Abstract
The interaction between the surface of stainless steel and Bacillus cereus was studied in terms of the characteristics of interfacial interaction determined from the measurement of the contact angle of the surface of B. cereus and stainless steel in the presence or absence of B. cereus adherence. The microtopographies and the roughness of the surface of stainless steel and stainless steel adhered by B. cereus were evaluated with the help of atomic force microscopy and perfilometry. The strain of B. cereus studied was considered hydrophilic, whereas the stainless steel was considered hydrophobic. The adhesion was not thermodynamically favorable (ΔGadhesion > 0) between the stainless steel and the strain of B. cereus studied. Thus, the interaction between them was not favored by the thermodynamic aspect of adhesion. There was no difference (p > 0.05) in the roughness of the surfaces of stainless steel adhered by B. cereus when analyzed by atomic force microscope and perfilometry.
Keywords: hydrophobicity, roughness, Bacillus cereus, dairy plant, adhesion
INTRODUCTION
Bacillus cereus, a spore forming bacterium, is an inevitable low-grade contaminant of a wide variety of foods, including cereals, food additives and processed milk products (11, 14). Due to high incidence in these products, some outbreaks have been reported in which milk or milk-related products containing B. cereus were suggested to be the cause of disease (2, 14). Also, B. cereus isolates has been observed in a high number of samples collected from food processing contact surfaces in the dairy industry (2). The occurrence of these microorganisms in pasteurized milk can be explained by the presence of their heat-resistance spores in raw milk or by milk recontamination, due to inadequately cleaned and sanitized surfaces (3, 9, 13). The main consequence of B. cereus contamination in milk is the decreasing of shelf life and the occurrence of an off-flavor (9). B. cereus produces thermoresistant extracellular proteases and phospholipases that cause sweet coagulation and bitterness defects in milk.
Bacterial adhesion of B. cereus and other microorganisms to food processing contact surfaces is affected by the interaction of physicochemical characteristics of the microorganism surface and the contact surfaces. Specific linkages between microorganisms and food processing surfaces depend on the chemical composition of the both surfaces (17). Surfaces characteristics including cell surface hydrophobicity relative surface charge have been reported to affect the adhesion of bacteria. The prediction of bacterial adhesion based on physicochemical factors was initially studied using the DLVO theory, first proposed by Derjaguin and Landau in 1941 and complemented by Verwey and Overbeek in 1948 (15). DLVO theory was proposed for liophobic colloidal particles, considering only long forces such as van der Waals forces. In 1994, van Oss and co-workers (19) proposed the extended DLVO theory that includes the influence of short forces such as Born repulsion forces, hydration forces, hydrophobic interactions and polymer bridges. However, it is important to mention that cellular structures such as flagella, fimbriae, and pili, and that the production of extracellular polysaccharides plays an important role in the adhesion process. In addition, environmental conditions such as pH, ionic forces, temperature, exposure time and cellular concentration of microorganisms strongly influence the adhesion process (6). Better understanding of the role of physicochemical properties in the adhesion process and biofilm formation on food processing contact surfaces can aid in the control of bacterial growth in environmental milk processing at dairy plants.
In the present study, the characteristics of interfacial tension of an isolated B. cereus strain from a dairy plant, a stainless steel surface and a stainless steel surface adhered by B. cereus were examined by measurements of contact angles between the surfaces and applying the data to the extended-DLVO theory. In addition, the roughness and the microtopography of stainless steel surfaces were evaluated in the presence or absence of B. cereus adherence.
MATERIALS AND METHODS
Microorganism and Surfaces
Studies were conducted using suspensions a B. cereus strain isolated from the surface of a stainless steel pasteurized milk packaging machine that was identified as Ribo 1 222 173 S4 . Surfaces of coupons (10 mm × 10 mm × 0.5 mm) of stainless steel AISI 304, #4, in the presence or absence of B. cereus adherence, were used for bacterial adherence studies. One milliliter of the culture was stored at -80°C in nutrient broth (Merck, Sao Paulo, Brazil) containing glycerol (80:20). A working culture of the strain containing approximately 107 cfu/mL was prepared by inoculation of 100 µL of frozen culture into 10 mL of Brain Hearth Infusion (BHI, Merck, São Paulo, Brazil), followed by incubation at 32°C for 24 h. The culture was sub-cultured two times before use. The number of microorganisms in each suspension was obtained by total count using Plate Count Agar (PCA, Merck, São Paulo, Brazil) at 32°C for 24 h.
