Abstract
Designing newer drugs, vaccines, and diagnostic techniques is dependent on better understanding of M. tuberculosis virulence mechanism. In this study the prevalence of pcaA gene was determined in M. tuberculosis strains typed by spoligotyping. The associated risk factors among patients with different nationalities residing in Iran were also determined. The isolated M. tuberculosis strains have been characterized by performing susceptibility tests against four first-line antituberculosis drugs and were then subjected to spoligotyping characterization. PCR was used for detection of pcaA gene and its nucleotide sequence was also determined. Spoligotyping of M. tuberculosis strains resulted in 140 different patterns. One hundred twenty two (87.1%) of these spoligotype isolates were unique and reported for the first time. The remaining18 (12.8%) spoligotype patterns were previously reported from other geographical regions of the world. Haarlem family was most prevalent than other genotype. Antibiotic resistances were higher in those isolated from the Iranian patients. The pcaA gene was detected in M. tuberculosis clinical isolates but not in saprophyte strains such as M. kansasi. The results showed that, spread of M. tuberculosis strains belonging to the Beijing family among Iranian patients has to be considered seriously. This study confirmed the widespread existence of pcaA gene in almost all the clinical isolates. It is also important to undertake studies to identify which factors are the most significant to consider in tuberculosis control program.
Keywords: tuberculosis, resistance, drugs, spoligotyping, pcaA
INTRODUCTION
Molecular technology applied to understand the basis of transmission patterns of TB in the world (38). The most extensively used molecular epidemiology technique is Restriction Fragment Length Polymorphism (RFLP) typing, which uses the insertion sequence IS6110 to differentiate clinical isolates (5,37). Polymerase Chain Reaction (PCR) is the most sensitive method in the diagnosis of clinically suspected tuberculosis (1,8,25). New typing methods based on the PCR, such as spoligotyping (18), and mycobacterial interspersed repetitive units (MIRU) typing have also been described (34).
Spoligotyping, developed by Kamerbeek et al., in 1997 (18) can simultaneously detect and type M. tuberculsis strains. This method is based on DNA polymorphism within the direct repeat (DR) locus of M. tubeculosis. This locus contains multiple, well conserved 39-bp DRs interspersed with nonrepetitive spacer sequences which are 34 to 41 bp long. Strains vary in the number of DRs and in the presence or absence of particular spacers. This technique requires minimal quantities ofDNA and has the potential to be used directly on clinical specimens without the need for prior culture.
Mycobacterium tuberculosis cells have a complex structure that contains many unique lipids and glycolipids (3,42). The bacterium synthesizes mycolic acids, very long chain α-alkyl, β-hydroxyl fatty acids, in three different classes that predominantly located in cell envelope. The three mycolic acids includ α-, methoxy-, and keto-mycolates (35,43). The resident cyclopropane rings and methyl branches can be modified through the combined action of a large family of S-adenosyl methionine (SAM)-dependent methyl transferases (2). The double bonds in the meromycolate chain are then modified resulting in the exhibition of exquisite substrate specificity (14).
Recently a mutant of M. tuberculosis have been created that failed to produce the rope-like (corded) colony structures characteristic of virulent strains and was unable to establish chronic infections in mice. The disrupted gene in the mutant strain coded for the enzyme, cyclopropane synthetase, which modifies mycolic acids moieties. The enzyme catalyzes the formation of a three-membered carbon ring structure on alpha mycolate. The gene was named pcaA for “proximal cyclopropanation of alpha mycolate” (15). PcaA was recently shown to be crucial to M. tuberculosis persistence and virulence in vivo (14). In this work, the frequency of pcaA gene occurrence was determined among the clinical M. tuberculosis isolates in Iran.
MATERIAL AND METHODS
This study involved a total of 523 patients that referred to the National Research Institute of Tuberculosis and Lung Disease (NRILTD), the referral tuberculosis center in Iran during March 21st -2004 to March 21st -2005.Laboratory procedures for determining drug resistance were performed by the indirect proportion method (30).
DNA extracts for spoligotyping were prepared by using the classical cetyltrimethylammonium bromide method (39). Spoligotyping by use of a set of 43 spacers was carried out as described by Kamerbeek et al. (18). Spoligotype patterns were designated with hexadecimal codes and/or arbitrary database numbers as described by Dale et al. (7). Typing results were analyzed and compared with world spoligotyping database as described by Sola et al. (4).
