Abstract
Defatted soy flour (DSF), soy protein isolate (SPI), hemp protein isolate (HPI), medium roast peanut flour (MPF) and pea protein isolate (PPI) stably bind and concentrate cranberry (CB) polyphenols, creating protein/polyphenol-enriched matrices. Proanthocyanidins (PAC) in the enriched matrices ranged from 20.75 mg/g (CB-HPI) to 10.68 mg/g (CB-SPI). Anthocyanins (ANC) ranged from 3.19 mg/g (CB-DSF) to 1.68 mg/g (CB-SPI), while total phenolics (TP) ranged from 37.61 mg/g (CB-HPI) to 21.29 mg/g (CB-SPI). LC-MS indicated that the enriched matrices contained all identifiable ANC, PAC and flavonols present in CB juice. Complexation with SPI stabilized and preserved the integrity of the CB polyphenolic components for at least 15 weeks at 37 °C. PAC isolated from enriched matrices demonstrated comparable anti-adhesion bioactivity to PAC isolated directly from CB juice (MIC 0.4 to 0.16 mg/mL), indicating their potential utility for maintenance of urinary tract health. Approximately 1.0 g of polyphenol-enriched matrix delivered the same amount of PAC available in one cup (300 mL) of commercial CB juice cocktail; which has been shown clinically to be the prophylactic dose for reducing recurring urinary tract infections. CB-SPI inhibited gram- positive and gram-negative bacterial growth. Nutritional and sensory analyses indicated that the targeted CB-matrix combinations have high potential for incorporation in functional food formulations.
Keywords: A-type proanthocyanidins, polyphenols, Vaccinium macrocarpon, anti-adhesion, antimicrobial, shelf-stability
INTRODUCTION
Cranberry (Vaccinium macrocarpon Ait) is one of very few exclusively North American indigenous fruits. As recently as a two decades ago, this berry fruit was essentially unknown to consumers outside of Canada and the United States, and mostly relegated to use as a Thanksgiving holiday condiment to compliment a turkey entree. However, due to the relatively recent discovery of efficacious anti-infective phytochemicals uniquely found in cranberry and some related fruits in the genus Vaccinium, cranberry products now command a strong global market presence including Europe and Australasia.1 It is the now widespread recognition that consumption of cranberry juice provides consumers with a natural, proactive, and highly-effective means to prevent urinary tract infections (UTI), that is the strongest impetus behind current market demand.2–6 Although the precise mechanisms of action are still under investigation, consumption of cranberry products inhibits adherence of infective bacteria to the bladder epithelium, so that the bacteria are safely discarded in the urine without colonizing and subsequently causing infection.2,7. Research has attributed this bioactivity to the presence of PAC containing A-type linkage.8,9 These same cranberry components are effective agents against dental caries and periodontitis, since they inhibit the attachment and biofilm formation by Streptococcus mutans, and inhibit host inflammatory responses.10,11 In addition to the anti-microbial properties, cranberry polyphenols are responsible for a variety of potential health benefits, including anticancer activity,12,13 antioxidant capacity that can prevent lipoprotein oxidation and platelet aggregation,14,15 and protection against cardiovascular diseases.16
Daily consumption of cranberry juice has proven to be an effective preventive strategy for reducing recurring UTI, as well as an excellent prophylactic practice.17 However, cranberry fruits are well-known for their tart, astringent flavor, and cranberry juice is unpalatable for most consumers unless high levels of sugar are added, as is typical for commercially-available juices. This contributes to the relatively high caloric count associated with cranberry juice consumption. Concentrated cranberry supplements in pill form are also now widely available for UTI prevention, but the heating required to spray-dry the capsule contents has the potential to disrupt the bioactivity of the ingredients and reduce shelf-life of the products. Research has indicated that processing of cranberry into these non-food supplements can impact the PAC composition, which is vulnerable to heat or oxidation.18,19 Thus, even though some supplementation can be effective at prevention of bacterial adhesion, it is difficult for consumers to know which ones are efficacious. We recently developed a technique that enables a rapid and streamlined one-step separation of the active health-beneficial flavonoids in cranberry (bioflavonoids) from other extraneous or high caloric components of cranberry juice, including sugars, pectins, and the large volumes of water typically present. This process binds and concentrates cranberry compounds onto an edible protein matrix, creating a unique, stable dry powder ingredient, amenable to delivery in a variety of functional food applications for human consumers.20
The purpose of this study was to develop an advanced functional food ingredient which is low-sugar/low glycemic index, compact, lightweight, portable (minimal water content), nutritive (delivered in a naturally-protein-rich matrix), appetizing (amenable to incorporation into a variety of snack food formats), and a concentrated source of natural cranberry bioflavonoids, mainly A-type PAC. We investigated alternative protein-enriched food substrates for their capacity to bind bioflavonoids from cranberry juice concentrate and evaluated their ability to retain anti-UTI and antimicrobial activity. We also evaluated the nutritional value, shelf stability, and sensory characteristics of representative samples of cranberry-enriched matrices for use in functional food products.
MATERIALS AND METHODS
Materials
Cranberry juice concentrate (CJC) 50.2 °Brix (Ocean Spray Cranberries Inc., Lakeville-Middleboro, MA) was donated by The Cranberry Network, LLC (Wisconsin Rapids, WI). Defatted soy flour (DSF, 50% protein, Hodgson Mill Inc., Effingham, IL), hemp pro 70% protein isolate (HPI, Manitoba Harvest Winnipeg, MB, Canada), and pea protein isolate (PPI, 85% protein, Roquette, France) were bought from a local market. Soy protein isolate (SPI, 90% protein, ADM, Decatur, IL), and partially defatted peanut flour 12% fat, medium roast (MPF, Virginia type, 50% protein, Golden Peanut Company, Alpharetta, GA) were donated by the manufacturers. Reference compounds procyanidin A2 (PAC-A2), procyanidin B2 (PAC-B2), catechin and epicatechin were purchased from Chromadex (Irvine, CA). 4-Dimethylaminocinnamaldehyde and Folin-Ciocalteu reagent were purchased from Sigma-Aldrich Inc. (St. Louis, MO). All organic solvents were HPLC grade and obtained from VWR International (Suwanee, GA). Staphylococcus aureus (ATCC 13301) and Escherichia coli K12 (ATCC 29425) were purchased from the American Type Culture Collection, ATCC (Manassas, VA).
