Summary
The present study was undertaken to evaluate the hypothesis that the antihypertensive action of soluble epoxide hydrolase (sEH) inhibition is mediated by an increased availability in intrarenal epoxyeicosatrienoic acids (EETs) with consequent improvement of renal haemodynamic autoregulatory efficiency and of the pressure-natriuresis relationship.
Ren-2 transgenic rats (TGR), a model of angiotensin II (ANG II)-dependent hypertension, and normotensive transgene-negative Hannover Sprague-Dawley (HanSD) rats were treated with the sEH inhibitor cis-4-[4-(3-adamantan-1-yl-ureido)cyclohexyloxy]benzoic acid (c-AUCB) for 48 hours. The effects on blood pressure (BP), autoregulation of renal blood flow (RBF) and glomerular filtration rate (GFR) and on the pressure-natriuresis relationship in response to stepwise reductions in renal arterial pressure (RAP) were determined.
Treatment with c-AUCB did not significantly change BP, renal autoregulation or pressure-natriuresis in normotensive HanSD rats. In contrast, treatment with c-AUCB significantly reduced BP, increased intrarenal bioavailability of EETs, significantly suppressed ANG II levels in TGR. However, treatment with c-AUCB did not significantly improve the autoregulatory efficiency of RBF and GFR in response to reductions of RAP and to restore the blunted pressure-natriuresis relationship in TGR.
Taken together, our present data indicate that antihypertensive actions of sEH inhibition in TGR are predominantly mediated via significant suppression of the intrarenal renin-angiotensin system activity.
Keywords: hypertension, pressure-natriureis, renal blood flow, glomerular filtration rate, cytochrome P450 metabolites, epoxyeicosatrienoic acids, soluble epoxide hydrolase, renin-angiotensin system
INTRODUCTION
Although the development of hypertension in the Ren-2 renin transgenic rat strain (TGR) is clearly related to the insertion of a mouse Ren-2 renin gene into the genome of normotensive Hannover Sprague-Dawley (HanSD) rats, the exact pathophysiological mechanism(s) responsible for the development and maintenance of hypertension in this monogenetic model of hypertension remain still unknown1. We and others have found that plasma and kidney angiotensin II (ANG II) levels are elevated in conscious heterozygous TGR during the developmental phase of hypertension when compared with age-matched HanSD rats2-4. In addition, it has been demonstrated that TGR also exhibit an enhanced peripheral and renal vascular and tubular responsiveness to ANG II as compared with HanSD rats5,6. Although these findings suggest that the inappropriately activated circulating and intrarenal renin-angiotensin system (RAS) is a critical mechanism responsible for the development of hypertension in this model, recent findings have indicated that the disturbed interaction of the RAS with other vasoactive systems instead of an isolated activation of the RAS might play a crucial role in the pathophysiology of hypertension in this model7-10.
According to this concept, special attention has been addressed to cytochrome P450 (CYP)-dependent metabolites including epoxyeicosatrienoic acids (EETs), because an increasing body of evidence indicates that lipid mediators play an important role in the regulation of renal tubular ion transport and renal and systemic vascular tone11-13. It has been proposed that EETs serve as a compensatory system with protective effects against enhanced RAS activity13-15. In addition, it has been recently demonstrated that 2K1C Goldblatt hypertensive rats and Cyp1a1-Ren-2 transgenic rats, two different models of ANG II-dependent hypertension that depend on the enhanced endogenous activity of the RAS, exhibit reduced intrarenal availability of EETs. This is the result of increased conversion of EETs to biologically inactive dihydroxyeicosatrienoic acids (DHETEs) by increased activity of soluble epoxide hydrolase (sEH)16-18. Moreover, it has been shown that chronic pharmacological blockade of sEH in these models results in an antihypertensive action that is associated with increased intrarenal availability of EETs and an improvement of renal hemodynamic autoregulatory efficiency and of the pressure-natriuresis relationship17,18. Since TGR reveal a well-documented impairment of the autoregulation of renal haemodynamic and pressure-natriuresis relationship19,20 and in view of our recent finding that TGR exhibit a reduced tissue availability of EETs21, we hypothesized that inhibition of sEH may exhibit an antihypertensive action that is mediated by an increase in EETs bioavailability with consequent improvement of renal haemodynamic autoregulation and of the impaired pressure-natriuresis relationship.
To test this hypothesis, we evaluated the effects of treatment with the sEH inhibitor cis-4-[4-(3-adamantan-1-yl-ureido)cyclohexyloxy]benzoic acid (c-AUCB) on blood pressure (BP) and on the autoregulation of renal haemodynamic and the pressure-natriuresis relationship in adult TGR with established hypertension.
Furthermore, to gain a more detailed insight into the role of intrarenal interactions of CYP-derived metabolites with the RAS in the regulation of renal function, we determined the renal concentrations of ANG II, EETs, DHETEs and 20-hydroxyeicosatrienoic acids (20-HETE) in untreated and c-AUCB-treated TGR and HanSD rats. In addition, the protein expression of sEH as well as of CYP2C3 enzyme that is the enzyme predominantly responsible for EETs’ formation in the kidney was assessed.
Methods
Ethical approval and animals
The studies were performed in accordance with guidelines and practices established by the Animal Care and Use Committee of the Institute for Clinical and Experimental Medicine, Prague, which are in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. All animals used in the present study were bred at the Center of Experimental Medicine of this Institute, from stock animals supplied by the Max Delbrück Center for Molecular Medicine, Berlin, Germany, which is accredited by the Czech Association for Accreditation of Laboratory Animal Care. Heterozygous TGR were generated by breeding male homozygous TGR with female homozygous HanSD rats as described and justified in the original study1 and age-matched HanSD rats served as transgene-negative normotensive controls. The animals were kept on a 12-hour/12-hour light/dark cycle. Throughout the experiments rats were fed a normal salt, normal protein diet (0.45% NaCl, 19-21% protein) produced by SEMED (Prague, Czech Republic) and had free access to tap water.
Chemicals
The sEH inhibitor c-AUCB was given in drinking water prepared freshly as described previously16-18 at the dose of 26 mg/L. Briefly, the crystalline c-AUCB (26 mg) was dissolved in ethanol (5 ml) and (2-hydroxyprolyl)-ß-cyclodextrin (150 mg) after 5 min sonication and this solution was added to one liter of tap water. Hydrogen carbonate sodium (3 ml/L) was given to ensure that the water did not become acidic since low pH can cause the compound to precipitate. This dose of c-AUCB was used in our recent studies and we found that it exhibited maximal antihypertensive action and substantially increased tissue concentrations of EETs16-18.