The pcaA gene was amplified by PCR, using two sets of primers; pcaA-1 and pcaA-2. To determine the sequence of pcaA (GenBank accession no. BX842573) from various strains of Mycobacteria isolated from humans, primer pcaA -1 (5’ACGCCGCATTTTGGAAAC -3’), correspondingto bp 1-18 of the M. tuberculosis pcaA gene, and primer pcaA-2 (5’TTTTCCAGTGTGAACTGGTCG -3’), corresponding to bp 824-845 of the same gene were used. PCR was performed using a reaction buffer composed of 10 mM Tris/HCl, pH 8.8; 50 mM KCl; 1.5 mMMgCl2; 0.125 μM of the above-mentioned primers; 0.125 mM dNTPs; 1.25 units Taq DNA polymerase and 10-100 ng DNA. PCR amplification was performed in a DNA thermalcycler, set for 5 min at 94ºC denaturation step, followed by 30 cycles at 94ºC for 0.5 min, 58ºC for 0.5 min,and 72ºC for 1 min and followed by a 10-min extension at 72ºC. PCR products were electrophoretically fractionated in 0.8% agarose gel (1x Trisborate-EDTA [pH 8.3]) and then visualized under UV after ethidium bromide staining. PCR products were purified with the PCR purification kit (Roch-Germany) and sequences of these gene products were determined by automated dideoxy sequencing method.
RESULTS
We enrolled 523 patients with TB, who presented to the NRITLD during the March 21st -2004 to March 21st -2005. Of the 523 patients, 338(65.6%) were Iranians and 185(35.4%) were Afghan patients. There was a striking difference in the prevalence of drug resistance in response to different drugs (Fig. 1). The rate of MDR strains was higher in the Iranian patients relative to those isolated from the Afghani patients, as shown in (Fig. 2). Microscopic smear for acid fast bacilli (AFB) were done on sputum and other specimens from 523 patients. Of these, 405(77.4%) had smear positive pulmonary tuberculosis (Table 1). One hundred forty distinct genotypes of M. tuberculosis were identified which belonged to three evolutionary groups (I, II, and III). There were 122 (87.1%) unique spoligotype patterns. The remaining isolates 18(12.8%) belonged to spoligotype clusters that shared with world spoligotyping database and were also reported from other geographical regions of the world. (Table 2) shows the number of patients in each cluster. Group III is more predominantly seen in Iranians but was less prevalent in Afghan patients (Table 1). Haarlem family was the most prevalent family in this study. T family and EAI family were in the second and third level (Table 2). The Beijing strains were most common in the Beijing province of China, accounting for 92% of strains (28,40). These strains were also observed in some other Asian countries (16). Our study indicated that 6.4% of the strains belonged to the Beijing Family (Table 1). The pcaA gene was detected in all clinical M. tuberculosis isolates but it was not seen in any of the environmental Mycobacterial isolates such as in M. kansas I (Fig. 3). Also, the sequence of PCR fragment was determined and compared with Gene Data Bank (GenBank accession no. BX842573).
Figure 1.
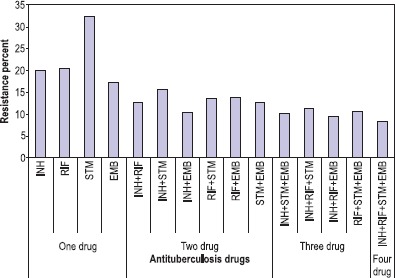
Resistance patterns to antituberculosis drugs among M. tuberculosis isolates from 523 TB patients (P<0.002).
Figure 2.
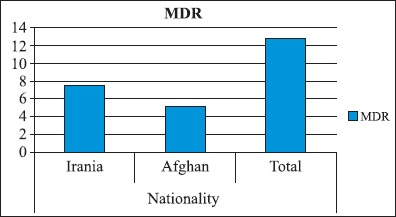
The distribution pattern of Multidrug-Resistance (MDR) strains in reference to patient’s nationalities (P<0.005).
Table 1.