Sorption of Cranberry Polyphenols to Protein-rich Matrices
The methods for sorption of CJC to protein rich flours or isolates were previously reported.20 Briefly, CJC was diluted with water (1:4 v/v) before addition of the protein-rich matrices (DSF, SPI, HPI, MPF or PPI) at a concentration of 30 g/L (unless indicated elsewhere). Dilution was required because of the high viscosity of the 50 °Brix CJC which interfered with effective mixing, sorption and separation from the protein matrices. The mixture was stirred for 15 minutes, centrifuged and the solid pelleted material was freeze-dried and stored at −20 °C. The dried cranberry (CB) polyphenol-enriched matrices were subjected to an anti-adhesion assay, nutritional analysis, and descriptive sensory analysis as described below. Measurements of polyphenols sorbed by the matrices were calculated by two methods: (1) indirect: by subtraction of the polyphenol concentration still remaining in the supernatant, after the sorption/centrifugation with matrix, from the polyphenol concentration in CJC prior to treatment, divided by the weight of the dried pelleted material, 20 (2) direct: by measurement of polyphenols eluted from the dried pelleted material. Although results from both methods were similar, measurement of polyphenols was standardized using the second more direct method which extracts polyphenols from the dried pelleted material before measurement.
Elution of Phytochemicals Bound to the Matrices
Three aliquots (0.5 g) from each CB polyphenol-enriched matrix were eluted with 8 mL of 1% acetic acid in 80% methanol in water by with sonication for 5 min at 55 °C, centrifugation for 10 min and collection of the supernatant in a 25-mL volumetric flask. The process was repeated on the pellet two more times, and the eluates were pooled together and brought to 25 mL with the extraction solvent. This eluted solution was used to determine total phenolics (TP), anthocyanins (ANC) and PAC content, and for HPLC and LC-MS analyses.
Total Phenolics, Anthocyanins and Proanthocyanidins in Eluates
The eluates extracted from the enriched matrices were diluted to appropriate concentrations for analysis. TP were determined with Folin-Ciocalteu reagent by the method of Singleton.21 Concentrations were expressed as mg/L gallic acid equivalents based on a created gallic acid standard curve. Total monomeric ANC content was measured by the pH differential spectrophotometric method described by Giusti and Wrolstad,22 using a Shimadzu UV-2450 (Shimadzu, Kyoto, Japan) spectrophotometer. The eluates with predetermined dilution were added to 0.025 mol/L potassium chloride buffer, pH 1.0, and 0.4 mol/L sodium acetate buffer, pH 4.5. Absorbances at 520 and 700 nm were measured after 30 min of incubation in the dark at room temperature. The absorbance (A) of the diluted sample was then calculated as (A520 – A700)pH1.0 – (A520 – A700)pH4.5. The total monomeric ANC concentration was calculated as mg/L cyanidin 3-O-glucoside equivalents according to the formula: A(MW)(DF)1000/(ε × 1), where the molecular weight (MW, 449 g/mol) of cyanidin 3-O-glucoside was used, the molar absorptivity (ε) was 26,900, DF was the dilution factor, 1000 was the conversion factor from grams to milligrams, and A was absorbance. Total PAC concentration was determined colorimetrically using the 4-Dimethylaminoinnamaldehyde (DMAC) method in a 96-well plate as previously described.23 A series of dilutions of standard procyanidin A2 dimer were prepared in 80% ethanol ranging from 1–100 μg/mL. Blank, standard and diluted samples were analyzed in triplicates. The plate reader protocol was set to read the absorbance (640 nm) of each well in the plate every minute for 30 min (SpectraMax® M3, Sunnyvale, CA). Concentration of PAC in the solution was expressed as mg/L procyanidin A2 equivalent.
Reversed Phase HPLC and LC-ESI-MS Analysis
HPLC analyses for ANC were conducted using an Agilent 1200 HPLC (Agilent Technologies, Santa Clara, CA). Separation was performed using a RP Supelcosil-LC-18 column, 250 mm × 4.6 mm × 5 μm (Supelco, Bellefonte, PA). The mobile phase consisted of 5% formic acid in H2O (A) and 100% methanol (B). The flow rate was 1 mL/min with a step gradient of 10%, 15%, 20%, 25%, 30%, 60%, 10%, and 10% of solvent B at 0, 5, 15, 20, 25, 45, 47, and 60 min, respectively at constant temperature (30 °C). LC-MS analysis was performed using a Shimatdzu LC-MS-IT-TOF instrument equipped with a Prominence HPLC system. The LC separation was performed using a Shim-pack XR-ODS column (50 mm × 3.0 mm × 2.2 μm) at 40 °C with a binary solvent system comprising 0.1% formic acid in water (A), and methanol (B). Compounds were eluted into the ion source at a flow rate of 0.35 mL/min with a step gradient of B of 5–8% (0–5 min), 8–14% (10 min), 14% (15 min), 20% (25 min), 25% (85 min), 5% (35 min) and 5% (40 min). Ionization was performed using ESI source in the positive and negative modes. Compounds were characterized and identified by their MS, MS/MS spectra and LC retention times and by comparison with available reference samples and our previous analyses.20
Normal Phase HPLC-fluorescence Analysis of Proanthocyanidins
HPLC analyses were conducted using an Agilent 1200 HPLC with fluorescence detector (FLD) and photodiode array detector (DAD) (Agilent Technologies, Englewood, CO). PAC separation was performed according to Wallace and Giusti,24 a method adapted from Brownmiller25 using a Develosil Diol column, 250 mm × 4.6 mm × 5 μm (Phenomenex, Torrance, CA). The binary mobile phase consisted of (A) acetonitrile:acetic acid (98:2, v/v) and (B) methanol:water:acetic acid (95:3:2, v/v/v). Separation was accomplished using a linear gradient at 35 °C with 0.8 mL/min flow rate as follows 0–35 min, 0–40% B; 35–40 min, 40–100% B; isocratic 100% B, 45 min; 100-0% B, 50 min, and 0% B to 55 min. The column was re-equilibrated for 5 min between samples. Eluate was monitored by fluorescence detection with excitation at 230 nm and emission at 321 nm as well at 280 nm with the DAD detector. Samples were filtered through 0.2 μm PTFE filters (Fisher Scientific, Pittsburg, PA) before injecting 5 μL onto the HPLC column. Four concentrations of PAC-A2 standard reference were prepared at 0.5, 0.25, 0.125, and 0.05 mg/mL and 5 μL was injected as an external standard. PAC were identified by comparison with available standards, our previous analyses,20 reported literature,22, 23 and LC-ESI-MS. Quantification of PAC was calculated using peak areas and a calibration curve for PAC-A2, and amounts were expressed as PAC-A2 equivalents.