Attachment of Cells
Coupons were first cleaned by washing them with neutral liquid detergent and water, followed by rinsing with distilled water and immersion in 70% ethyl alcohol for 1 h to remove fat. Subsequently, coupons were rinsed with distilled water, air-dried, and sterilized at 121°C for 15 min (12). The cleaned and sanitized coupons were added to 250 mL flasks containing 100 mL of nutrient broth, which were previously inoculated with suspensions of B. cereus. The initial number of cells was approximately 103 cfu/mL, and the flasks were incubated statically for different times and at different temperatures according to the full factorial at two levels (Table 1).
Table 1.
Temperature and time of adherence of Bacillus cereus on the surface of stainless steel.
| Experiments | Time (days) | Temperature (°C) |
|---|---|---|
| E1 | 1 | 4°C |
| E2 | 1 | 10°C |
| E3 | 1 | 25°C |
| E4 | 1 | 35°C |
| E5 | 5.5 | 7°C |
| E6 | 5.5 | 15°C |
| E7 | 5.5 | 20°C |
| E8 | 5.5 | 30°C |
| E9 | 10 | 4°C |
| E10 | 10 | 10°C |
| E11 | 10 | 25°C |
| E12 | 10 | 35°C |
At the proper times, coupons were removed and rinsed for 1 min in tubes containing 10 mL of sterilized 0.1 % peptone water to remove the planktonic cells. Afterward, each coupon was placed into Petri plates and analyzed for contact angle and roughness.
Contact Angle Measurement
For stainless steel AISI 304, #4 and stainless steel AISI 304 # 4 adhered by B. cereus, the contact angles between the surfaces and water, formamide, and α-bromonaphthalene were determined using a goniometer (Kruss, Germany). Measurements of the contact angle of one 2.0 µL drop were taken each second for 30 s for all liquids and surfaces. Experiments were conducted in triplicate. The contact angle for the microorganism surface was determined on a layer of vegetative cells using the drop method (5). First, the strain of B. cereus was activated twice in BHI broth (Merck, Sao Paulo, Brazil), creating suspensions with approximately 107 CFU/mL. The suspensions were centrifuged at 12.000 g for 10 min and washed three times in 0.1 M phosphate buffered saline (PBS). The cell pellet was suspended in the same buffer, and the suspension was filtered using an acetate cellulose membrane (0.45 µm pore size, 27 mm in diameter) using negative pressure. During the filtration, 30 mL of pure water (Milli-Q) were added. The membranes were transferred to Petri plates containing 1 % (v/v) agar and 10 % (v/v) glycerol. The membranes were cut into three parts for contact angle measurements with water, formamide, and α- bromonaphthalene.
Determination of the Total Interfacial Tension (γtot)
The equation of Young-Good-Girifalco-Fowkes (Equation 1) relates the contact angle formed by the liquid above a solid surface with the components of interfacial tension of liquids (γl LW, γl + , γl -) and of the surface (γs LW,γs +, γs -) as follows:
| Equation (1) |
where γtot is the total interfacial tension of the surface, γLWis the interfacial tension of the interactions of the Lifshitz-van der Waals forces, γABis the polar component of the Lewis acid-base interaction, γ+ is the interfacial tension of the electron acceptor component of the acid-base component, γ –is the interfacial tension of electron donor component of the acid-base component, ΘB is the contact angle obtained with α-bromonaphthalene and s and l indicate surface and liquid, respectively (19).
The three components of the interfacial tension of the surfaces were determined from the contact angles obtained from three liquids with different polarities, whose interfacial tensions are known as shown in Table 2.