Correlation between nationality and direct smear, sex, sample source, Beijing family and Major group
| Nationality | |||||
|---|---|---|---|---|---|
| Iranian | Afghani | ||||
| Count | % | Count | % | ||
| SMEAR | + | 268 | 51.2 | 137 | 26.2 |
| - | 70 | 13.4 | 48 | 9.2 | |
| Sample source | Sputum | 285 | 54.5 | 173 | 33.1 |
| Bronchial | 21 | 4 | 6 | 1.1 | |
| Pleural fluid | 1 | 0.2 | 2 | 0.4 | |
| Urine | 2 | 0.4 | 1 | 0.2 | |
| Lymph node | 2 | 0.4 | 1 | 0.2 | |
| Biopsy | 11 | 2.1 | 2 | 0.4 | |
| Abscess | 1 | 0.2 | 0 | 0 | |
| G/W | 8 | 1.5 | 0 | 0 | |
| BAL | 7 | 1.3 | 0 | 0 | |
| SEX | Male | 216 | 41.3 | 128 | 24.5 |
| Female | 122 | 23.3 | 57 | 10.9 | |
| Family | Non-Beijing | 321 | 61.4 | 170 | 32.5 |
| Beijin | 17 | 3.5 | 15 | 2.9 | |
| Major group | Group 1 | 181 | 34.6 | 150 | 28.7 |
| Group 2 | 104 | 19.9 | 29 | 5.5 | |
| Group 3 | 53 | 10.1 | 6 | 1.1 | |
Table 2.
Octal presentation of the clinical M. tuberculosis strains isolates Spoligotype.
| Row Labels | No. of patients | octal code | |
|---|---|---|---|
| AFRI | 1 | 570071740030400 | |
| CAS1 | 18 | 703777740003771 | |
| EAI | 31 | 777777775410771 | |
| Haarlem | 75 | 777777775420771 | |
| LAM2 | 1 | 670000777760531 | |
| Manu | 4 | 777777777423571 | |
| T | 3 | 707757177760771 | |
| T1 | 34 | 777777777760771 | |
| T2 | 2 | 777777777760731 | |
| undefined | 37 | 777777777777771 | |
| W-Beijing | 14 | 000000000003771 | |
| Total | 220 |
Figure 3.
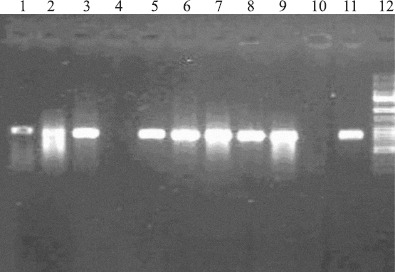
Gel electrophoresis of the PCR amplified pcaA gene products (M. tuberculosis lanes 1, 2, 3, 5, 6, 7, 8, 9, and 11; M. Kansasi lane 4; Negative control lane 10, lane 12 marker 50–1000bp).
DISCUSSION
Mycobacterium tuberculosis currently infects one-third of the world population, and cause 2.9 million deaths annually (11,24). It is reported that tuberculosis has an incidence of 8 million new cases annually in the world with >19,000 cases/year in Iran (41). Therefore, development of new drug and vaccines is necessary for treatment and prevention of the disease. The primary aim of this study was: 1) determination of drug susceptibility in clinical isolates; 2) determination of spoligotyping pattern of the clinical strain isolated from Iranian and Afghani patients; 3) determination of the extent of Beijing strains prevalence among the clinical isolates; 4) detecting the prevalence of pcaA gene by PCR amplification with specific primers among the isolated strains.
The resistance rate for Isoniazid (INH), Rifampicin (RIF), Streptomycin (STM) and Ethambutol (EMB) were 20%, 20.5%, 32.4% and 17.4% respectively (Fig. 1). In a similar study conducted in Turkey, resistance rate were reported to be 16.2% for STM, and 11.6% for INH (19). In a Taiwanese study; 19.0% of the strains were resistant to INH, 6.1% to RIF, 15.7% to EMB, and 10.0% to STM (21). In a Brazilian study 13.8% of the strains were resistant to INH and 8.6% for STM (22).
We detected almost similar resistance rate to EMB, but much higher rate towards STM. The higher STM resistance rate may be due to the more widespread usage of these antituberculosis drugs in Iran. In comparison to the results of a similar study reported previously in Iran (23), we observed a much higher resistance rate to one drug as well as to a combination of two or more drugs.