Stability of Polyphenols at 37 °C
The CB polyphenol-enriched SPI was made by mixing diluted CJC (1:3 v/v with water) with SPI at a ratio of 100 g/L (the concentration most suitable for commercial scale preparation). Multiple two-gram samples of freeze-dried material were aliquoted into 50 mL screw-cap vials and placed in a 37 °C incubator. At regular intervals over a 15-week period, triplicate sets of samples (2 g) were removed (0, 1, 2, 3, 5, 7, 9, 11, 13 and 15 weeks) and eluted 3 times with 20 mL volumes of methanol:water:acetic acid (75:20:5). Total monomeric ANC, PAC, and TP eluted from the matrices were quantified using the pH differential assay, DMAC assay and Folin Ciocalteu assay for total phenolics, respectively, and results were expressed as percentages of the original amounts that were measured on day 0.
Stability of Polyphenols at 80 °C
The CB polyphenol-enriched DSF was prepared at the same ratio used for 37 °C stability experiment (4×, 100 g/L). Multiple two-gram samples of freeze-dried CB polyphenol-enriched DSF were aliquoted into 50 mL screw-cap vials and incubated at 80 °C. Triplicate sets of samples (2 g) were removed at 0, 30, 60 and 90 minutes and eluted 3 times with 20 mL volumes of acidic methanol (methanol: water: acetic acid, 75:20:5). Total monomeric ANC, PAC, and TP in eluates were quantified as described in the stability test above.
Nutritional Evaluation of CB Polyphenol-enriched SPI and PPI
Samples of CB polyphenol-enriched SPI or PPI, and untreated SPI and PPI were packaged in plastic sample bags and shipped frozen to Medallion Laboratories (Minneapolis, MN). Analyses were performed in accordance with AOAC methods for total carbohydrates, ash, moisture, proteins, dietary fiber, fat, and calories per 100 g sample. Methods for these calculations are detailed on the Medallion Labs website (www.medlabs.com).
Descriptive Sensory Analysis of CB Polyphenol-enriched SPI
CB polyphenol-enriched SPI, and untreated SPI were packaged in plastic sample bags and delivered to Sensory Spectrum Inc. (Kannapolis, NC). Samples were held refrigerated and removed from refrigerator approximately 30 minutes prior to evaluation. Panelists were provided approximately 1 teaspoon of each product in a 1 oz. cup, with more available if needed. Panelists evaluated the organoleptic properties of the samples as a dry powder and were allowed to sip water to combine in the mouth if desired.
Anti-adhesion Assay and Isolation of Proanthocyanidins
The human red blood cells (HRBC) hemagglutination in vitro assay was used to compare the bacterial anti-adhesion activity of CB polyphenol-enriched matrices, PAC isolated from the matrices, and CJC.
PAC were extracted from CB polyphenol-enriched matrices and separated using solid-phase chromatography according to a well-established method for PAC isolation.26 Briefly, the powdered matrices (4.0 g) were homogenized with 70% aqueous acetone (40 mL × 3), filtered and the sediment discarded. The collected extracts were concentrated under reduced pressure to remove acetone, suspended in water, and applied to preconditioned C-18 solid phase chromatography columns. Columns were washed with water to remove sugars, followed by acidified 20% aqueous methanol to remove organic acids. The polyphenolic fractions containing ANC, flavonol glycosides and PAC were eluted with 100% methanol and dried under reduced pressure. These fractions were suspended in 50% ethanol, and applied to pre-conditioned Sephadex LH-20 columns which were washed with 50% ethanol to remove low molecular weight ANC and flavonol glycosides. PAC adsorbed to the LH-20 were eluted from the column with 70% aqueous acetone, and monitored using diode array detection at 280 nm. The absence of absorption at 360 nm and 450 nm confirmed that ANC and flavonol glycosides were removed. Acetone was removed under reduced pressure and the resulting purified PAC fraction was freeze-dried and weighed. Methods including 13C NMR, electrospray mass spectrometry, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and acid catalyzed degradation with phloroglucinol have previously been utilized to confirm the presence and purity of PAC routinely isolated in the extract.8,9,26,27
Both CB polyphenol-enriched matrices and isolated PAC were tested for in vitro bacterial anti-adhesion activity on a per weight basis. Whole product samples were suspended in phosphate buffer solution (PBS) at 60 mg/ml, and isolated PAC at 5 mg/mL. All samples were then neutralized with 1 N NaOH, diluted serially (2-fold), and tested for bacterial anti-adhesion activity utilizing a mannose-resistant HRBC (A1, Rh+) hemagglutination assay specific for uropathogenic P-fimbriated E. coli according to Foo.8,9 A 30-μL drop of each dilution was incubated with 10 μL of bacterial suspension on a 24-well polystyrene plate for 10 min at room temperature on a rotary shaker. Freshly drawn HRBCs (A1, Rh+) were suspended (3%) in PBS and added separately (10-μL drops) to test suspensions, which were then incubated for 20 min on a rotary shaker at room temperature and evaluated microscopically for the ability to prevent agglutination. The concentration at which hemagglutination activity was suppressed by 50% was recorded as the endpoint for the assay and was considered the minimum inhibitory concentration (MIC); the lower the MIC, the higher the anti-adhesion activity of the sample. Negative controls included wells containing bacteria + PBS, HRBC + bacteria + test material. A well containing bacteria + HRBC served as a positive control for agglutination. A standard sample was run with each batch (a frozen sample of Ocean Spray 90 MX powder at a starting concentration of 60 mg/mL was serially diluted with an expected MIC of 7.5 mg/mL).
Antibacterial Assay
For the antibacterial assay, CB polyphenol-enriched SPI was prepared with CJC diluted 1:3 v/v and sorbed to SPI at a ratio of 10 g/L. This ratio minimized the volume of base that would be needed to bring the pH of the media to pH 5. The CB polyphenol-enriched SPI was screened against Staphylococcus aureus and Escherichia coli K12. Bacterial strains were maintained on solid MHA medium (Mueller Hinton Agar 2, Sigma). For assay preparation, bacteria were inoculated into 30 mL of liquid MHB medium (Mueller Hinton Broth 2, Sigma) and grown overnight at 37 °C, with shaking at 130 rpm. Overnight bacterial cultures (100–200 μL) were transferred into 10 mL MHB to obtain optical densities equal to McFarland Turbidity Standard No 0.5, which gives a density of 1.5 × 108 cells/mL. Optical densities were measured at 590 nm on a Synergy HT Multi-Detection Microplate Reader (BioTek, Winooski, VT). The bacterial suspensions were then diluted to 5 × 105 cells/mL for antibacterial assays. CB polyphenol-enriched SPI matrix was added to sterile plastic tubes at concentrations of 0, 0.1%, 0.5%, 1%, 1.5%, 2% and 3% in MHB. Media was adjusted to pH 5–6, if needed, with 6 M HCl or 5 M NaOH in a duplicate set of tubes and the volume recorded. The same volume of acid or base was added to the sterile tubes to adjust pH without introducing contaminants. Bacterial suspensions (600 μL) were added to each tube and incubated for 24 h at 37 °C, with shaking at 130 rpm. Subsequently, 100 μL liquid medium was removed from each tube and spread on the surface of the MHA containing Petri plates. After overnight incubation at 37 °C, the number of bacterial colonies on Petri plates was recorded.
