thank colleagues Hasan Jafri, MD, George McCracken, MD, and Robert Ward, MD, and their staff in the Pediatric Pharmacology Research Unit network for their support. The technical assistance of Maurits de Rotte, PharmD, in developing the IMPDH assay is gratefully acknowledged.
Parts of this work were presented in abstract form at Clinical Pharmacology and Nephrology meetings in 2007 and 2008.
AAV was a recipient of an investigator-initiated research grant from Roche Laboratories, Inc (Nutley, New Jersey). The sponsor reviewed and approved the manuscript.
References
- 1.Filler G, Zimmering M, Mai I. Pharmacokinetics of nolate mofetil are influenced by concomitant immunosuppression. Pediatr Nephrol. 2000;14:100–104. doi: 10.1007/s004670050021. [DOI] [PubMed] [Google Scholar]
- 2.Oellerich M, Shipkova M, Schutz E, et al. Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit. 2000;22:20–26. doi: 10.1097/00007691-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Weber LT, Hoecker B, Armstrong VW, Oellerich M, Tönshoff B. Long-term pharmacokinetics of mycophenolic acid in pediatric renal transplant recipients over 3 years posttransplant. Ther Drug Monit. 2008;30:570–575. doi: 10.1097/FTD.0b013e31818752d9. [DOI] [PubMed] [Google Scholar]
- 4.Allison AC, Kowalski WJ, Muller CD, Eugui EM. Mechanisms of action of mycophenolic acid. Ann N Y Acad Sci. 1993;696:63–87. doi: 10.1111/j.1749-6632.1993.tb17143.x. [DOI] [PubMed] [Google Scholar]
- 5.Fulton B, Markham A. Mycophenolate mofetil: a review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996;51:278–298. doi: 10.2165/00003495-199651020-00007. [DOI] [PubMed] [Google Scholar]
- 6.North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Annual Report 2008. https://web.emmes.com/study/ped/annlrept/annlrept.html.
- 7.Ettenger R, Cohen A, Nast C, Moulton L, Marik J, Gales B. Mycophenolate mofetil as maintenance immunosuppression in pediatric renal transplantation. Transplant Proc. 1997;29:340–341. doi: 10.1016/s0041-1345(96)00296-5. [DOI] [PubMed] [Google Scholar]
- 8.CellCept[prescribing information] Basel, Switzerland: Roche; 2009. http://www.rocheusa.com/products/cellcept/ [Google Scholar]
- 9.Filler G, Feber J, Lepage N, Weiler G, Mai I. Universal approach to pharmacokinetic monitoring of immunosuppressive agents in children. Pediatr Transplant. 2002;6:411–418. doi: 10.1034/j.1399-3046.2002.02039.x. [DOI] [PubMed] [Google Scholar]
- 10.Weber LT, Shipkova M, Armstrong VW, et al. The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: a report of the German Study Group on Mycophenolate Mofetil Therapy. J Am Soc Nephrol. 2002;13:759–768. doi: 10.1681/ASN.V133759. [DOI] [PubMed] [Google Scholar]
- 11.David-Neto E, Pereira Araujo LM, Sumita NM, et al. Mycophenolic acid pharmacokinetics in stable pediatric renal transplantation. Pediatr Nephrol. 2003;18:266–272. doi: 10.1007/s00467-002-1057-1. [DOI] [PubMed] [Google Scholar]
- 12.van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261–266. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 13.Cox VC, Ensom MH. Mycophenolate mofetil for solid organ transplantation: does the evidence support the need for clinical pharmacokinetic monitoring? Ther Drug Monit. 2003;25:137–157. doi: 10.1097/00007691-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Le Meur Y, Buchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 15.van Gelder T, Silva HT, de Fijter JW, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation. 2008;86:1043–1051. doi: 10.1097/TP.0b013e318186f98a. [DOI] [PubMed] [Google Scholar]
- 16.Shaw LM, Korecka M, Venkataramanan R, Goldberg L, Bloom R, Brayman KL. Mycophenolic acid pharmacodynamics and pharmacokinetics provide a basis for rational monitoring strategies - Am J Transplant. 2003;3:534–542. doi: 10.1034/j.1600-6143.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 17.van Hest RM, van Gelder T, Bouw R, et al. Time-dependent clearance of mycophenolic acid in renal transplant recipients. Br J Clin Pharmacol. 2007;63:741–752. doi: 10.1111/j.1365-2125.2006.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic-pharmacodynamic relationship for myco-phenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998;64:672–683. doi: 10.1016/S0009-9236(98)90058-3. [DOI] [PubMed] [Google Scholar]