Experimental design
Series 1: responses of renal blood flow, glomerular filtration rate and renal sodium excretion to decreases in renal arterial pressure
In this series, heterozygous male TGR with established hypertension (at age 85 to 90 days) and age-matched male HanSD rats were employed. Rats were treated with c-AUCB for 48 hours, because our recent studies have shown that 48-hour treatment with c-AUCB elicited the peak decrease in BP in ANG II dependent models of hypertension16-18. Thereafter, rats were anesthetized with sodium thiopental (60 mg/kg, i.p.) and placed on a thermoregulated surgical table to maintain body temperature at 37 °C. A tracheostomy was performed and a PE-240 tube was inserted to maintain a patent airway and the exterior end of the tracheal cannula was placed inside a small plastic chamber in which a humidified 95% oxygen/5% carbon dioxide mixture was continuously passed, which has been shown to improve the stability of arterial BP of barbiturate-anesthetized rat22. In this regard, it is important to note that even if barbiturate anaesthesia exhibits some negative effects on BP, we found in our previous studies that values obtained in anesthetized animals reflect correct BP values that closely correlate with values obtained in conscious animals in which BP was measured by tail-pletysmography via tail-cuff apparatus2,6,9. The right jugular vein was catheterized for fluid infusion and aesthetic administration as needed. The left femoral artery was catheterized with a PE-50 catheter to allow continuous monitoring of arterial BP and blood sampling. Mean arterial pressure was monitored with a pressure transducer (model MLT 1050) and recorded on the computer using a computerized data-acquisition system (Power Lab/4SP, AD Instruments, and UK). The left kidney was exposed via a flank incision, isolated from the surrounding tissue and placed in a lucite cup, and the ureter was then cannulated with a PE-10 catheter. The aortic clamp was placed on the aorta above the junction of the left renal artery to regulate the level of renal arterial pressure (RAP). In addition, an ultrasonic transient-time flow probe (1RB, Transonic Systems, Altron Medical Electronic GmbH, Germany) connected to a Transonic flowmeter was placed around the left renal artery and RBF was recorded using a computerized acquisition system. At the end of the experiment, zero value was established by complete occlusion of aorta. During surgery, an isotonic saline solution containing bovine serum albumin (6%, Sigma Chemical Co., Prague, Czech Republic) was infused at a rate of 40 μl/min. After the surgery, isotonic saline solution containing albumin (1%) and polyfructosan inulin (7.5%, Inutest, Laevosan, Linz, Austria) was infused at the same infusion rate. After completion of the surgical procedures, an equilibration period of 50-min was allowed for the animals to establish steady state before initiating one 30-min control urine collection at physiological level of RAP. In groups to whom the control protocol was applied, three additional 30-min urine collection periods at physiological level of RAP were performed. In groups in whom the experimental protocol was applied, a first 30-min urine collection at the physiological level of RAP was performed. Thereafter, 30–min urine collections were performed at reduced RAPs of 105, 90 and 80 mmHg. Five minutes of equilibration time were allowed after each step of reduction in RAP. Blood samples were collected after the second and fourth urine collection to allow determination of GFR and renal sodium excretion. This experimental procedure is identical to that used by Wang et al.23 in ANG II-infused hypertensive rats and that recently employed in our lab in Cyp1a1-Ren-2 transgenic rats and 2K1C hypertensive rats17,18,24.
Urine volume was measured gravimetrically. Urinary sodium concentration was determined by flame photometry. Polyfructosan in plasma and urine was measured colorimetrically. Polyfructosan clearance was used as an estimate of GFR. Values were calculated per gram of kidney weight. Fractional sodium excretion was calculated using standard formula. The autoregulatory index (AI) of RBF was calculated by the method of Semple and de Wardener25 using the following formula: AI = [(RBF2 – RBF1)/RBF1]/[(RAP2 – RAP1)/RAP1]. The same formula was employed for calculation of the AI of GFR. An AI of zero indicates ideal autoregulation and higher values indicate impairment of the autoregulation.
The following experimental groups were examined:
HanSD rats + vehicle + control protocol (n = 10)
HanSD rats + c-AUCB + control protocol (n = 9)
HanSD rats + vehicle + experimental protocol (n = 10)
HanSD rats + c-AUCB + experimental protocol (n = 11)
TGR + vehicle + control protocol (n = 9)
TGR + c-AUCB + control protocol (n = 9)
TGR + vehicle + experimental protocol (n = 12)
TGR + c-AUCB + experimental protocol (n = 12)
Series 2: assessment of ANG II, EETs, DHETEs and 20-HETE concentrations and western blot analysis for renal cortical CYP2C23 and sEH protein expression
Animals were divided into the following experimental groups and were exposed to the same treatment protocol as in series 1:
HanSD rats + vehicle (n = 8)
HanSD rats + c-AUCB (n = 8)
TGR + vehicle (n = 9)
TGR + c-AUCB (n = 10)
Since it is now well-recognized that plasma and tissue ANG II concentrations in anesthetized animals are higher than those obtained from decapitated conscious rats and that normotensive animals exhibit greater increases in renin secretion in response to anaesthesia and surgery than ANG II-dependent hypertensive intrarenal renin-depleted animals2,26, in the present study rats from each experimental group were euthanized by decapitation and plasma and tissue samples were collected. Plasma and whole kidney ANG II levels were measured by radioimmunoassay as described previously2,26. This approach is routinely used in our laboratory, allows comparison of the present results with those of our previous studies to evaluate the role of the RAS in the pathophysiology of hypertension and tissue damage2,3,6,8,9,15,16. The levels of the CYP metabolites EETs, DHETEs and 20-HETE were measured in the kidney cortex. Samples were extracted, separated by reverse-phase high performance liquid chromatography and analysed by negative-mode electrospray ionization and tandem mass spectroscopy as described previously16,21. Specifically, 8,9-EETs; 11,12-EETs and 14,15-EETs and DHETEs, respectively, were measured separately and were then pooled for clear presentation. Thus, data are shown as total concentrations of EETs and of DHETEs, respectively, because it is well recognized that these metabolites are the most biologically active products formed in the CYP epoxygenase enzymatic pathway13. Western blot analyses for protein expression of CYP2C23 and sEH in the renal cortex were performed as described in detail previously and were normalized for ß-actin16,27.