Statistics
Statistical analysis was performed with GraphPad Prism v6 (GraphPad Software, Inc., La Jolla, CA). A one-way ANOVA analysis and a Tukey post-hoc multiple comparison test were done with a significance threshold of 0.05.
RESULTS AND DISCUSSION
Capacity of Protein-rich Matrices to Sorb CB Polyphenols
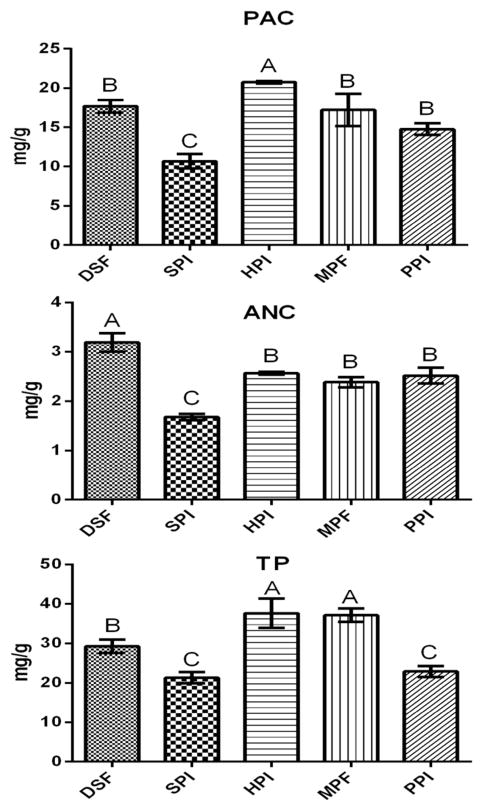
Due to the high viscosity of the CJC (50 °Brix) which interferes with the sorption process, it was diluted 1:4 v/v with water (11.9 °Brix) before complexation with the matrices. Five protein-rich matrices (DSF, SPI, HPI, MPF and PPI) were added to the diluted CJC at a concentration of 30 g/L, mixed, centrifuged, and the pelleted material was freeze-dried. PAC, ANC and TP were measured before and after separation of the pelleted matrices from liquid using DMAC, pH differential and Folin Ciocalteu assays, respectively. Matrices were able to sorb and concentrate between 60% – 83% of PAC, 6% – 46% of ANC, and 45–71% of TP from the 5× CJC, at 30 g/L (Supplemental Figures 1–3). CB polyphenol-enriched matrices were eluted with acidified methanol and these same assays were conducted on the eluates. The highest PAC concentration was found in the CB-HPI matrix (20.75 mg/g), followed by CB-DSF (17.67 mg/g) and CB-MPF (17.22 mg/g). The highest total polyphenolic content was found in CB-HPI and CB-MPF (37.61 and 37.12 mg/g, respectively), followed by CB-DSF (29.24 mg/g). The lowest PAC and TP contents were eluted from the CB-SPI (10.68 and 21.29 mg/g, respectively). The highest ANC concentration was achieved in the CB-DSF (3.19 mg/g) and the lowest in CB-SPI (1.68 mg/g) (Figure 1). Matrices differ in affinities to cranberry polyphenols in ways which are not completely understood, but which may be a consequence of the distinctive physicochemical properties of their protein components, differences in particle size, surface area, degree of solubility in the juice and the fact that other components of the matrices, such as carbohydrates, can also bind polyphenols. 20
Figure 1.
Proanthocyanidins (PAC), anthocyanins (ANC) and total phenolics (TP) in the cranberry enriched matrices calculated as mg/g. CB = cranberry, DSF = defatted soy flour, SPI = soy protein isolate, HPI = hemp protein isolate, MPF = partially defatted medium roast peanut flour, PPI = pea protein isolate. Means with different letters are significantly different (Tukey’s post-hoc test, p < 0.05).
Characterization of Polyphenols in the Enriched Matrices
Compounds eluted from the CB polyphenol-enriched matrices were characterized by HPLC, LC-ESI-MS, and MS/MS. LC-ESI-MS confirmed a series of masses corresponding to CB polyphenolic components, in both the negative and positive modes, indicating that the protein-rich matrices efficiently captured a broad range of polyphenolic compounds. Table 1 exemplifies the main compounds eluted from CB-HPI, including ANC, PAC oligomers, and flavonols.
Table 1.
Compounds identified in the cranberry polyphenol-enriched hemp protein isolate (HPI).