Table 2.
Interfacial tension components of liquids at 25 °C.
| Liquid | Interfacial tension (mJ/m2) | |||
|---|---|---|---|---|
| γl Tot | γl LW | γl + | γl - | |
| α-Bromonaphthalene | 44.4 | 44.4 | 0.0 | 0.0 |
| Water | 72.8 | 21.8 | 25.5 | 25.5 |
| Formamide | 58.0 | 39.0 | 2.28 | 39,6 |
Source: (18)
The interfacial tension is equal to the sum of the two components (γs LWand γs AB):
| Equation (2) |
| Equation (3) |
| Equation (4) |
Total Free Energy of Interaction (∆Gsws TOT)
The total free energy of interaction among molecules of the surface(s) immersed in water (w) is determined by the sum of the apolar and polar free energies of interaction, ∆Gsws LW and ∆Gsws AB, respectively.
| Equation (5) |
| Equation (6) |
| Equation (7) |
When ∆Gsws TOT >, 0 the surface is considered hydrophilic. Conversely, if ∆Gsws TOT< 0, the surface is considered hydrophobic.
Determination of the Total Free Energy of Adhesion (∆Gadhesion )
From the values of the components of the interfacial tensions, it is possible to determine the ∆Gadhesion between two surfaces (microbial cells (b) and food processing surfaces (s)):
| Equation (8) |
| Equation (9) |
| Equation (10) |
When free energy is related to the interfacial tension, then ∆Gadhesion can be represented by the following:
| Equation (11) |
| Equation (12) |
| Equation (13) |
where γbs is the interfacial tension between the bacterial surfaces and the adhesion surface, γbl isthe interfacial tension between the bacterial surfaces and the liquid, and γsl isthe interfacial tension between the adhesion surfaces and the liquid. The ∆Gadhesion values allow for evaluation of the thermodynamics of the adhesion process: if ∆Gadhesion < 0, the process is favorable, but if ∆Gadhesion > 0, the process is unfavorable.
Surface Roughness
The microtopography of the stainless surface in the presence or absence of B. cereus adherence was evaluated using atomic force microscopy (AFM)that analyzed areas of 100 µm2and 10 µm2 (Universal SPM System Ntegra Prima/NT-MDT) and using a Perthometer (Ambios Technology, XP1) that analyzed one line of 1 mm in each coupon. The experiment was conducted with three repetitions. The roughness of the surfaces was compared before and after bacterial adhesion by different numbers of B. cereus by regression analysis at 5% of probability. The means of the roughness were submitted to Tukey’s test (α = 0.05) by using the Statistical Analysis System (SAS), version 9.1.
RESULTS AND DISCUSSION
Adhesion of B. cereus
The numbers (log cfu/cm2) of Bacillus cereus cells adhered to stainless steel AISI 304 #4 in different experiments are shown in Table 3.
Table 3.
Number (log cfu/cm2) of Bacillus cereus cells adhered to stainless steel AISI 304 #4 in different experiments.
| Experiments | Log cfu/cm2* |
|---|---|
| E1 (4 °C/1 d) | 0.91 ± 0.66 |
| E2 (10 °C/1 d) | 1.40 ± 1.00 |
| E3 (25 °C/1 d) | 3.21 ± 0.67 |
| E4 (35 °C/1 d) | 4.01 ± 0.80 |
| E5 (7 °C/5.5 d) | 0.50 ± 0.42 |
| E6 (15 °C/5.5 d) | 3.32 ± 0.43 |
| E7 (20 °C/5.5 d) | 3.15 ± 0.21 |
| E8 (30 °C/5.5 d) | 3.66 ± 0.61 |
| E9 (4 °C/10 d) | 0.31 ±0.61 |
| E10 (10 °C/10 d) | 1.16 ± 0.95 |
| E11 (25 °C/10 d) | 3.43 ± 0.81 |
| E12 (35 °C/10 d) | 4.43 ± 0.77 |
Mean of three repetitions.