Statistical Analysis
All values are expressed as means ± SEM. With Graph-Pad Prism software (Graph Pad Software, San Diego, CA, USA), statistical analysis was performed using Student’s t-test, Wilcoxon’s signed-rank test for unpaired data, or one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test when appropriate. ANOVA for repeated measurements, followed by Tukey-Kramer multiple comparisons test was performed for the analysis within groups (e.g. for the analysis of autoregulation capacity of RBF and GFR). Values exceeding the 95% probability limits (p<0.05, two-sided) were considered statistically significant.
Results
Series 1: responses of renal blood flow, glomerular filtration rate and renal sodium excretion to stepwise decreases in renal arterial pressure
Basal values [average values obtained from the first clearance periods performed at a physiological level of renal arterial pressure (RAP) were pooled from groups exposed to control and experimental protocols] of mean arterial pressure (MAP), body weight, RBF, GFR, urine flow, absolute and fractional sodium excretion are summarized in Table 1.
Table 1.
Pooled basal values of mean arterial pressure, renal function and electrolyte excretion from groups exposed to control and experimental protocols.
| HanSD | HanSD | TGR | TGR | |
|---|---|---|---|---|
| + | + | + | + | |
| Parameter | untreated | c-AUCB | untreated | c-AUCB |
| Body Weight (g) | 327 ± 10 | 332 ± 9 | 340 ± 11 | 345 ± 12 |
| MAP (mmHg) | 110 ± 3 | 113 ± 2 | 149 ± 3@ | 123 ± 3* |
| GFR (ml/min per g) | 1.61 ± 0.17 | 1.83 ± 0.19 | 1.93 ± 0.27 | 1.85 ± 0.14 |
| RBF (ml/min per g) | 6.18 ± 0.22 | 5.91 ± 0.45 | 7.42 ± 0.62 | 7.39 ± 0.61 |
| UNaV (μmol/min per g) | 1.09 ± 0.37 | 0.98 ± 0.35 | 1.75 ± 0.36 | 0.54 ± 0.09* |
| FENa (%) | 0.91 ± 0.26 | 0.84 ± 0.22 | 1.27 ± 0.26 | 0.49 ± 0.08* |
| Urine flow (μl/min per g) | 11.86 ± 2.94 | 9.98 ± 2.65 | 18.42 ± 2.42* | 9.12 ± 1.49 |
HanSD, transgene-negative Hannover Sprague-Dawley rats (HanSD); TGR, Ren-2 transgenic rats; c-AUCB, treatment with the soluble epoxide hydrolase inhibitor; MAP; mean arterial pressure; GFR, glomerular filtration rate; RBF, renal blood flow; UNaV, absolute sodium excretion; FENa, fractional sodium excretion.
P<0.05 vs. unmarked values.
P<0.05 vs. all values (statistical analysis was performed by employing one-way ANOVA followed by Tukey-Kramer multiple comparisons test).
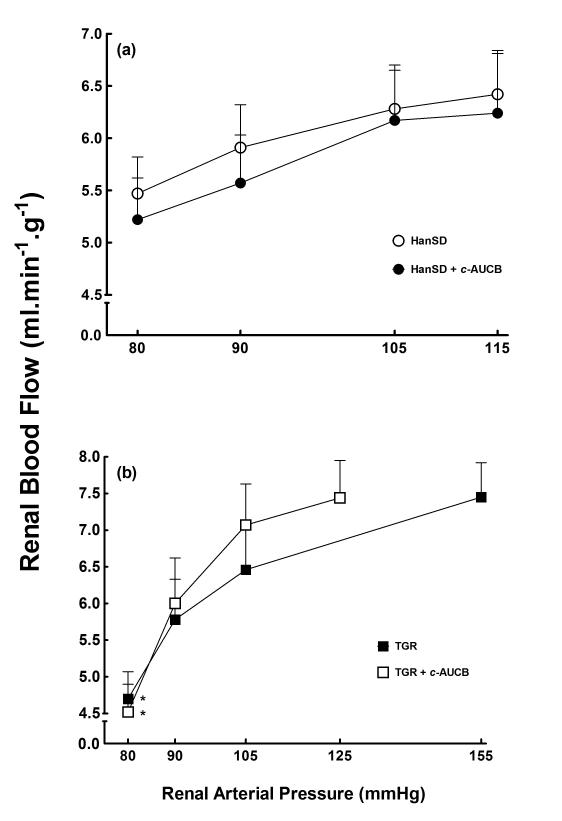
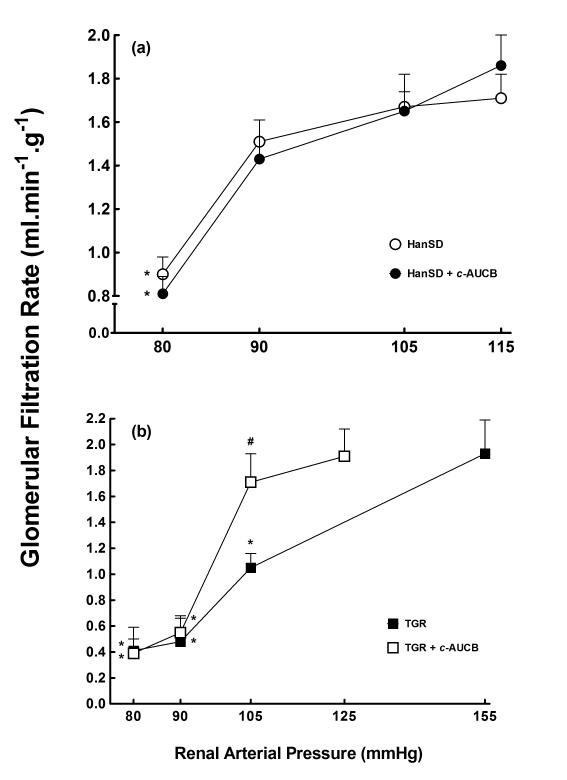
As shown in Figures 1A and 2A, HanSD rats maintained autoregulatory efficiency of RBF and GFR in response to reduction of RAP and only the reduction to the lowest level of RAP (80 mmHg) elicited a significant decrease in GFR from 1.71 ± 0.11 to 0.90 ± 0.08 ml.min−1.g−1 (p<0.05) and the treatment with c-AUCB did not significantly change autoregulatory efficiency of RBF and GFR in HanSD rats. As shown in Figure 1B and 2B, untreated TGR exhibited impaired autoregulatory efficiency of RBF and GFR as compared with HanSD rats. As shown reduction in RAP to 80 mmHg elicited significant decreases in RBF in untreated TGR from 7.45 ± 0.47 to 4.69 ± 0.37 ml.min−1.g−1 (p<0.05) and significant decreases in GFR were already observed at the level of RAP of 105 mmHg in untreated TGR. Treatment with c-AUCB did not significantly improve autoregulatory efficiency of RBF and GFR in TGR.