| Compound | MS m/z | MS/MS m/z | UV Nm | RT (min) |
|---|---|---|---|---|
|
| ||||
| Cyn-3-gal | 449 [M]+ | 287 | 524, 279 | 14.71 |
| Cyn-3-glc | 449 [M]+ | 287 | 524, 277 | 15.35 |
| Cyn-3-arb | 419 [M]+ | 287 | 524, 277 | 18.51 |
| Peo-3-gal | 463 [M]+ | 301 | 517, 279 | 19.61 |
| Peo-3-glc | 463 [M]+ | 301 | 515, 279 | 20.61 |
| Peo-3-arb | 433 [M]+ | 301 | 520, 279 | 21.67 |
|
| ||||
| Qur-3-gal | 465 [M+1]+ | 303 | 351, 254 | 24.26 |
| Qur-3-ara | 435 [M+1]+ | 303 | 358, 255 | 24.54 |
| Qur-3-rha | 449 [M+1]+ | 303 | 355, 260 | 24.78 |
| Myr-3-gal | 481 [M+1]+ | 319 | 356, 268, 240 | 23.6 |
| Qur | 303 [M+1]+ | 229 | 363, 257 | 25.39 |
|
| ||||
| Catechin | 289 [M-1]− | 245 | 279 | 10.81 |
| Epicatechin | 289 [M-1]− | 245 | 279 | 17.69 |
| PAC dimer-A2 | 575 [M-1]− | 423, 287 | 279, 232 | 23.1 |
| PAC dimer-B2 | 577 [M-1]− | 425, 287 | 279, 232 | 13.5 |
| PAC trimer-A | 863 [M-1]− | 575 | 277, 240 | 20.7, 21.4, 23.3 |
| PAC trimer-B | 865 [M-1]− | 577 | 279, 241 | 19.147 |
| PAC tetramer-A | 1151 [M-1]− | 423 | 277, 239 | 23.35 |
Cyn = cyanidin, Peo = peonidin, Qur = quercetin, Myr = myricitin, PAC = proanthocyanidin, gal = galactoside, glc = glucoside, arb = arabinoside, rha = rhamnoside
Quantification of PAC by Normal Phase HPLC-fluorescent Detection
Separation of CB PAC monomers and oligomers was achieved by HPLC coupled to a fluorescence detector using a Develiosil Diol column.24 Compounds were successfully separated according to their degree of polymerization, showing strong signals for monomers through tetramers. Smaller but clearly identifiable signals were also obtained for pentamers and hexamers (Figure 2). PAC were identified with reference to standard compounds (catechin, epicatechin, PAC-A2 and PAC-B2), and with the aid of the literature.24 Monomeric and oligomeric PAC components were quantified as PAC-A2 equivalent (Table 2). CJC contained both PAC A- and B-type dimers to tetramers with higher levels of A-type. As shown in Table 2, all of the protein-rich matrices successfully captured those PAC from the CJC at different levels. Representative HPLC chromatograms of cranberry juice concentrate and the eluate from CB-SPI are illustrated in Figure 4. Parallel to the results obtained from the DMAC assay, the best matrices for capturing monomeric and lower oligomeric PAC were MPF, HPI and DSF (Table 2).
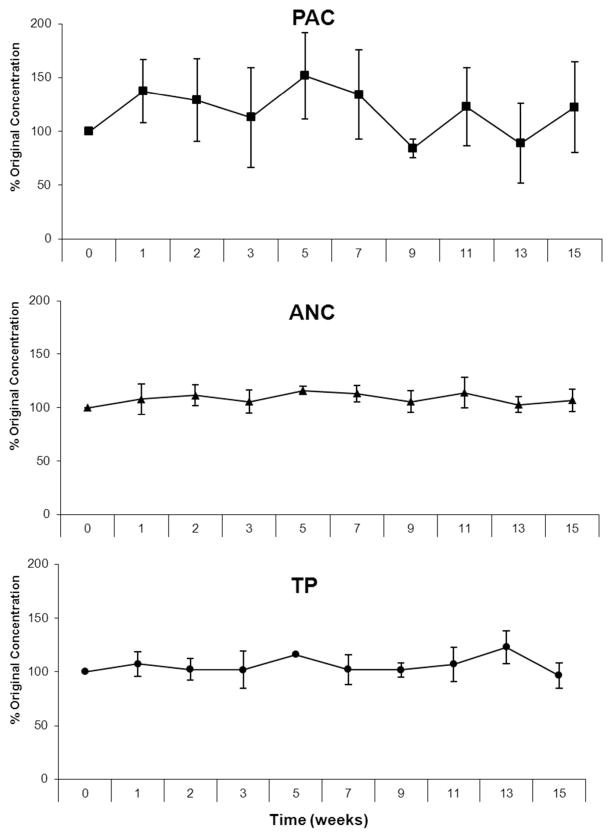
Figure 2.
Stability of cranberry proanthocyanidins (PAC), anthocyanins (ANC) and total phenolics (TP) bound to soy protein isolate at 37 °C for 15 weeks. There was no significant difference between times of measurement at P > 0.05.
Table 2.
Proanthocyanidin monomers to tetramers in the cranberry polyphenol-enriched protein-rich matrix eluates (mg/g), using fluorescence HPLC detection, quantified as procyanidin A2 (mean ± SD).
| Proanthocyanidin | CB-DSF mg/g | CB-SPI mg/g | CB-HPI mg/g | CB-MPF mg/g | CB-PPI mg/g |
|---|---|---|---|---|---|
| Monomers | 0.70 ± 0.00 | 0.61 ± 0.2 | 0.78 ± 0.01 | 0.74 ± 0.01 | 0.58 ± 0.00 |
| Dimer-A2 | 1.64 ± 0.01 | 1.24 ± 0.04 | 1.73 ± 0.01 | 1.71 ± 0.01 | 1.40 ± 0.02 |
| Dimer-B2 | 0.55 ± 0.00 | 0.5 ± 0.01 | 0.54 ± 0.00 | 0.60 ± 0.00 | 0.51 ± 0.01 |
| Trimer A-type | 1.00 ± 0.00 | 0.74 ± 0.02 | 0.94 ± 0.01 | 1.05 ± 0.00 | 0.90 ± 0.01 |
| Trimer B-type | 0.41 ± 0.17 | 0.38 ± 0.02 | 0.45 ± 0.00 | 0.45 ± 0.23 | 0.35 ± 0.01 |
| Tetramers | 0.75 ± 0.02 | 0.53 ± 0.00 | 0.80 ± 0.01 | 0.80 ± 0.00 | 0.67 ± 0.02 |
| Total (monomers –tetramers) | 5.03 ± 0.04 | 4.00 ± 0.10 | 5.24 ± 0.05 | 5.33 ± 0.04 | 4.37 ± 0.07 |
CB = cranberry; DSF = defatted soy flour; HPI= hemp protein isolate; MPF = medium roast peanut flour; PPI = pea protein isolate
Figure 4.
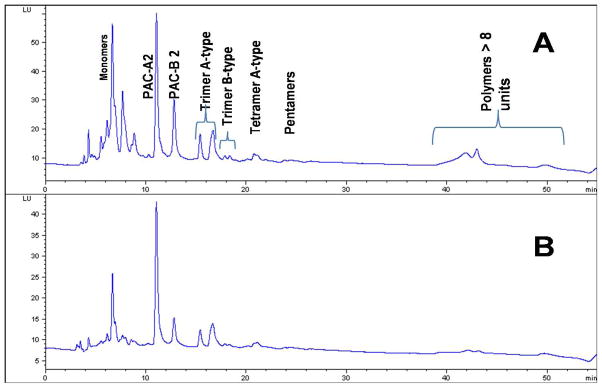
Fluorescence-HPLC of 5× cranberry juice concentrate (A), and eluted proanthocyanidins from cranberry polyphenol-enriched soy protein isolate (B). HPLC conditions: Develiosil Diol column, Solvent A: acidic acetonitrile; Solvent B: aqueous acidic methanol, fluorescence excitation and emission wavelengths 230 and 321 nm.