- 19.Pape L, Ehrich JH, Offner G. Long-term follow-up of pediatric transplant recipients: mycophenolic acid trough levels are not a good indicator for long-term graft function. Clin Transplant. 2004;18:576–579. doi: 10.1111/j.1399-0012.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Glander P, Braun KP, Hambach P, et al. Non-radioactive determination of inosine 5'-monophosphate dehydrogenase (IMPDH) in peripheral mononuclear cells. Clin Biochem. 2001;34:543–549. doi: 10.1016/s0009-9120(01)00267-3. [DOI] [PubMed] [Google Scholar]
- 21.Vethe NT, Bergan S. Determination of inosine monophosphate dehydrogenase activity in human CD4+ cells isolated from whole blood during mycophenolic acid therapy. Ther Drug Monit. 2006;28:608–613. doi: 10.1097/01.ftd.0000245680.38143.ca. [DOI] [PubMed] [Google Scholar]
- 22.Glander P, Sombogaard F, Budde K, et al. Improved assay for the nonradioactive determination of inosine 5'-monophos-phate dehydrogenase activity in peripheral blood mononu-clear cells. Ther Drug Monit. 2009;31:351–359. doi: 10.1097/FTD.0b013e31819c3f3d. [DOI] [PubMed] [Google Scholar]
- 23.Budde K, Glander P, Braun KP, et al. Pharmacodynamic monitoring of mycophenolate mofetil in renal allograft recipients. Transplant Proc. 2001;33:3313–3315. doi: 10.1016/s0041-1345(01)02407-1. [DOI] [PubMed] [Google Scholar]
- 24.Glander P, Hambach P, Braun KP, et al. Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant. 2004;4:2045–2051. doi: 10.1111/j.1600-6143.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 25.Weimert NA, Derotte M, Alloway RR, et al. Monitoring of ino-sine monophosphate dehydrogenase activity as a biomarker for mycophenolic acid effect: potential clinical implications. Ther Drug Monit. 2007;29:141–149. doi: 10.1097/FTD.0b013e31803d37b6. [DOI] [PubMed] [Google Scholar]
- 26.Kamar N, Glander P, Nolting J, et al. Pharmacodynamic evaluation of the first dose of mycophenolate mofetil before kidney transplantation. Clin J Am Soc Nephrol. 2009;4:936–942. doi: 10.2215/CJN.04860908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hocker B, Weber LT, Feneberg R, et al. Prospective, randomized trial on late steroid withdrawal in pediatric renal transplant recipients under cyclosporine microemulsion and mycophenolate mofetil. Transplantation. 2009;87:934–941. doi: 10.1097/TP.0b013e31819b6d4a. [DOI] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration. Guidance for Industry: E11 Clinical Investigation of Medicinal Product in the Pediatric Population. Washington, DC: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CBER); 2000. [Google Scholar]
- 29.Premaud A, Le Meur Y, Debord J, et al. Maximum a posteriori Bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit. 2005;27:354–361. doi: 10.1097/01.ftd.0000162231.90811.38. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 31.Cox S, Cabovska B, Vinks AA. Determination of mycophe-nolic acid and mycophenolic acid glucuronide in human plasma using solid phase extraction and HPLC. Ther Drug Monit. 2005;27:215–216. [Google Scholar]
- 32.Shipkova M, Niedmann PD, Armstrong VW, Niedmann PD, Oellerich M, Wieland E. Simultaneous determination of mycophenolic acid and its glucuronide in human plasma using a simple high-performance liquid chromatography procedure. Clin Chem. 1998;44:1481–1488. [PubMed] [Google Scholar]
- 33.Nilsson C, Aboud S, Karlen K, Hejdeman B, Urassa W, Biberfeld G. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin Vaccine Immunol. 2008;15:585–589. doi: 10.1128/CVI.00161-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Hest RM, Mathot RA, Vulto AG, Ijzermans JN, van Gelder T. Within-patient variability of mycophenolic acid exposure: therapeutic drug monitoring from a clinical point of view. Ther Drug Monit. 2006;28:31–34. doi: 10.1097/01.ftd.0000194504.62892.b2. [DOI] [PubMed] [Google Scholar]