Figure 1.
Relationship between renal arterial pressure and renal blood flow in transgene-negative Hannover Sprague-Dawley (HanSD) rats (a) and Ren-2 transgenic rats (TGR) (b) treated with c-AUCB or untreated. * P<0.05 versus basal values (statistical analysis was performed by employing repeated measures ANOVA followed by Tukey-Kramer multiple comparisons test).
Figure 2.
Relationship between renal arterial pressure and glomerular filtration rate in transgene-negative Hannover Sprague-Dawley (HanSD) rats (a) and Ren-2 transgenic rats (TGR) (b) treated with c-AUCB or untreated. * P<0.05 versus basal values (statistical analysis was performed by employing repeated measures ANOVA followed by Tukey-Kramer multiple comparisons test). # P<0.05 versus corresponding values from untreated rats (statistical analysis was performed by employing unpaired Student’s t-test for unpaired data).
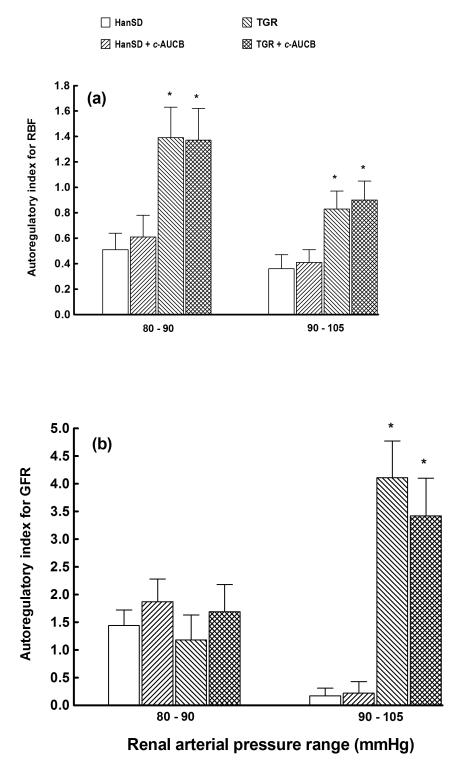
Figure 3 shows the autoregulatory efficiency of RBF and GFR using autoregulatory indices calculated by the method of Semple and de Wardener25. Results of autoregulatory indices show that untreated TGR exhibited substantial impairment of the autoregulatory efficiency of RBF and GFR already at the reduction of RAP from 105 to 90 mmHg. Treatment with c-AUCB did not restore autoregulatory indices of RBF and GFR in TGR.
Figure 3.
Autoregulatory indices calculated by the method of Semple and de Wardener for renal blood flow (RBF) (a) and glomerular filtration rate (GFR) (b) responses to reduction of renal arterial pressure from 105 to 90 and from 90 to 80 mmHg in in in transgene-negative Hannover Sprague-Dawley (HanSD) rats and Ren-2 transgenic rats (TGR) treated with c-AUCB or untreated. * P<0.05 versus unmarked values at the same level of renal arterial pressure (statistical analysis was performed by employing one-way ANOVA followed by Tukey-Kramer multiple comparisons test). .
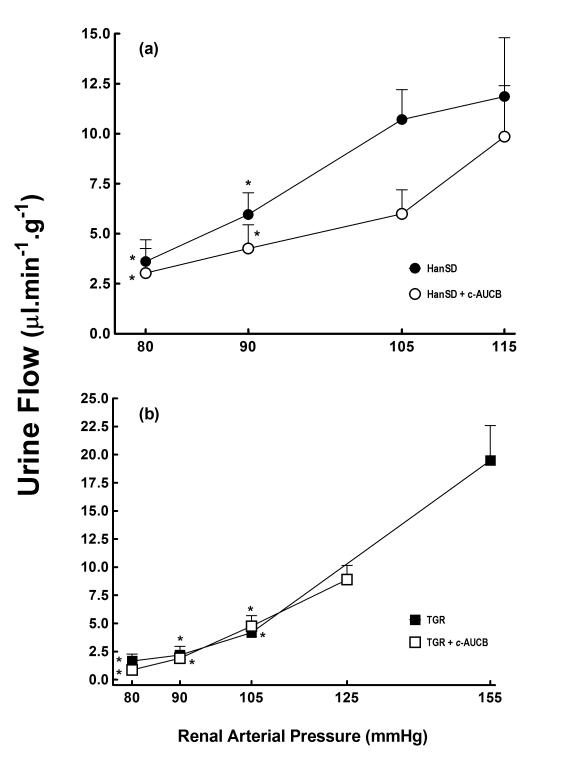
As shown in Figures 4A and 5A, treatment with c-AUCB had no significant effect on urine flow and absolute and fractional (not shown) sodium excretion at the spontaneous levels of RAP in HanSD rats. Reduction in RAP elicited similar responses in both groups of HanSD rats.
Figure 4.
Relationship between renal arterial pressure and urine flow in transgene-negative Hannover Sprague-Dawley (HanSD) rats (a) and Ren-2 transgenic rats (TGR) (b) treated with c-AUCB or untreated. * P<0.05 versus basal values (statistical analysis was performed by employing repeated measures ANOVA followed by Tukey-Kramer multiple comparisons test). .
Figure 5.