Stability of Polyphenols Bound to Matrices
The efficiency of the sorption process to stabilize cranberry polyphenolic components was evaluated by measuring polyphenolic content in two representative matrices (CB-SPI and CB-DSF) under storage conditions. CB-SPI matrix was subjected to a stability test by incubation at 37 °C for 15 weeks. Prior to incubation (0 time), samples were eluted for polyphenolics and quantified for PAC, ANC and TP. Aliquots of CB-SPI were removed after each incubation interval and polyphenols were quantified as percentage of the original amounts that were eluted on day 0. Levels of PAC, ANC and TP in the CB-SPI were remarkably stable for 15 weeks of incubation at 37 °C with no significant differences. For PAC analysis, it was noticed that the readings from one of the three replicates was consistently an outlier that caused flocculation in the stability curve, but the PAC data over the entire period for each of the triplicate samples were consistent with stability (Figure 2).
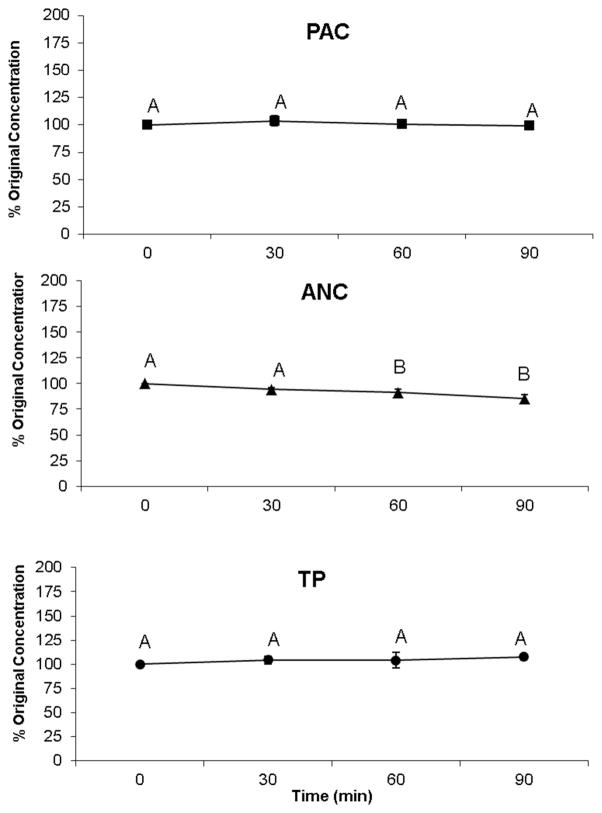
Another short-term stability test was performed on CB-DSF to study the effect of high temperature on the PAC, ANC and TP in the matrix. The CB enriched matrix was incubated at 80 °C for 90 min. Aliquot samples were analyzed at 30, 60 and 90 min, and polyphenols were represented as percentage of the concentration at 0 min (Figure 3). Both PAC and TP were remarkably stable during the 80 °C treatment. ANC levels appeared to be stable during the first 30 min, then slightly declined in concentration; with a significant decline by the 60 min time point, followed by another phase of stability for the next 30 min.
Figure 3.
Stability of cranberry proanthocyanidins (PAC), anthocyanins (ANC) and total phenolics (TP) bound to at 80 °C for 90 minutes. Means with different letters are significantly different (Tukey’s post-hoc test, p < 0.05).
Both stability experiments suggest that CB polyphenol-complexation with SPI or DSF preserved the integrity of the polyphenolic components and protected them from degradation when stored for up to 15 weeks of incubation at 37 °C. ANC was the only group which showed slight decline at 80 °C treatment, and this phenomenon is well known for ANC at high temperatures.28 These results agree with our previous stability tests performed on DSF complexed with blueberry polyphenols.20
Nutritional and Sensory Evaluations
A representative subset of the CB polyphenol-enriched SPI and PPI matrices was subjected to nutritional analysis to evaluate the effect of the complexation process on proximates (the macronutrients that describe the food matrix itself). The food matrices were evaluated before and after treatment with diluted CJC. An increase in the carbohydrates and sugar percentages can be explained by the sorption of some sugars from the CJC to the matrix. In spite of the increase in carbohydrate and sugar contents, the increase in calories per 100 g powder was very small (~5%). Protein content decreased by ~ 25–35% which can be explained by some solubility of the matrix in the aqueous juice. Results are summarized in Table 3.
Table 3.
Nutritional evaluation of the cranberry polyphenol-enriched soy protein isolate and pea protein isolate per 100 g powders
| Assay | Untreated Soy Protein Isolate | Cranberry/ Soy Protein isolate | Untreated Pea Protein isolate | Cranberry/ Pea Protein isolate |
|---|---|---|---|---|
|
| ||||
| Carbohydrates % | 0.00% | 26.40 | 0.50 | 35.10 |
| Calories/100g | 384 | 403 | 391 | 415 |
| Calories from Fat/100g | 32.0 | 41.0 | 72.0 | 70.0 |
| Ash% | 3.04 | 2.05 | 4.35 | 2.07 |
| Moisture% | 6.24 | 2.92 | 7.81 | 3.95 |
| Protein% | 87.9 | 64.00 | 79.40 | 51.00 |
| Total Fat % | 3.59 | 3.59 | 7.96 | 7.83 |
| Sugars % | ||||
| Fructose | < 0.1 | 1.38 | < 0.1 | 2.32 |
| Glucose | < 0.1 | 6.81 | < 0.1 | 11.50 |
| Sucrose | < 0.1 | < 0.1 | 0.37 | < 0.1 |
| Totals Sugars % | 0.00 | 8.20 | 0.37 | 13.82 |
Descriptive sensory analysis was performed on one representative sample to understand the magnitude and types of differences between an untreated matrix (SPI) and the same matrix enriched with CB phytochemicals. The degree of difference score (DOD) gave qualitative information about distinguishing features and any differences seen. DOD scale is a 0 to 10 rating indicating how different a product is from a reference product or control, with 0 meaning no difference and 10 being extremely different. Results are summarized in Table 4. The difference was very noticeable with a DOD of 9. One panelist likened the CB-SPI to a Sweet-Tart™ or War Head™ candy (Candy Warehouse, CA), another to freeze-dried strawberries (more sour than berry). Sensory Spectrum experts determined that CB-SPI could have application for berry flavored smoothies, protein bars, or similar formulations. Sweeteners would be needed to balance the sour and astringent flavors.