Analyses of the Contact Angles
The contact angle with water (θW ) measured for B. cereus was lower than 50 °, (Table 4) indicating that it is a hydrophilic surface according to the classification system proposed by Azeredo (1). According to this author, surfaces with a θW less than 50 ° are classified as hydrophilic, whereas surfaces with a θW greater than 50° are classified as hydrophobic. The stainless steel surfaces adhered by B. cereus at 20°C/5.5 d and 10°C/10 d with θW values lower than 50° also were considered hydrophilic. The surfaces were evaluated in others conditions and were classified as hydrophobic. The stainless steel surface without cells adhered was hydrophobic. The contact angle with water is a qualitative criterion used to classify the hydrophobicity of food processing or microorganism surfaces. Faille et al. (7) found contact angles (θW ) for stainless steel of 75°, confirming the hydrophobic characteristics of this material.
Table 4.
Measurements of the contact angles (θ) of the cells of Bacillus cereus and stainless steel in the presence or absence of Bacillus cereus adherence with water (θA), formamide (θF) and α-bromonaphthalene (θB).
| Contact angles (º)* | |||
|---|---|---|---|
| ΘW | ΘF | ΘB | |
| Stainless steel | 70.77 ± 7.9 | 53.36 ± 9.4 | 28.1 ± 3.1 |
| B. cereus | 24.52 ± 2.8 | 15.37 ± 1.3 | 45.17 ± 0.5 |
| E1 (4 ºC/1 d) | 72.1 ± 2.6 | 56.1 ± 8.6 | 34.6 ± 6.7 |
| E2 (10 ºC/1 d) | 73.2 ± 0.5 | 57.0 ± 7.2 | 30.5 ±5.9 |
| E3 (25 ºC/1 d) | 57.3 ± 11.9 | 57.5 ± 6.7 | 25.3 ±6.7 |
| E4 (35 ºC/1 d) | 60.1 ± 10.5 | 40.5 ± 1.5 | 32.0 ± 3.5 |
| E5 (7 ºC/5.5 d) | 69.6 ± 1.4 | 52.2 ± 4.2 | 35.6 ± 4.7 |
| E6 (15 ºC/5.5 d) | 56.7 ± 10.7 | 56.7 ± 7.0 | 32.2 ±4.3 |
| E7 (20 ºC/5.5 d) | 49.3 ± 6.1 | 44.2 ± 2.6 | 39.0 ± 7.3 |
| E8 (30 ºC/5.5 d) | 73.7 ± 5.0 | 53.5 ±8.3 | 34.5 ± 0.2 |
| E9 (4 ºC/10 d) | 77.6 ± 8.9 | 57.6 ± 6.0 | 33.6 ± 6.7 |
| E10 (10 ºC/10 d) | 48.9 ± 14.8 | 19.2 ± 3.2 | 34.2 ± 3.3 |
| E11 (25 ºC/10 d) | 62.8 ± 8.2 | 43.2 ± 6.0 | 31.5 ± 1.6 |
| E12 (35 ºC/ 10 d) | 58.9 ± 12.7 | 39.4 ± 11.9 | 32.8 ± 6.3 |
Mean of three repetitions.
Thermodynamic Parameters of the Surfaces
The contact angle measurements for the three substances were used to calculate the components of interfacial tension and levels of hydrophobicity (Table 5). It is possible to estimate the hydrophilic or hydrophobic character of the surfaces by components of interfacial tension. With increasing γ LWvalues, the apolarity of a surface increases, which results in lower affinity of that surface for polar liquids. A high γAB component value means more water of hydration on the surface and increased hydrophilicity. According to these criteria, B. cereus surfaces and stainless steel surfaces adhered by B. cereus at 10°C/10 d are considered to hydrophilic because their γAB values are higher than those of the other surfaces. This result does not agree with those obtained by contact angle measurement with water (θW ) that also classified as hydrophilic the surfaces of stainless steel adhered by B. cereus at 20°C/5.5 d.
Table 5.