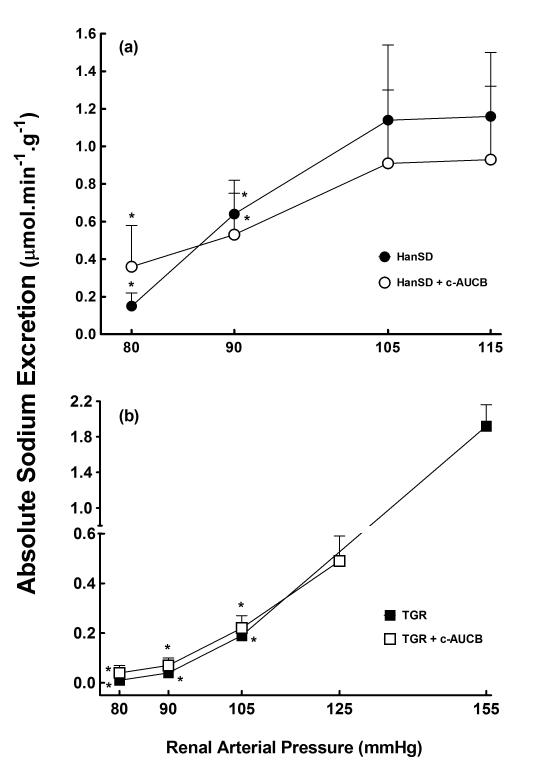
Relationship between renal arterial pressure and absolute sodium excretion in transgene-negative Hannover Sprague-Dawley (HanSD) rats (a) and Ren-2 transgenic rats (TGR) (b) treated with c-AUCB or untreated. * P<0.05 versus basal values (statistical analysis was performed by employing repeated measures ANOVA followed by Tukey-Kramer multiple comparisons test).
As shown in Figures 4B and 5B, the reduction in RAP resulted in significantly greater decreases in urine flow and absolute sodium excretion in untreated TGR as compared with HanSD rats, the differences are significant at the level of RAP of 105 mmHg and are further pronounced at the levels of 90 and 80 mmHg. Treatment of TGR with c-AUCB had no significant effect on the responses of urine flow and absolute sodium excretion to reductions in RAP in TGR.
Untreated and c-AUCB-treated TGR and HanSD rats exposed to the control protocol did not show any significant changes in renal hemodynamic or renal sodium excretion throughout the experiment and therefore data are not presented.
Series 2: assessment of ANG II, EETs, DHETEs and 20-HETEs concentrations and Western blot analysis for renal cortical CYP2C23 and sEH protein expression
Densitometric analysis revealed that, when normalized for ß-actin, there were no significant differences in CYP2C3 or sEH protein expression in the renal cortex between TGR and HanSD rats with or without c-AUCB treatment. In addition, there were no significant differences in renal concentrations of 20-HETEs between untreated TGR and untreated HanSD rats (4098 ± 198 vs. 4127 ± 322 ng/ g of protein) and treatment with c-AUCB did not significantly change 20-HETEs concentrations either in TGR or HanSD rats.
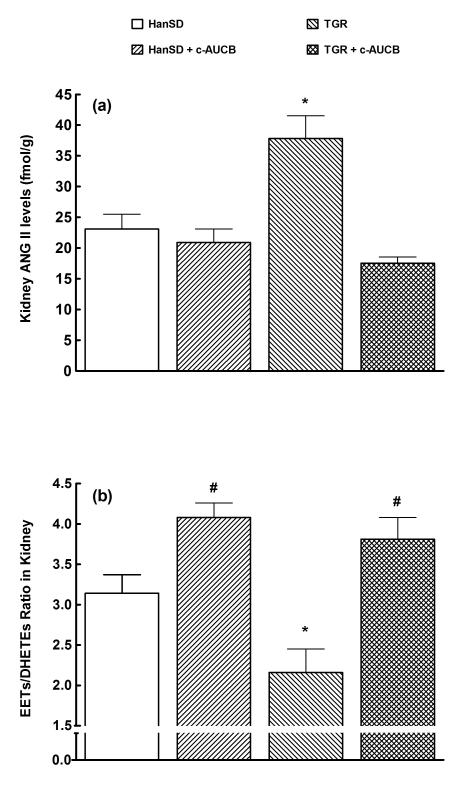
Plasma ANG II levels were higher in untreated TGR than in untreated HanSD rats (30 ± 2 vs. 9 ± 1 fmol/ml, p<0.05) and treatment with c-AUCB did not significantly change plasma ANG II levels in HanSD rats, but significantly decreased them in TGR (to 18 ± 1 fmol/ml, p<0.05). As shown in Figure 6A, kidney ANG II levels were significantly higher in untreated TGR than in untreated HanSD rats (38 ± 4 vs. 23 ± 2 fmol/g of tissue, p<0.05). Treatment with c-AUCB significantly decreased kidney ANG II levels in TGR but not in HanSD rats.
Figure 6.
Kidney ANG II levels (a) and renal ratio of EETs to DHETEs (b) in transgene-negative Hannover Sprague-Dawley (HanSD) rats and Ren-2 transgenic rats (TGR) treated with c-AUCB or untreated. * P<0.05 versus unmarked values. # P<0.05 versus all other values (statistical analysis was performed by employing one-way ANOVA followed by Tukey-Kramer multiple comparisons test). .
As shown in Figure 6B, untreated TGR revealed significantly lower intrarenal availability of biologically active epoxygenase metabolites, when expressed as EETs/DHETEs ratio, than untreated HanSD rats (2.16 ± 0.29 vs. 3.14 ± 0.23, p<0.05). Treatment with c-AUCB significantly increased this ratio in both TGR and HanSD rats, when compared with basal values (p<0.05 in both strains).
Discussion
The results of our present study demonstrate that treatment with c-AUCB significantly reduced BP in TGR and was associated with significant increases in the availability of biologically active epoxygenase metabolites assessed as the ratio of EETs to DHETEs. However, in contrast to our hypothesis these actions were not accompanied by an improvement of autoregulatory efficiency of RBF and GFR or the slope of the pressure-natriuresis relationship. In addition, to our surprise treatment with c-AUCB resulted in a significant reduction of plasma ANG II level and normalization of kidney ANG II concentrations in TGR to the level observed in untreated HanSD rats. Moreover, our data show that treatment with c-AUCB did not alter ANG II level in HanSD rats.
Since our present data strongly oppose our hypothesis that the antihypertensive action of c-AUCB treatment is due to EETs-mediated improvement of the autoregulatory efficiency of renal haemodynamic and of the blunted pressure-natriuresis relationship in TGR, the critically important issue of the present study is related to the following question: what is the mechanism(s) responsible for the BP-lowering effects of c-AUCB treatment in TGR?
In this regard, it is important to realize that studies performed during the last three decades have revealed that EETs exert important biological actions on the regulation of vascular tone and especially on the control of renal tubular transport of sodium11-13. EETs have been consistently shown to cause vasodilatation through stimulation of the large-conductance calcium-activated potassium channels11,12,28. They have also been identified as an endothelium-derived hyperpolarizing factor that mediates nitric oxide- and prostaglandin-independent vasodilatation, and have been found to oppose the vasoconstrictor actions of ANG II14,15,29-31. On the basis of these results, it is still conceivable that one potential mechanism of BP-lowering actions of c-AUCB treatment in TGR could be EETs-mediated attenuation of the previously well-documented selective peripheral and renal vascular responsiveness to ANG II5,6.