Multidrug-resistance TB, defined as simultaneous resistance to the two most important drugs, INH and RIF, is a potential threat to tuberculosis control (29). Patients infected with strains resistant to multiple drugs are extremely difficult to cure, and the necessary treatment is much more toxic and expensive (27). Resistance to INH+RIF was 12.7% (Fig. 2). Other investigators reported INH+RIF resistance rates to be 11.4% (12), 8.9% (10) and 5.1% (21). Therefore, the MDR rate detected in this investigation was within the MDR isolation range observed elsewhere in the world.
Molecular epidemiology techniques can help to better trace infectious disease transmission, and monitor their mode of spread. We employed spoligotyping technique which is a rapid PCR-based identification system in order to differentiate between clinical M. tuberculosis isolates. Spoligotyping can be used to simultaneously detect and type M. tuberculosis present in clinical specimens, such as sputum, tissue, or bronchoalveolar lavage, a procedure that can be performed in less than 2 days. In addition, this method was used to type M. tuberculosis in sections of 40-years- old paraffin- embedded tissues and characterization of members of the Mycobacterium tuberculosis complex (MTB) in historic tissue samples (18,32,44). Evolutionary investigations indicated that, three genetic groups of M. tuberculosis are separable based on polymorphic nucleotides in katG codon 465 and gyrA codon 95 (33). Bacteria belonging to spoligotype major Group 2 and 3 failed to hybridize with spacer 33 to 36 (33). With respect to the world spoligotyping database and analysis of our spoligotyping patterns, 140 spoligotype patterns were observed which belonged to three major groups (Table 2). Additionally, 220 strains were subclassified into previously described families; 34% belong to the Haarlem family, 17.1% belong to the T family, 14% to the EAI familly, 8.6% to the CASI family, 6.3% to the W-Beijing family, and 1.3% to the Manu family (Table2). Although W-Beijing, Central Asian (CAS), and East-African-Indian (EAI) genotype families belong to principal genetic group 1; whereas, X (european-low banders), Latino-American and Mediterranean (LAM), Haarlem (H), and T families belong to principal genetic groups 2 and 3 (31). Ferdinand et al. (9) reported that 23.5% of isolates belonged to the EAI family, 9.1% to the CAS family, 4% to the Beijing family and 2.7% to the Manu family. In other study, 11.7% of the isolates belonged to T family, 7.3% to Haarlem family, and 3.2% to Beijing family (20). In the two mentioned investigations, most of the isolates were belong to EAI and T family whereas in this study, the majority belonged to the Haarlem family which indicates clonal spread of M. tuberculosis strains.
Beijing strains of M. tuberculosis are located in group 1 and are associated with MDR strains reported from most parts of the world (16). Strains of the Beijing genotype were first described in China and were already highly prevalent in 17 different areas around Beijing from 1956 to 1960 (40). These strains were also disseminated to the neighboring Asian countries, such as Mongolia, Thailand, South Korea, and Vietnam (40). Other far away places were later contaminated by these strains. Strains of the Beijing genotype have also been detected in South Africa, Colombia, and Gran Canaria Island (26,30,36). Spoligotyping seems to be both sensitive and specific for the Beijing family and is also easily comparable between different studies. IS6110 fingerprinting can also be used to detect this genotype family with results that correlate closely with the spoligotyping technique (6). In this study, 6.4% of the strains belonged to Beijing family (Table 1). Beijing family isolation rate in many Asian countries were reported to be >50% (32). This may be indicative of the fact that these Beijing strains are not local in origin and have entered Iran via Afghani immigrations from its Eastern border. Drug resistance is a growing problem in TB treatment and control. Furthermore, MDR isolates have become a major worldwide predicament (30). The TB strains isolated from the Iranian patients showed higher levels of drug resistance relative to these isolated from the Afghani group (Fig. 2). Thirty four percent of Iranian isolates belonged to Group 1; whereas, 28.7% of Afghani isolates were within this group (Table 1). As noted earlier, drug resistance in group 1 is higher than the other two groups. Group 1 is also mostly associated with MDR strains (26,36).