Values of the interfacial tension components (γLW, γ+, γ-, γAB,γTOT) of the cells of the Bacillus cereus, stainless steel and stainless steel adhered by cells of B. cereus.
| Interfacial tension (mJ/m2) | |||||
|---|---|---|---|---|---|
| Surfaces | γLW | γ+ | γ- | γAB | γTOT |
| Stainless steel | 39.3206 | 0.0899 | 6.3333 | 1.5091 | 40.8297 |
| B. cereus | 32.2682 | 3.2454 | 45.2929 | 24.2482 | 56.5164 |
| E1 (4 °C/1 d) | 36.8944 | 0.0956 | 12.2929 | 2.1681 | 39.0625 |
| E2 (10 °C/1 d) | 38.4688 | 0.0207 | 11.6946 | 0.9840 | 39.4528 |
| E3 (25 °C/1 d) | 40.2433 | 0.3842 | 0.4922 | 0.8697 | 41.1130 |
| E4 (35 °C/1 d) | 37.9096 | 1.0378 | 16.8009 | 8.3510 | 46.2606 |
| E5 (7 °C/5.5 d) | 36.4894 | 0.3478 | 12.4987 | 4.1699 | 40.6593 |
| E6(15 °C/5.5 d) | 37.8335 | 0.1378 | 7.6388 | 2.0519 | 39.8854 |
| E7(20 °C/5.5 d) | 35.0565 | 0.4573 | 32.1712 | 7.6712 | 42.7277 |
| E8(30 °C/5.5 d) | 36.9345 | 0.3508 | 9.1703 | 3.5871 | 40.5216 |
| E9 (4 °C/10 d) | 37.2915 | 0.1260 | 7.6212 | 1.9598 | 39.2513 |
| E10(10 °C/10 d) | 37.0542 | 3.3996 | 20.0995 | 16.5324 | 53.5866 |
| E11(25 °C/10 d) | 38.0982 | 0.8236 | 15.1795 | 7.0715 | 45.1697 |
| E12(35 °C/10 d) | 37.6033 | 1.1625 | 17.5686 | 9.0384 | 46.6417 |
The component γ− can also be a semi-quantitative measure of hydrophobicity γ− values ≤ 25.5 mJ/m2 indicate a hydrophobic surface regardless of the value of the apolar component. The γ− values between 25 mJ/m2 and 35 mJ/m2 suggest that the hydrophobicity is dependent upon the apolar component. In these cases, the surfaces are hydrophilic when γLW≤ 45 mJ/m2and hydrophobic when γLW ≥ 46 mJ/m2(1). According to the results, B. cereus is an electron donor because the γ− values are higher than the γ+ values. Strevett and Chen (15) demonstrated that γ− is always higher than γ+ forE. coli, P. fluorescens, B. subtilis and P. aeruginosa, confirming the electron donor characteristics of these bacterial cells. All biosurfaces are predominantly electron donors because of the presence of oxygen in the atmosphere and the hydration of microbial cells (18). Stainless steel surfaces not adhered by B. cereus also act as electron donors. Similar to our findings, Chaves (6) observed that the surface of stainless steel AISI 316 predominately acts as an electron, similar to most solid surfaces. Furthermore, B. cereus surfaces and stainless steel surfaces adhered by cells of B. cereus at 20°C/5.5 d are considered hydrophilic because they did not present γ− ≤ 25.5 mJ/m2. The other surfaces, including stainless steel surfaces adhered by B. cereus at 10°C/10 d, are considered hydrophobic.