However, most of the available evidence so far has indicated that the EETs’ antihypertensive properties are related with their action on renal sodium excretion12,13, because at the kidney level it has been shown that EETs inhibit sodium reabsorption in the proximal tubule by blocking the sodium-hydrogen exchanger32 and also decrease sodium reabsorption in the cortical collecting duct by blocking the epithelial sodium channels33. In addition, it has been demonstrated that EETs play an important role in the regulation of the afferent arteriole autoregulatory responses to changes in perfusion pressure by limiting pressure-mediated vasoconstriction34. It was therefore conceivable to assume that net intrarenal deficiency of EETs in TGR contributes to the well-known impairment of the pressure-natriuresis relationship19,20 and in accordance with the concept originally proposed by Guyton et al.35 and supported by findings of several other groups23,36-39, this impairment is the critical mechanism responsible for the pathophysiology of hypertension. This notion has been further supported by our recent findings that chronic inhibition of sEH normalized the intrarenal EETs bioavailability and improved the pressure-natriuresis relationship in 2K1C Goldblatt hypertensive and Cyp1a1-Ren-2 transgenic rats which was associated with significant BP-lowering effects17,18. However, our current data clearly indicate that this is not the underlying mechanism of the BP-lowering actions of c-AUCB treatment in TGR. We cannot offer a fully satisfactory explanation, why in two other different models of ANG II-dependent hypertension, which likewise depend on the enhanced endogenous activity of the RAS, the EETs’ actions at the kidney level were responsible for the antihypertensive actions of the same sEH inhibitor (c-AUCB) which, in addition, was employed at the same dose.
Nevertheless, treatment with c-AUCB in TGR markedly decreased plasma and normalized intrarenal ANG II to the level observed in HanSD rats. In view of our findings it seems therefore reasonable to assume that suppression of ANG II levels is the main underlying mechanism responsible for the BP-lowering action c-AUCB treatment. This notion is in good agreement with previous studies showing that the inappropriate activation of the intrarenal RAS is the main contributor to the pathophysiology of ANG II-dependent form of hypertension40. In addition, our current findings are also in agreement with studies showing that only chronic and not acute blockade of the RAS restored the pressure-natriuresis relationship, indicating long-term modulatory actions of elevated ANG II levels on the slope of pressure-natriuresis relationship and suggesting that acute BP-lowering effects of RAS inhibition are primarily attributable to changes in the total peripheral resistance23,41. Furthermore, this notion is also supported by a recent study in homozygous as well as heterozygous TGR made by Vaněčková et al.42, showing that acute RAS inhibition (by acute intravenous administration of the angiotensin-converting enzyme (ACE) inhibitor captopril) resulted in acute profound BP decreases that were predominantly mediated by the decrease in the total peripheral resistance. However, it is difficult to reconcile our present findings in TGR with our recent findings in ANG II-dependent models of hypertension that demonstrated that treatment with c-AUCB (at the same dose) did not alter circulating and renal RAS activity16-18. However, one possible explanation might be offered; Heinrich et al.43 have found that 14,15-EETs did not alter basal renin release, but exhibited marked inhibitory effect renin release stimulated by isoproterenol in renal cortical slices and therefore it is conceivable to assume enhanced intrarenal tissue availability of EETs could suppress activity of the mouse Ren-2 renin gene and that could lead the suppression of ANG II concentrations in TGR without altering ANG II levels in transgene-negative HanSD rats.
Nevertheless, it is important to recognize that Bohlender et al.44 have shown that the regulation of the renin concentrations and activities in TGR are extremely complicated because one must consider the mouse as well as the rat renin concentrations (and activities) in these TGR. Therefore, it is obvious that additional studies, which are beyond the scope of the present study, will be necessary to address this issue and currently we cannot offer a fully satisfactory explanation(s) for these discrepancies in our findings.
However, in this regard, it is important to recognize that previous studies have demonstrated that increased intrarenal ANG II concentrations in ANG II-dependent models of hypertension are the result of a combination of enhanced production of ANG II from endogenous intrarenal components and the uptake of circulating ANG II by ANG II type 1 (AT1) receptors40,45. Therefore, it is conceivable to assume that the normalization of intrarenal ANG II concentrations in c-AUCB-treated TGR is the consequence EETs-mediated suppression of intrarenal ANG II production due to suppression of intrarenal renin activity combined with decreased AT1 receptor-dependent uptake of ANG II from the circulation as the result of decreased plasma ANG II in c-AUCB-treated TGR. This notion could be fully corroborated by studies employing an experimental protocol by which co-administration of c-AUCB and an AT1 receptor antagonist will be used. In addition, since it is well-known that oxidative stress play an important role in the pathogenesis of ANG II-dependent forms of hypertension46.. Thus, we have recently reported that TGR exhibit increased intrarenal oxidative stress8 that exerts renal vasoconstrictor and antinatriuretic effects and modulates renal function in prehypertensive TGR9. It is therefore possible that normalization of intrarenal ANG II concentrations in c-AUCB-treated TGR might reduce the increased oxidative stress and may at least partly contribute to the antihypertensive actions of c-AUCB in those animals. However, to corroborate these notion will require further studies in the future which again are beyond the aim of current study.
Collectively, with the above-discussed observations in mind we suggest that the underlying mechanism(s) responsible for the BP-lowering effect of 48-hour treatment with the sEH inhibitor c-AUCB in TGR are related to the combination of enhanced bioavailability of EETs with their effects at the vascular level with the marked suppression of the RAS activity. We suggest that main underlying mechanism responsible for the BP-lowering actions of c-AUCB treatment in TGR in this study is a decrease in peripheral vascular resistance that is mediated by increased bioavailability of vasodilatory EETs and decreased concentrations of vasoconstrictor ANG II.
In conclusion, our findings show, first, that treatment with the sEH inhibitor c-AUCB significantly reduced BP in TGR and elicited substantial increases in the intrarenal availability of endogenous biologically active epoxygenase metabolites; second, that treatment with the sEH inhibitor c-AUCB did not improve the autoregulatory efficiency of renal hemodynamic and of the blunted pressure-natriuresis mechanism in TGR; third, that the treatment with the sEH inhibitor c-AUCB resulted in a marked suppression of plasma ANG II levels and normalization of renal ANG II concentrations in TGR to the level observed in normotensive HanSD rats.