Designing newer drugs, vaccines, and diagnostic techniques is dependent on better understanding of M. tuberculosis virulence mechanism. Since it appears that TB virulence mechanism is multi-gene dependent, we sought to determine the prevalence of one of these virulence genes, namely pcaA, in clinical isolates. The cyclopropane moiety of mycolic acids is a unique lipid structure that greatly affects the pathogenesis of M. tuberculosis (15). The bacterium uses a family of Sadenosylmethionine-dependent methyltransferases to modify the mycolic acids of its cell envelope with a variety of stereochemistries and positions for cyclopropyl groups (13). There are at least three mycolic acid cyclopropane synthases (PcaA, CmaA1, and CmaA2) that are responsible for these site-specific modifications of mycolic acids (17). PcaA act as a proximal cis-cyclopropane synthetase for -mycolate molecule that is essential for M. tuberculosis virulence (13,15). PcaA was identified as a gene necessary for the morphology of cording in M. tuberculosis. PcaA has clearly been shown to be essential for maintenance of a chronic infection (15). This suggests that the family of mycolic acid methyltransferases may have particular importance in virulence and persistence of M. tuberculosis. Since there is no known cyclopropanated lipid in mammals, it is conceivable that these structures together with biochemical assays will provide a foundation for the rational development of new anti-tuberculosis drugs. Moreover, the substrate similarity for the active sites of these enzymes points to the possibility of one potential inhibitor acting upon multiple targets. This reduces the potential for drug resistance which is a favorable feature for any new drug in the fight against tuberculosis. In conclusion, we confirmed that pcaA gene widespread existence in almost all the clinical isolates (Fig. 3) can be a potential target for drug, vaccine and diagnostic test designs. It is also important to undertake studies to identify which factors are the most significant to consider in tuberculosis control program.
Acknowledgments
I am acknowledging Dr. J. Kamerbeek for providing us with Spoligotyping protocol and I wish to thank all those who have contributed in some way to this work specially TB laboratory staff (Mr. Kamran, Mr. Geidi and Mr. Gadiri) for excellent laboratory assistance and Mr. Visheh for film development.
This research was partially sponsored by the Research Deputy of Iran university of Medical Science, Kurdistan university of Medical Science and Iranian National Reference TB Laboratory, Shahid Beheshti university of Medical Science.
RESUMO
Caracterização do complexo Mycobacterium tuberculosis isolado de pacientes do Irã e Afeganistão pelo método de spoligotyping
O desenvolvimento de novas drogas, vacinas e técnicas de diagnóstico depende de uma melhor compreensão dos mecanismos de virulência de Mycobacterium tuberculosis. Neste estudo, a prevalência do gene pcaA em cepas de M. tuberculosis foi avaliada através de da técnica de spoligotyping. Os fatores de risco associados nos pacientes de diferentes nacionalidades vivendo no Irã foram também determinados. As cepas de M. tuberculosis isoladas foram submetidas a testes de sensibilidade a quatro drogas anti-tuberculose de primeira linha e à caracterização por spoligotyping. Empregou-se PCR para detectar o gene pcaA, determinado-se também a sequencia de nucleotidios. A espoligotipagem resultou em 140 grupos diferentes, sendo 120 (87,1%) reportados pela primeira vez. Os demais espoligotipos (12,8%) já foram descritos em outras regiões geográficas no mundo. A família Haarlem foi mais comum que os demais genótipos. A resistencia a antibióticos foi maior nas cepas isoladas dos pacientes iranianos. O gene pcaA foi detectado em isolados clínicos de M. tuberculosis mas não em cepas saprófitas, como M. kansasi. Os resultados indicaram a existência de M. tuberculosis pertencente à família Beijing nos pacientes iranianos. Este estudo confirmou a presença do gene pcaA em quase todos os isolados clínicos. Estudos que identifiquem os fatores mais significantes nos programas de controle da tuberculose são necessários.