Total Free Energy of Interaction (∆Gsws TOT)
According to the quantitative criteria, the B. cereus strain and the stainless steel surfaces adhered by B. cereus at 20 °C/5.5 d were considered hydrophilic because the ∆Gsws TOT values for both surfaces were > 0 (Table 6). The other surfaces were hydrophobic (∆Gsws TOT < 0). Similar results were found using the semiquantitative γ−value. However, these results are different from those determined by qualitative criteria using contact angles with water (θw ), which considered stainless surfaces adhered by B. cereus at 10 °C/10 d as hydrophilic. The ΔGsws LW values are generally negative in the bacterial interactions, indicating the Lifshitz van der Waals forces are predominantly attractive, whereas the ΔGsws AB can be positive or negative, indicating repulsion or attraction, respectively (4). In our experiment, ΔGsws AB values indicated attraction for most of the surfaces evaluated (Table 6). Only the surfaces considered hydrophilic showed ΔGsws AB > 0, demonstrating that hydrophobicity is predominantly determined by polar forces of attraction. The component ΔGsws AB represents the hydration degree of surfaces, which means that high ΔGsws AB values equal low hydrophobicity of the surfaces. Therefore, the qualitative and quantitative criteria used to evaluate hydrophobicity showed a slight divergence from the results of the hydrophobicity of the surfaces of the stainless steel adhered by B. cereus at 10 °C/10 d (Tables 4 and 6). For the other evaluations, the results for both criteria were in agreement. The B. cereus surface was classified as hydrophilic and stainless steel surfaces were classified as hydrophobic. A possible explanation for the hydrophilicity of stainless steel surfaces adhered by microorganisms as classified by quantitative criteria is the random distribution of the drops of water that were placed on the surfaces to measure the contact angles. These drops can be placed in regions with different concentrations of adhered cells. Therefore, the contact angles measurements can be related to the hydrophobicity of the stainless steel surfaces or the cells, reflecting the characteristics of only one surface.
Table 6.
Values of the apolar (∆GswsLW) and polar (∆GswsAB) components of the total free energy of interaction (∆GswsTOT) of B. cereus surfaces and stainless steel surfaces in the presence or absence of B. cereus adherence.
| Total free energy of interaction (mJ/m2) | |||
|---|---|---|---|
| ∆Gsws LW | ∆Gsws AB | ∆Gsws TOT | |
| Stainless steel | -5.1300 | -23.6028 | -28.7328 |
| B. cereus | -2.0468 | 21.8664 | 19.8195 |
| E1 (4 °C/1 d) | -3.9462 | -29.2732 | -33.2194 |
| E2(10 °C/1 d) | -4.7024 | -31.9868 | -36.6892 |
| E3(25 °C/1 d) | -5.6075 | -77.0592 | -82.6668 |
| E4(35 °C/1 d) | -4.4238 | -15.3336 | -19.7574 |
| E5(7 °C/5.5 d) | -3.7673 | -27.0336 | -30.8009 |
| E6(15 °C/5.5 d) | -4.3900 | -42.7792 | -47.1692 |
| E7(20 °C/5.5 d) | -3.1313 | 10.8848 | 7.7535 |
| E8(30 °C/5.5 d) | -3.9668 | -36.0436 | -40.0104 |
| E9(4 °C/10 d) | -4.1335 | -42.9876 | -47.1211 |
| E10(10 °C/10 d) | -4.0224 | -7.2648 | -11.2872 |
| E11(25 °C/10 d) | -4.5199 | -19.1152 | -23.6351 |
| E12(35 °C/10 d) | -4.2813 | -13.6348 | -17.9161 |
Global Free Energy of Adhesion (∆Gadhesion )
According to the thermodynamic theory of adhesion, if attractive forces are higher than repulsive forces, the interaction of short reach plays an important role in bacterial adhesion to surfaces. Such forces include polar and apolar interactions. Bacterial adhesion is favorable if the interactions lead to a decrease in the free energy of adhesion (∆Gadhesion < 0) (6). In our experiment, the free energy of adhesion between stainless steel and B. cereus was positive (∆Gadhesion > 0) (Table 7), being thermodynamically unfavorable. This finding was similar those observed by Teixeira et al. (16), in which strains of Pseudomonas aeruginosa and Staphylococcus sciuri isolated from a milk machine were hydrophilic, the stainless steel surface was hydrophobic and the free energy of adhesion was positive. According to van Oss (20), it is well known that bacterial adhesion in an aqueous solution is favorable between hydrophobic surfaces, which can remove the water among them. However, it should be emphasized that adhesion between hydrophobic and hydrophilic surfaces or two hydrophilic surfaces can occur.