- 35.Bunchman T, Navarro M, Broyer M, et al. The use of mycophenolate mofetil suspension in pediatric renal allograft recipients. Pediatr Nephrol. 2001;16:978–984. doi: 10.1007/s004670100006. [DOI] [PubMed] [Google Scholar]
- 36.Ettenger R, Sarwal MM. Mycophenolate mofetil in pediatric renal transplantation. Transplantation. 2005;80(suppl):S201–S210. doi: 10.1097/01.tp.0000186957.32801.c0. [DOI] [PubMed] [Google Scholar]
- 37.Shaw LM, Figurski M, Milone MC, Trofe J, Bloom RD. Therapeutic drug monitoring of mycophenolic acid. Clin J Am Soc Nephrol. 2007;2:1062–1072. doi: 10.2215/CJN.03861106. [DOI] [PubMed] [Google Scholar]
- 38.Filler G. Value of therapeutic drug monitoring of MMF therapy in pediatric transplantation. Pediatr Transplant. 2006;10:707–711. doi: 10.1111/j.1399-3046.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 39.Shaw LM, Kaplan B, DeNofrio D, et al. Pharmacokinetics and concentration-control investigations of mycophenolic acid in adults after transplantation. Ther Drug Monit. 2000;22:14–19. doi: 10.1097/00007691-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Naesens M, de Loor H, Vanrenterghem Y, et al. The impact of renal allograft function on exposure and elimination of mycophenolic acid (MPA) and its metabolite MPA 7-O-glucuronide. Transplantation. 2007;84:362–373. doi: 10.1097/01.tp.0000276936.14041.6c. [DOI] [PubMed] [Google Scholar]
- 41.Filler G, Bendrick-Peart J, Christians U. Pharmacokinetics of mycophenolate mofetil and sirolimus in children. Ther Drug Monit. 2008;30:138–142. doi: 10.1097/FTD.0b013e31816ba73a. [DOI] [PubMed] [Google Scholar]
- 42.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmaco-dynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46:13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 43.Jamei M, Marciniak S, Feng K, et al. The Simcyp® population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5:211–223. doi: 10.1517/17425250802691074. [DOI] [PubMed] [Google Scholar]
- 44.Bolger MB, Lukacova V, Woltosz WS. Simulations of the nonlinear dose dependence for substrates of influx and efflux transporters in the human intestine. AAPS J. 2009;11:353–363. doi: 10.1208/s12248-009-9111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuypers DR, Naesens M, Vermeire S, et al. The impact of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther. 2005;78:351–361. doi: 10.1016/j.clpt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Baldelli S, Merlini S, Perico N, et al. C-440T/T-331C polymorphisms in the UGT1A9 gene affect the pharmacokinetics of mycophenolic acid in kidney transplantation. Pharmacogenomics. 2007;8:1127–1141. doi: 10.2217/14622416.8.9.1127. [DOI] [PubMed] [Google Scholar]
- 47.Levesque E, Delage R, Benoit-Biancamano MO, et al. The impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther. 2007;81:392–400. doi: 10.1038/sj.clpt.6100073. [DOI] [PubMed] [Google Scholar]
- 48.Shaw LM, Holt DW, Oellerich M, Meiser B, van Gelder T. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit. 2001;23:305–315. doi: 10.1097/00007691-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Budde K, Glander P, Kramer BK, et al. Conversion from myco-phenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic outcomes. Transplantation. 2007;83:417–424. doi: 10.1097/01.tp.0000251969.72691.ea. [DOI] [PubMed] [Google Scholar]
- 50.Sombogaard F, Peeters AM, Baan CC, et al. Inosine monophos-phate dehydrogenase messenger RNA expression is correlated to clinical outcomes in mycophenolate mofetil-treated kidney transplant patients, whereas inosine monophosphate dehydrogenase activity is not. Ther Drug Monit. 2009;31:549–556. doi: 10.1097/FTD.0b013e3181b7a9d0. [DOI] [PubMed] [Google Scholar]