Taken together, these findings indicate that the antihypertensive action of sEH inhibition by c-AUCB in TGR rats is predominantly mediated by suppression of systemic and intrarenal RAS activity.
Acknowledgments
This study was supported by grant No. NT/12171-5 awarded to Z.H. awarded by the Internal Grant Agency of the Ministry of Health of the Czech Republic. S.V. is supported by the project of Ministry of Health of the Czech Republic for the development of research organization 00023001 (IKEM) – institutional support. L.Č. is a recipient of grant No. MŠMT-Kontakt LH 11116 from the Ministry of Education, Youth and Sports for the support of the international research cooperation and K.K. was supported by this grant during his stay in Prague when he participated on this project.
H.J.K. is supported by grants from the German Research Foundation (DFG), Bonn (Kra 436/14-2 and 436 TSE 113/57/0-1). Z.H. is supported by grant No. 305/08/P053 awarded by GAČR. This study was also supported by the financial support from the EU by the Operational Program Prague – Competitiveness; project “CEVKOON” (#CZ.2.16/3.1.00/22126). J.D.I. is an Established Investigator of the American Heart Association and these studies were supported by NIH grants HL59699 and DK38226. S.H.H. was supported in part by the Howard Hughes Foundation and a fellowship from the NIEHS Supported Basic Research Program. Partial support was provided by NIEHS Grant R01 ES02710, R01 Es013933 and P42 Es013933 and NIH Grant R01 HL059699 awarded to B.D.H. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
References
- 1.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harboring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 2.Husková Z, Kramer HJ, Vaňourková Z, Červenka L. Effects of changes in sodium balance on plasma and kidney angiotensin II levels in anesthetized and conscious Ren-2 transgenic rats. J. Hypertens. 2006;24:517–527. doi: 10.1097/01.hjh.0000209988.51606.c7. [DOI] [PubMed] [Google Scholar]
- 3.Kujal P, Čertíková Chábová V, Vernerová Z, et al. Similar renoprotection after renin-angiotensin-dependent and –independent antihypertensive therapy in 5/6-nephrectomized Ren-2 transgenic rats: are there blood pressure-independent effects? Clin. Exp. Pharmacol. Physiol. 2010;37:1159–1169. doi: 10.1111/j.1440-1681.2010.05453.x. [DOI] [PubMed] [Google Scholar]
- 4.Hartner A, Porst M, Klanke B, Cordasic N, Veelken R, Hilgers KF. Angiotensin II formation in the kidney and nephrosclerosis in Ren-2 hypertensive rats. Nephrol. Dial. Transplant. 2006;21:1778–1785. doi: 10.1093/ndt/gfl065. [DOI] [PubMed] [Google Scholar]
- 5.Jacinto SM, Mullins JJ, Mitchell KD. Enhanced renal vascular responsiveness to angiotensin II in hypertensive ren-2 transgenic rats. Am. J. Physiol. 1999;276:F315–F322. doi: 10.1152/ajprenal.1999.276.2.F315. [DOI] [PubMed] [Google Scholar]
- 6.Kopkan L, Kramer HJ, Huskova Z, Vaňourková Z, Škaroupková P, Thumová M, Červenka L. The role of intrarenal angiotensin II in the development of hypertension in Ren-2 transgenic rats. J. Hypertens. 2005;23:1531–1539. doi: 10.1097/01.hjh.0000174972.46663.5e. [DOI] [PubMed] [Google Scholar]
- 7.Lee MA, Böhm M, Paul M, Bader M, Ganten U, Ganten D. Physiological characterization of the hypertensive transgenic rat TGR(mRen2)27. Am. J. Physiol. 1990;270:E919–E929. doi: 10.1152/ajpendo.1996.270.6.E919. [DOI] [PubMed] [Google Scholar]
- 8.Kopkan L, Husková Z, Vaňourková Z, et al. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascular. Pharmacol. 2009;51:175–181. doi: 10.1016/j.vph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Kopkan L, Husková Z, Vanourková Z, Thumová M, Skaroupková P, Cervenka L, Majid DS. Superoxide and its interaction with nitric oxide modulates renal function in prehypertensive Ren-2 transgenic rats. J Hypertens. 2007;25:2257–2265. doi: 10.1097/HJH.0b013e3282efb195. 2007. [DOI] [PubMed] [Google Scholar]
- 10.Vernerová Z, Kujal P, Kramer HJ, Bäcker A, Červenka L, Vaněčková I. End-organ damage in hypertensive transgenic Ren-2 rats: influence of early and late endothelin receptor blockade. Physiol. Res. 2009;58(Suppl 2):S69–S78. doi: 10.33549/physiolres.931640. [DOI] [PubMed] [Google Scholar]
- 11.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent response. Pfugers. Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 13.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal mircovascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J. Hypertens. 2001;19:983–992. doi: 10.1097/00004872-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Lee CR, Imig JD, Edin ML, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB. J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honetschlägerová Z, Husková Z, Vaňourková Z, et al. Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J. Physiol. 2011;589:207–219. doi: 10.1113/jphysiol.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honetschlägerová Z, Sporková A, Kopkan L, et al. Inhibition of soluble epoxide hydrolyse improves the impaired pressure-natriuresis relationship and attenuates the development of hypertension and hypertension-associated end-organ damage in Cyp1a1-Ren-2 transgenic rats. J. Hypertens. 2011;29:1590–1601. doi: 10.1097/HJH.0b013e328349062f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sporková A, Kopkan L, Varcabová Š, et al. Role of cytochrome P450 metabolites in the regulation of renal function and blood pressure in 2-kidney, 1-clip hypertensive rats. Am. J. Physiol. 2011;300:R1468–R1475. doi: 10.1152/ajpregu.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springate J, Van Liew J, Ganten D. Enalapril and pressure-diuresis in hypertensive rats transgenic for mouse renin gene. Kidney. Blood. Press. Res. 1997;20:1–5. doi: 10.1159/000174116. [DOI] [PubMed] [Google Scholar]
- 20.Lippoldt A, Gross V, Bohlender J, Ganten U, Luft FC. Lifelong angiotensin-converting enzyme inhibition, pressure natriuresis, and renin-angiotensin system gene expression in transgenic (mRen-2)27 rats. J. Am. Soc. Nephrol. 1996;7:2119–2129. doi: 10.1681/ASN.V7102119. [DOI] [PubMed] [Google Scholar]