Palavras-chave: tuberculose, resistência, drogas, spoligotyping, pcaA
REFERENCES
- 1.Barouni A.S., Saridakis H.O., Vidotto M.C. Detection of Mycobacterium in clinical samples by multiprimer polymerase chain reaction. Braz. J. Microbiol. 2004;35:29–32. [Google Scholar]
- 2.Berger B.J., Knodel M.H. Characterisation of methionine adenosyltransferase from Mycobacterium smegmatis and M. tuberculosis. BMC Microbiol. 2003;3:12. doi: 10.1186/1471-2180-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan P.J., Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 4.Brudey K., Driscoll J.R., Rigouts L., Prodinger W.M., Gori A., Al-Hajoj S.A., Allix C., Aristimuno L., Arora J., Baumanis V., Binder L., Cafrune P., Cataldi A., Cheong S., Diel R., Ellermeier C., Evans J.T., Fauville-Dufaux M., Ferdinand S., Garcia de Viedma D., Garzelli C., Gazzola L., Gomes H.M., Guttierez M.C., Hawkey P.M., van Helden P.D., Kadival G.V., Kreiswirth B.N., Kremer K., Kubin M., Kulkarni S.P., Liens B., Lillebaek T., Ho M.L., Martin C., Martin C., Mokrousov I., Narvskaia O., Ngeow Y.F., Naumann L., Niemann S., Parwati I., Rahim Z., Rasolofo-Razanamparany V., Rasolonavalona T., Rossetti M.L., Rusch-Gerdes S., Sajduda A., Samper S., Shemyakin I.G., Singh U.B., Somoskovi A., Skuce R.A., van Soolingen D., Streicher E.M., Suffys P.N., Tortoli E., Tracevska T., Vincent V., Victor T.C., Warren R.M., Yap S.F., Zaman K., Portaels F., Rastogi N., Sola C. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6:23. doi: 10.1186/1471-2180-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalcanti H.R., Marques E., Fonseca, L.d.S.Saad M.H.F. Do DNA extraction methods and Taq polimerase quality improve the double repetitive element (DRE) PCR typing method for Mycobacterium tuberculosis strains? Braz. J. Microbiol. 2007;38:409–412. [Google Scholar]
- 6.Dale J.W., Al-Ghusein H., Al-Hashmi S., Butcher P., Dickens A.L., Drobniewski F., Forbes K.J., Gillespie S.H., Lamprecht D., McHugh T.D., Pitman R., Rastogi N., Smith A.T., Sola, C.Yesilkaya H. Evolutionary relationships among strains of Mycobacterium tuberculosis with few copies of IS6110. J. Bacteriol. 2003;185(8):2555–2562. doi: 10.1128/JB.185.8.2555-2562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale J.W., Brittain D., Cataldi A.A., Cousins D., Crawford J.T., Driscoll J., Heersma H., Lillebaek T., Quitugua T., Rastogi N., Skuce R.A., Sola C., Van Soolingen D., Vincent V. Spacer oligonucleotide typing of bacteria of the Mycobacterium tuberculosis complex: recommendations for standardised nomenclature. J. Tuberc. Lung Dis. 2001;5(3):216–9. [PubMed] [Google Scholar]
- 8.Dil A., Mir A.W., Kirmani A., Shakeel ul R., Eachkoti R., Siddiqi M.A. Improved diagnosis of central nervous system tuberculosis by MPB64-Target PCR. Braz. J. Microbiol. 2008;39:209–213. doi: 10.1590/S1517-83822008000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferdinand S., Sola C., Chanteau S., Ramarokoto H., Rasolonavalona T., Rasolofo-Razanamparany V., Rastogi N. A study of spoligotyping-defined Mycobacterium tuberculosis clades in relation to the origin of peopling and the demographic history in Madagascar. Infect Genet Evol. 2005;5(4):340–8. doi: 10.1016/j.meegid.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Ferdinand S., Sola C., Verdol B., Legrand E., Goh K.S., Berchel M., Aubery A., Timothee M., Joseph P., Pape J.W., Rastogi N. Molecular characterization and drug resistance patterns of strains of Mycobacterium tuberculosis isolated from patients in an AIDS counseling center in Port-au-Prince, Haiti: a 1-year study. J. Clin. Microbiol. 2003;41(2):694–702. doi: 10.1128/JCM.41.2.694-702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes B.A., Sahm D.F., Weissfeld A.S., Scott E.G., Bailley W.R. Diagnostic microbiology; Mosby. 2002 [Google Scholar]
- 12.Githui W.A., Meme H.K., Juma E.S., Kinyanjui P., Karimi F., Chakaya J.M., Kangangi J., Kutwa A. Isolation of multidrugresistant tuberculosis strains in patients from private and public health care facilities in Nairobi, Kenya. J. Tuberc. Lung Dis. 2004;8(7):837–41. [PubMed] [Google Scholar]