Table 4.
Descriptive sensory analyses of soy protein isolate (SPI) and cranberry polyphenol-enriched SPI.
| Sample | SPI | CB-SPI |
|---|---|---|
| Degree of Difference (DOD) | - | DOD* = 9 (driven by flavor) |
| Appearance | Beige, fine powder | Intense Pink, fine powder (finer) |
| Flavor | Low flavor intensity, nutty-roasted; Roasted beany; Cardboard; Bitter taste Typical for roasted soy powder | Almost no soy flavor; Very Sour Very Astringent; Late general berry flavor – not specifically cranberry; (Note: has berry aroma) |
| Texture | Fine powdery texture with some grit | Finer powdery texture, may be more soluble in liquid |
DOD scale is a 0 to 10 rating indicating how different a product is from a reference product or control, with 0 meaning no difference and 10 being extremely different
Anti-adhesion/Anti-UTI Activity
Agglutination of human red blood cells (HRBCs) by uropathogenic E. coli is used as a model for bacterial adhesion to uroepithelial cells.26 The CB polyphenol enriched matrices were evaluated for their bacterial anti-adhesion/anti-UTI properties in vitro in a 2-fold dilution series as a whole product at a starting concentration of 60 mg/mL. Results indicated weak activities of all CB enriched matrices due to insolubility of the powders in the test solutions. To determine if CB polyphenol enriched matrices retained the anti-adhesion activity, PAC components were isolated from the enriched matrices, measured gravimetrically, and then tested for their anti-adhesion activity in comparison to PAC isolated from CJC. PAC isolated from all matrices were effective in inhibiting HRBC agglutination at MIC of 0.04–0.08 mg/mL with no significant differences. The MIC of PAC isolated from CJC was 0.16 mg/mL with no significant difference from the PAC isolated from the matrices, indicating that all tested protein-rich matrices were effective in preserving the activity of the PAC components. Table 5 summarizes the anti-adhesion activity of enriched matrices, PAC isolated gravimetrically and anti-adhesion activity of isolated PAC. In addition, the quantity of dry enriched matrix equivalent to 300 mL CB juice cocktail (containing 36.0 g PAC) in terms of PAC amounts is also included for reference, and ranged from 0.9 to 1.2 g CB polyphenol-enriched matrix (Table 5).
Table 5.
Anti-adhesion activity of cranberry polyphenol-enriched matrices, proanthocyanidins (PAC) levels measured gravimetrically after isolation, equivalencies of matrices to PAC content in 300 mL cranberry juice cocktail, and anti-adhesion activity isolated PAC.
| Sample | Anti-adhesion activity of whole sample MIC mg/mL | PAC level (Gravimetric) | Amount of matrix equivalent to 300 mL CB cocktail (g) | Anti-adhesion activity of isolated PAC MIC mg/mL* |
|---|---|---|---|---|
|
| ||||
| CB-DSF | 120 | 43.3 mg/g | 1.2 | 0.04 |
| CB-SPI | 60 | 33.9 mg/g | 0.9 | 0.04 |
| CB-HPI | 120 | 42.7 mg/g | 1.2 | 0.08 |
| CB-MPF | 120 | 43.3 mg/g | 1.2 | 0.08 |
| CB-PPI | 60 | 30.8 mg/g | 0.9 | 0.08 |
| CJC (1×) | 9.9 | 6.5 mg/mL | NA | 0.16 |
CB = cranberry, DSF = defatted soy flour; SPI = soy protein isolate; HPI = hemp protein isolate; MPF = medium roast peanut flour; PPI = pea protein isolate, CJC (1×) = cranberry juice concentrate (undiluted), MIC = minimum inhibitory concentration.
No significant differences between results
Anti-bacterial Activity
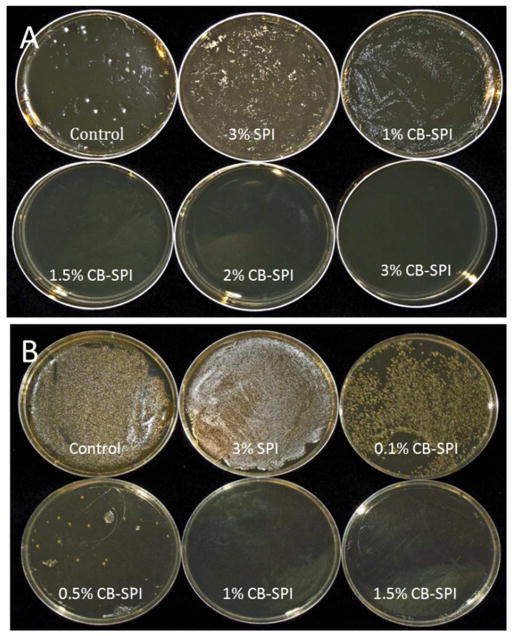
The antibacterial activities of CB-SPI, prepared with 4× dilution of CJC at 10 g/L (190 mg/g TP) were tested. Varied concentrations of CB-SPI were prepared in liquid MHB media-containing tubes, and bacteria were added to each tube. All tubes containing CB-SPI and bacteria were incubated in liquid MHB medium for 24 hours at 37 °C. For determination of bacterial growth, liquid culture aliquots were plated onto agar media. The lowest concentration of CB-SPI inhibiting any visual microbial growth was determined. For E. coli the MIC of CB-SPI was 1.5% (Figure 5A). In other experiments, the concentration of 1% CB-SPI was found to be sufficient for complete inhibition of S. aureus growth (Figure 5B). Therefore, CB-SPI demonstrated antibiotic activity against both gram-positive and gram-negative bacteria. CB polyphenol enriched matrix, as exemplified by CB-SPI, proved to have potent antimicrobial activity. The ability to inhibit bacterial growth suggests potential applications in foods and cosmetic industries, as a natural alternative to currently available antimicrobial compounds.
Figure 5.
Cranberry polyphenol-enriched soy protein isolate (CB-SPI) inhibited microbial growth of Escherichia coli (A) and Staphylococcus aureus (B). Visual microbial growth shows that the 1.5% CB-SPI completely inhibited the colony growth of E. coli, whereas 1% inhibited the growth of S. aureus.