- 21.Neckář J, Kopkan L, Husková Z, et al. Inhibition of soluble epoxide hydrolase by cis-4-[4-(3-adamantan-1-yl-ureido)cyclohexyloxy]benzoic acid exhibits antihypertensive and cardioprotective actions in transgenic rats with angiotensin II-dependent hypertension. Clin. Sci. 2012;122:513–525. doi: 10.1042/CS20110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervenka L, Wang CT, Navar LG. Effects of acute AT1 receptor blockade by candesartanu on arterial pressure and renal function in rats. Am. J. Physiol. 1998;274:F940–F945. doi: 10.1152/ajprenal.1998.274.5.F940. [DOI] [PubMed] [Google Scholar]
- 23.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 24.Erbanová M, Thumová M, Husková Z, et al. Impairment of the autoregulation of renal hemodynamics and of the pressure-natriuresis relationship precedes the development of hypertension in Cyp1a1-Ren-2 transgenic rats. J. Hypertens. 2009;27:575–586. doi: 10.1097/hjh.0b013e32831cbd5a. [DOI] [PubMed] [Google Scholar]
- 25.Semple SJ, de Wardener HE. Effect of increased renal venous pressure on circulatory autoregulation of isolated dog kidneys. Circ. Res. 1959;7:643–648. doi: 10.1161/01.res.7.4.643. [DOI] [PubMed] [Google Scholar]
- 26.Husková Z, Kramer HJ, Thumová M, Vaňourková Z, Bürgelová M, Teplan V, Červenka L. Effects of anaesthesia on plasma and kidney ANG II levels in normotensive and ANG II-dependent hypertensive rats. Kidney Blood Press Res. 2006;29:74–83. doi: 10.1159/000092981. [DOI] [PubMed] [Google Scholar]
- 27.Walkowska A, Škaroupková P, Husková Z, et al. Intrarenal cytochrome P-450 metabolites of arachidonic acid in the regulation of the nonclipped kidney function in two-kidney, one-clip Goldblatt hypertensive rats. J. Hypertens. 2010;28:582–593. doi: 10.1097/HJH.0b013e328334dfd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li PL, Campbell WB. Regulation of potassium channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ. Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Borrego-Conde LJ, Falck JR, Sharma KK, Wilcox CS, Umans JG. Contribution of nitric oxide, EDHF, and EETs to endothelium-dependent relaxation in renal afferent arterioles. Kidney. Int. 2003;63:2187–2193. doi: 10.1046/j.1523-1755.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 30.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 31.Kohagure K, Endo Y, Ito O, Arima S, Omata K, Ito S. Endogenous nitric oxide and epoxyeicosatrienoic acids modulate angiotensin II-induced constriction in the rabbit afferent arteriole. Acta. Physiol. Scand. 2000;168:107–112. doi: 10.1046/j.1365-201X.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- 32.Madhun ZT, Goldthwait DA, McKay D, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J. Clin. Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakairi Y, Jacobson HR, Noland DT, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am. J. Physiol. 1995;268:F931–F939. doi: 10.1152/ajprenal.1995.268.5.F931. [DOI] [PubMed] [Google Scholar]
- 34.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br. J. Pharmacol. 1999;127:1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyton AC, Hall JE, Coleman TG, Manning RD., Jr. The dominant role of the kidneys in the long term regulation of arterial pressure in normal and hypertensive states. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. Raven Press, Publishers; New York, NY: 1990. pp. 1029–1052. [Google Scholar]
- 36.Roman RJ, Cowley AW., Jr. Abnormal pressure-diuresis-natriuresis response in spontaneously hypertensive rats. Am J Physiol. 1985;248:F199–F205. doi: 10.1152/ajprenal.1985.248.2.F199. [DOI] [PubMed] [Google Scholar]
- 37.Miao CY, Liu KL, Benzoni D, Sassard J. Acute pressure-natriuresis function shows early impairment in Lyon hypertensive rats. J. Hypertens. 2005;23:1225–1231. doi: 10.1097/01.hjh.0000170386.84450.e3. [DOI] [PubMed] [Google Scholar]
- 38.Van der Mark J, Kline RL. Altered pressure natriuresis in chronic angiotensin II hypertension in rats. Am. J. Physiol. 1994;266:F739–F748. doi: 10.1152/ajpregu.1994.266.3.R739. [DOI] [PubMed] [Google Scholar]
- 39.Hall JE, Mizelle HL, Brands MV, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am. J. Physiol. 1992;262:R61–R71. doi: 10.1152/ajpregu.1992.262.1.R61. [DOI] [PubMed] [Google Scholar]
- 40.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 41.Kline RL, Liu F. Modification of pressure natriuresis by long-term losartan in spontaneously hypertensive rats. Hypertension. 1994;24:467–473. doi: 10.1161/01.hyp.24.4.467. [DOI] [PubMed] [Google Scholar]
- 42.Vaněčková I, Dobešová Z, Kuneš J, Zicha J. The effects of repeated delivery of angiotensin II AT1 receptor antisense on distinct vasoactive systems in Ren-2 transgenic rats: young vs. adult animals. Hypertens. Res. 2012 doi: 10.1038/hr.2012.29. doi: 10.1038/hr2012.39. [DOI] [PubMed] [Google Scholar]
- 43.Henrich WL, Falck JR, Campbell WB. Inhibition of renin release by 14,15-epoxyeicosatrienoic acid in renal cortical slices. Am. J. Physiol. 1990;258:E269–E274. doi: 10.1152/ajpendo.1990.258.2.E269. [DOI] [PubMed] [Google Scholar]
- 44.Bohlender J, Ménard J, Edling O, Ganten D, Luft FC. Mouse and rat plasma renin concentration and gene expression in (mRen2)27 transgenic rats. Am. J. Physiol. 1998;274:H1450–H1456. doi: 10.1152/ajpheart.1998.274.5.H1450. [DOI] [PubMed] [Google Scholar]
- 45.Červenka L, Vaněčková I, Husková Z, Vaňourková Z, Erbanová M, Thumová M, Škaroupková P, Opočenský M, Malý J, Čertíková Chábová V, Tesař V, Burgelova M, Viklický O, Teplan V, Želízko M, Kramer HJ, Navar LG. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: study in angiotensin II receptor subtype 1A knockout mice. J. Hypertens. 2008;26:1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch WJ. Angiotensin II-dependent superoxide. Effects on hypertension and vascular dysfunction. Hypertension. 2008;52:51–56. doi: 10.1161/HYPERTENSIONAHA.107.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]