- 13.Glickman M.S. The mmaA2 gene of Mycobacterium tuberculosis encodes the distal cyclopropane synthase of the alpha-mycolic acid. J. Biol. Chem. 2003;278(10):7844–9. doi: 10.1074/jbc.M212458200. [DOI] [PubMed] [Google Scholar]
- 14.Glickman M.S., Cahill S.M., Jacobs W.R.Jr. The Mycobacterium tuberculosis cmaA2 gene encodes a mycolic acid trans-cyclopropane synthetase. J. Biol. Chem. 2001;276(3):2228–33. doi: 10.1074/jbc.C000652200. [DOI] [PubMed] [Google Scholar]
- 15.Glickman M.S., Cox J.S., Jacobs W.R.Jr. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell. 2000;5(4):717–27. doi: 10.1016/s1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- 16.Glynn J.R., Whiteley J., Bifani P.J., Kremer K., van Soolingen D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 2002;8(8):843–9. doi: 10.3201/eid0808.020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C.C., Smith C.V., Glickman M.S., Jacobs W.R.Jr., Sacchettini J.C. Crystal structures of mycolic acid cyclopropane synthases from Mycobacterium tuberculosis. J. Biol. Chem. 2002;277(13):11559–69. doi: 10.1074/jbc.M111698200. [DOI] [PubMed] [Google Scholar]
- 18.Kamerbeek J., Schouls L., Kolk A., van Agterveld M., van Soolingen D., Kuijper S., Bunschoten A., Molhuizen H., Shaw R., Goyal M., van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 1997;35(4):907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kart L., Altin R., Tor M., Gulmez I., Oymak S.F., Atmaca H.M., Erdem F. Antituberculosis drug resistance patterns in two regions of Turkey: a retrospective analysis. Ann. Clin. Microbiol. Antimicrob. 2002;1:6. doi: 10.1186/1476-0711-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lari N., Rindi L., Sola C., Bonanni D., Rastogi N., Tortoli E.Garzelli, C. Genetic diversity, determined on the basis of katG463 and gyrA95 polymorphisms, Spoligotyping, and IS6110 typing, of Mycobacterium tuberculosis complex isolates from Italy. J. Clin. Microbiol. 2005;43(4):1617–24. doi: 10.1128/JCM.43.4.1617-1624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liaw Y.S., Hsueh P.R., Yu C.J., Wang S.K., Yang P.C., Luh K.T. Drug resistance pattern of Mycobacterium tuberculosis in a university hospital in Taiwan, 1998-2002. J. Formos Med. Assoc. 2004;103(9):671–7. [PubMed] [Google Scholar]
- 22.Lourenço M.C.d.S., Silva M.G.d., Fonseca L.d.S. Multidrugresistant tuberculosis among male inmates in Rio de Janeiro, Brazil. Braz. J. Microbiol. 2000;31:17–19. [Google Scholar]
- 23.Mansoori S.D.A.S., Mirabolhasani Z., Farnia P., Velayati A.A. The pattern of drug resistance among newly diagnosed and old cases of pulmonary tuberculosis in NRITLD. Arch. Iranian. Med. 2003;6(4):255–260. [Google Scholar]
- 24.Monteiro P.H.T., Martins M.C., Ueki S.Y.M., Giampaglia C.M.S., Telles M.A.d.S. Cord formation and colony morphology for the presumptive identification of Mycobacterium tuberculosis complex. Braz. J. Microbiol. 2003;34:171–174. [Google Scholar]
- 25.Ogusku M.M., Sadahiro A., Hirata M.H., Hirata R.D.C., Zaitz C., Salem J.I. PCR in the diagnosis of cutaneous tuberculosis. Braz. J. Microbiol. 2003;34:165–170. [Google Scholar]
- 26.Palittapongarnpim P., Luangsook P., Tansuphaswadikul S., Chuchottaworn C., Prachaktam R., Sathapatayavongs B. Restriction fragment length polymorphism study of Mycobacterium tuberculosis in Thailand using IS6110 as probe. Int. J. Tuberc. Lung Dis. 1997;1(4):370–6. [PubMed] [Google Scholar]
- 27.Pfyffer G.E. Drug-resistant tuberculosis: resistance mechanisms and rapid susceptibility testing. Schweiz Med. Wochenschr. 2000;130(49):1909–13. [PubMed] [Google Scholar]