Table 7.
Global free energy of adhesion values (∆Gadhesion) between Bacillus cereus (b) and the stainless steel surfaces of AISI 304 (s) in aqueous liquid media (l) and their apolar (∆GblsLW) and polar (∆GblsAB) components.
| Global free energy of adhesion (mJ/m2) | |||
|---|---|---|---|
| ∆Gbls LW | ∆Gbls AB | ∆Gadhesion | |
| B. cereus | -3.3353 | 5.8849 | 2.5496 |
Despite that the strain studied in this experiment is considered hydrophilic, some strains of Bacillus can produces highly hydrophobic spores that are able to adhere strongly to stainless steel, a surface recognized as hydrophobic. After adhesion, the process of spore germination can occur and vegetative cells may colonize the surfaces (10).
Roughness and Microtopography of the Surfaces
There is no difference (p ≥ 0.05) in the roughness of the surfaces analyzed by AFM and perfilometry (Figures 1 and 2). The increase of the number of adhered cells to surface coupons of stainless steel did not lead to an increase in surface roughness. These results can be explained due the low number of cells that adhered to the stainless steel in the different experiments, which reached approximately 104cfu/cm2. Thus, the measurements of roughness by AFM and perfilometry reflected the average roughness of the surface of the stainless steel.
Figure 1.
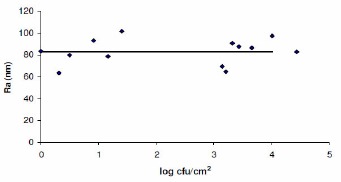
Means roughness (Ra) of the stainless steel adhered with different number of cells of Bacillus cereus as evaluated by AFM.
Figure 2.
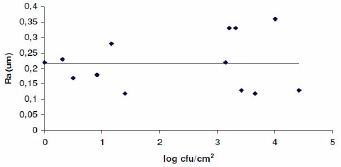
Means roughness (Ra) of the stainless steel adhered with different number of cells of Bacillus cereus as evaluated by Perthometer.
According to Flint et al. (8), the relation between roughness and bacterial adhesion is not clear. This divergence is probably related to the degree of roughness studied, the bacterial strains tested, physicochemical characteristics of the surfaces and the method for detecting bacteria. Flint et al. (8) did not find a relationship between thermoresistant streptococcus adhesion to stainless steel and the roughness of surfaces. However, it was observed that maximum adhesion occurred with mean roughness values close to mean length of the bacteria (0.9 µm). The authors suggested that some surface irregularities might provide protection to entrap cells in the cracks and crevices of the surface.
In 1 of 14 surfaces studied, a difference was observed between the qualitative criterion (contact angle measurement with water) and quantitative criterion (∆Gsws TOT – total free energy of interaction) to evaluate the hydrophobicity of the surfaces. The free energy of adhesion between stainless steel AISI 304 #4 and B. cereus was positive (∆Gadhesion > 0), being therefore thermodynamically unfavorable. Thus, an interaction between them was not favorable according to the thermodynamic aspect of adhesion. There was no difference (p ≥ 0.05) in the roughness of the surfaces analyzed by AFM and perfilometry.