In summary, the five food matrices tested in this study were able to sorb, concentrate and stabilize CB PAC, ANC and TP. The protein content in the tested matrices ranged from ~ 50% (DSF, MPF) to > 70% (HPI, SPI and PPI). All matrices efficiently sorbed high concentrations of polyphenolic components, especially HPI, DSF and MPF. There was no linear correlation between protein content and sorbing capacity, which indicated that other factors play a role in the capacity of each matrix to capture polyphenol components from the juice. CB PAC, ANC, flavonols could be eluted easily from the enriched matrices and identified chromatographically. Complexation with the protein matrices did not alter the chemical composition of CB phytochemicals. Our study showed that the five tested matrices retain their biological activity in preventing E. coli-mediated agglutination of HRBCs in vitro. In terms of PAC content, it is demonstrated that 0.9 to 1.2 g of polyphenol-enriched matrix is equivalent to one cup (300 mL) of commercial CB juice cocktail, which is the typically-recommended prophylactic dose for recurring UTIs. In addition, the prepared CB-SPI prototype showed both gram-positive and gram-negative antibacterial activities. The 15 weeks stability study proved that the complexation product stabilizes the polyphenols bound to SPI and results in a product with long shelf life. The nutritional analysis and sensory characteristics of the products suggest the potential for their commercial use in highly functional food products.
Supplementary Material
Acknowledgments
We thank The Cranberry Network, LLC (Wisconsin Rapids, WI) for providing the cranberry juice concentrate. This work was supported by an SBIR Phase 1 grant from National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number: 1R43DK092104-01A1.
Footnotes
Supporting Information Available: Supplementary Figures 1–3. This material is available free of charge via the Internet at http:pubs.acs.org.
References
- 1.Cassileth BR, Heitzer M, Wesa K. The public health impact of herbs and nutritional supplements. Pharm Biol. 2009;47:761–767. doi: 10.1080/13880200902991581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta K, Chou MY, Howell A, Wobbe C, Grady R, Stapleton AE. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urology. 2007;177:2357–2360. doi: 10.1016/j.juro.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell A, Botto H, Combescure C, Banc-Potard A-B, Gausa L, Matsumoto T, Tenke P, Sotto A, Lavigne J-P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multcentric randomized double blind study. BMC Infectious Diseases. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51:738–745. doi: 10.1002/mnfr.200600275. [DOI] [PubMed] [Google Scholar]
- 5.Lavigne JP, Bourg G, Combescure C, Botto H, Cotto A. In vitro and in vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin Microbiol Infect. 2008;14:350–355. doi: 10.1111/j.1469-0691.2007.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YL, Najm W, Owens J, Thrupp L, Baron S, Shanbrom E, Cesario T. Anti-microbial activity of urine after ingestion of cranberry: A pilot study. Evi-Based Compl Alt Med. 2010;7:227–232. doi: 10.1093/ecam/nem183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DasGupta R, Sullivan R, French G. Evidence-based prescription of antibiotics in urology: a 5-year review of microbiology. Brit J Urol Int. 2009;104:760–764. doi: 10.1111/j.1464-410X.2009.08779.x. [DOI] [PubMed] [Google Scholar]
- 8.Foo LY, Lu Y, Howell AB, Vorsa N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 9.Foo LY, Lu Y, Howell AB, Vorsa N. The structure of the cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173–181. doi: 10.1016/s0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 10.Bodet C, Grenier D, Chandad F, Ofek I, Steinberg D, Weiss EI. Potential oral health benefits of cranberry. Crit Rev Food Sci Nutr. 2008;48:672–80. doi: 10.1080/10408390701636211. [DOI] [PubMed] [Google Scholar]
- 11.La VD, Howell AB, Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob Agents Chemother. 2010;54:1778–1784. doi: 10.1128/AAC.01432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Liu RH. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2006;241:124–134. doi: 10.1016/j.canlet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC. 2002 Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon) J Agric Food Chem. 2002;50:5844–5849. doi: 10.1021/jf0202234. [DOI] [PubMed] [Google Scholar]
- 14.Porter ML, Krueger CG, Wiebe DA, Cunningham DG, Reed JD. Cranberry proanthocyanidins associate with low-density lipoprotein and inhibit in vitro Cu2+-induced oxidation. J Sci of Food Agric. 2001;81:1306–1313. [Google Scholar]
- 15.Wilson T, Porcari JP, Harbin D. Cranberry extract inhibits low density lipoprotein oxidation. Life Sci. 1998;62:A381–386. doi: 10.1016/s0024-3205(98)00204-5. [DOI] [PubMed] [Google Scholar]
- 16.Neto CC. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 17.Avorn J, Monane M, Gunvitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271:751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- 18.Grace MH, Massey AR, Mbeunkui F, Yousef GG, Lila MA. Comparison of health-relevant flavonoids in commonly consumed cranberry products. J Food Sci. 2012;77:H176–183. doi: 10.1111/j.1750-3841.2012.02788.x. [DOI] [PubMed] [Google Scholar]
- 19.Prior R, Lazarus S, Cao G, Muccitelli H, Hammerstone J. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem. 2001;49:1270–1276. doi: 10.1021/jf001211q. [DOI] [PubMed] [Google Scholar]
- 20.Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Poulev A, Howell A, Fridlender B, Lila MA, Raskin I. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 2012;131:1193–1200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999;299:152–178. [Google Scholar]
- 22.Giusti MM, Wrolstad RE. Unit F1.2: Anthocyanins. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE, editor. Current protocols in food analytical chemistry. John Wiley & Sons; New York: 2001. pp. 1–13. [Google Scholar]
- 23.Prior RL, Fan E, Ji H, Howell A, Nio C, Payne MJ, Reed J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric. 2010;90:1473–1478. doi: 10.1002/jsfa.3966. [DOI] [PubMed] [Google Scholar]
- 24.Wallace TC, Giusti MM. Extraction and normal-phase HPLC-fluorescence-electrospray MS characterization and quantification of procyanidins in cranberry extracts. J Food Sci. 2010;75:C690–C696. doi: 10.1111/j.1750-3841.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- 25.Brownmiller C, Howard LR, Prior RL. 2009 Processing and storage effects on procyanidin composition concentration of processed blueberry products. J Agric Food Chem. 2009;57:1896–902. doi: 10.1021/jf803015s. [DOI] [PubMed] [Google Scholar]
- 26.Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Reed JD, Krueger CG, Vestling MM. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry. 2005;66:2248–2263. doi: 10.1016/j.phytochem.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Castaneda-Ovando A, Pacheco-Hernandez MD, Paez-Hernandez ME, Rodriguez JA, Galan-Vidal CA. Chemical studies of anthocyanins: A review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.