- 28.Qian L., Van Embden J.D., Van Der Zanden A.G., Weltevreden E.F., Duanmu H., Douglas J.T. Retrospective analysis of the Beijing family of Mycobacterium tuberculosis in preserved lung tissues. J. Clin. Microbiol. 1999;37(2):471–4. doi: 10.1128/jcm.37.2.471-474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchotene K.O., von Groll A., Ramos D., Scholante A.B., Honscha G., Valença M., Scaini C.J., Silva P.E.A.d. Comparative evaluation of the Nitrate Reductase Assay and the Resazurin Microtitre Assay for drug susceptibility testing of Mycobacterium tuberculosis against first line anti-tuberculosis drugs. Braz. J. Microbiol. 2008;39:16–20. doi: 10.1590/S1517-83822008000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaaf H.S., Shean K., Donald P.R. Culture confirmed multidrug resistant tuberculosis: diagnostic delay, clinical features, and outcome. Arch. Dis. Child. 2003;88(12):1106–11. doi: 10.1136/adc.88.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebban M., Mokrousov I., Rastogi N., Sola C. A data-mining approach to spacer oligonucleotide typing of Mycobacterium tuberculosis. Bioinformatics. 2002;18(2):235–43. doi: 10.1093/bioinformatics/18.2.235. [DOI] [PubMed] [Google Scholar]
- 32.Soini H., Pan X., Amin A., Graviss E.A., Siddiqui A., Musser J.M. Characterization of Mycobacterium tuberculosis isolates from patients in Houston, Texas, by spoligotyping. J. Clin. Microbiol. 2000;38(2):669–76. doi: 10.1128/jcm.38.2.669-676.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sreevatsan S., Pan X., Stockbauer K.E., Connell N.D., Kreiswirth B.N., Whittam T.S., Musser J.M. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. U S A. 1997;94(18):9869–74. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supply P., Lesjean S., Savine E., Kremer K., van Soolingen D., Locht C. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 2001;39(10):3563–71. doi: 10.1128/JCM.39.10.3563-3571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toubiana R., Berlan J., Sato H., Strain M. Three types of mycolic acid from Mycobacterium tuberculosis Brevanne: implications for structure-function relationships in pathogenesis. J. Bacteriol. 1979;139(1):205–11. doi: 10.1128/jb.139.1.205-211.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toungoussova O.S., Sandven P., Mariandyshev A.O., Nizovtseva N.I., Bjune G., Caugant D.A. Spread of drug-resistant Mycobacterium tuberculosis strains of the Beijing genotype in the Archangel Oblast, Russia. J. Clin. Microbiol. 2002;40(6):1930–1937. doi: 10.1128/JCM.40.6.1930-1937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Embden J.D., Cave M.D., Crawford J.T., Dale J.W., Eisenach K.D., Gicquel B., Hermans P., Martin C., McAdam R., Shinnick T.M., et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 1993;31(2):406–9. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Soolingen D. Utility of molecular epidemiology of tuberculosis. Eur. Respir. J. 1998;11(4):795–7. doi: 10.1183/09031936.98.11040795. [DOI] [PubMed] [Google Scholar]
- 39.van Soolingen D., Hermans P.W., de Haas P.E., Soll D.R., van Embden J.D. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 1991;29(11):2578–86. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Soolingen D., Qian L., de Haas P.E., Douglas J.T., Traore H., Portaels F., Qing H.Z., Enkhsaikan D., Nymadawa P., van Embden J.D. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 1995;33(12):3234–8. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velayati A.A., Bakayev V.V., Bahrmand A.R. Use of PCR and culture for detection of Mycobacterium tuberculosis in specimens from patients with normal and slow responses to chemotherapy. Scand J. Infect. Dis. 2002;34(3):163–6. doi: 10.1080/00365540110080106. [DOI] [PubMed] [Google Scholar]
- 42.Yuan Y., Barry C.E. A common mechanism for the biosynthesis of methoxy and cyclopropyl mycolic acids in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U S A. 1996;93(23):12828–33. doi: 10.1073/pnas.93.23.12828. 3rd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Y., Crane D.C., Musser J.M., Sreevatsan S., Barry C.E. MMAS-1, the branch point between cis- and trans-cyclopropane-containing oxygenated mycolates in Mycobacterium tuberculosis. J. Biol. Chem. 1997;272(15):10041–9. doi: 10.1074/jbc.272.15.10041. 3rd. [DOI] [PubMed] [Google Scholar]
- 44.Zink A.R., Sola C., Reischl U., Grabner W., Rastogi N., Wolf N., Nerlich A.G. Molecular identification and characterization of Mycobacterium tuberculosis complex in ancient Egyptian mummies. Int. J. Osteoarchaeology. 2004;14(5):404–413. [Google Scholar]