REFERENCES
- 1.Azeredo J. Adesão de microrganismos e composição da matriz de bioagregados desenvolvimento de técnicas e estudo da influência de exopolímeros. Braga, Portugal: 1998. p. 231. D. Sc. Thesis. Departamento de Engenharia Biológica. Uminho. [Google Scholar]
- 2.Bartoszewicza M., Hansenb B.M., Swiecicka I. The members of the Bacillus cereus group are commonly present contaminants of fresh and heat-treated milk. Food Microbiol. 2008;25:588–596. doi: 10.1016/j.fm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Boor K.J. ADSA foundation scholar award—fluid dairy product quality and safety: looking to the future. J. Dairy Sci. 2001;84:1–11. doi: 10.3168/jds.s0022-0302(01)74445-1. [DOI] [PubMed] [Google Scholar]
- 4.Bos R., Van Der Mei H.C., Busscher H.J. Physico-chemistry of initial microbial adhesive interactions – its mechanisms and methods for study. FEMS Microbiol. Rev. 1999;23:79–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 5.Busscher H.J., Weerkamp A.H., Van Der Mei H.C., Van Pelt A.W., Jong H.P., Arends J. Measurement of the surface free energy of bacterial cell surface and its relevance for adhesion. Appl. Environ. Microbiol. 1984;48(5):980–983. doi: 10.1128/aem.48.5.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaves L.C.D. Portugal: 2004. Estudo da cinética da formação de biofilmes em superfícies em contato com água potável. Braga; p. 156. M. Sc. Dissertation. Departamento de Engenharia Biológica. Uminho. [Google Scholar]
- 7.Faille C., Fontaine F., Bénézech T. Potencial occurrence of adhering living Bacillus spores in milk product processing lines. J. Appl Microbiol. 2001;90:892–900. doi: 10.1046/j.1365-2672.2001.01321.x. [DOI] [PubMed] [Google Scholar]
- 8.Flint S.H., Brooks J.D., Bremer P.J. Properties of the stainless steel substrate, influencing the adhesion of thermo-resistant streptococci. J.Food Eng. 2000;43:235–242. [Google Scholar]
- 9.Fromm H.I., Boor K.J. Characterization of pasteurized fluid milk shelf-life attributes. J. Food Sci. 2004;69(8):207–214. [Google Scholar]
- 10.Hüsmark U., Rönner U. The influence of hydrophobic, electrostatic and morphologic properties on the adhesion of Bacillus spores. Biofoul. 1992;5:335–344. [Google Scholar]
- 11.Larsen H.D., Jørgensen K. The occurrence of Bacillus cereus in Danish pasteurized milk. Int. J. Food Microbiol. 1997;34:179–186. doi: 10.1016/s0168-1605(96)01182-8. [DOI] [PubMed] [Google Scholar]
- 12.Parizzi S.Q.F., Andrade N.J., Soares N.F.F., Silva C.A.S., Monteiro E.A.M. Bacterial adherence to different inert surfaces evaluated by epifluorescence microscopy and plate count method. Braz. Arch. Biol. Technol. 2004;47(1):77–83. [Google Scholar]
- 13.Peng J.S., Tsai W.C., Chou C.C. Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int. J. Food Microbiol. 2002;77:11–18. doi: 10.1016/s0168-1605(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 14.Reyes J.E., Bastías J.M., Gutiérrez M.R., Rodríguez M.O. Prevalence of Bacillus cereus in dried milk products used by Chilean School Feeding Program. Food Microbiol. 2007;24:1–6. doi: 10.1016/j.fm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Strevett K.A., Chen G. Microbial surface thermodynamics and applications. Res. Microbiol. 2003;154:329–335. doi: 10.1016/S0923-2508(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira P., Lopes Z., Azeredo J., Oliveira R., Vieira M.J. Physico-quimical surface characterization of a bacterial population isolated from milking machine. Food Microbiol. 2005;22:247–251. [Google Scholar]
- 17.Valcarce M.B., Busalmen S.R., Sánchez S.R. The influence of the surface condition on the adhesion of Pseudomonas fluorescens (ATCC 17552) to copper and aluminium brass. Int. Biodeter. Biodeg. 2002;50:61–66. [Google Scholar]
- 18.Van Der Mei H.C., Bos R., Busscher H.J. A reference guide to microbial cell surface hydrophobicity base don contact angles. Coll Surfac. 1998;11:213–221. [Google Scholar]
- 19.Van Oss C.J. Interfacial Forces in Aqueous Media. New York, N.Y: Marcel Dekker Inc; 1994. [Google Scholar]
- 20.Van Oss C.J. Hydrophobicity and hydrophilicity of biosurfactants. Curr. Opin. Coll. Int. Sci. 1997;2:503–512. [Google Scholar]


