Abstract
Progesterone (P4) may influence cognition in part through actions of its 5α-reduced metabolite, allopregnanolone. Ovariectomized mice that were C57BL/6 wildtype (WT), or deficient in the 5α-reductase Type 1 enzyme (5α-reductase knockout; 5αRKO), were administered vehicle, P4, allopregnanolone, or medroxyprogesterone acetate (MPA, 4mg/kg SC) after training in the object recognition or placement tasks. WT mice administered P4 or allopregnanolone performed significantly better in the object recognition and placement tasks than did WT mice administered vehicle or MPA. 5αRKO mice administered allopregnanolone, but not P4, MPA, or vehicle show enhanced performance in the object recognition and placement tasks. Levels of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex and hippocampus were lowest among mice administered MPA. Thus, some of P4’s effects to enhance cognitive performance may be incumbent upon its 5α-reduction.
Keywords: cognition, learning, memory, neurosteroids, hormone replacement therapy, brain derived neurotrophic factor (BDNF)
Introduction
Progesterone (P4) and its metabolite, allopregnanolone, may influence cognitive performance. Early studies of cognitive performance across pregnancy, a state of very high P4 and allopregnanolone levels, suggest these high levels of progestogens may have disorganizing effects on cognition [14]. Yet, a large, recent study comparing performance of the same participant at a baseline test and later during pregnancy did not demonstrate deficits in working memory, immediate, or delayed recall tasks [5]. P4 levels are higher during the luteal phase of the menstrual cycle, than during the follicular phase, when women and men have similar low levels of P4 [24]. During the luteal phase, attention and verbal and visual memory is enhanced and positively correlate with endogenous P4 levels [25,32]. Luteal increases in activity of the hippocampus assessed by Functional Magnetic Resonance Imaging (fMRI) are related to increased salivary levels of P4 [1]. Thus, cognitive function can be altered in association with endogenous changes in P4 levels; however, questions remain about the effects of exogenous administration of P4, with or without estrogens, which naturally co-vary with progestogens.
Exogenous P4 has been reported to positively and negatively influence cognitive performance of women on various measures. In a study of women with dementia that investigated the effects of estrogen-replacement therapy (ERT; estrogens only) or hormone-replacement therapy (HRT; estrogens combined with a synthetic progestin), the authors concluded that there were short term influences of ERT [15]. In a study of 10 healthy, postmenopausal women (54–70 years of age), micronized P4 enhanced sleep quality (i.e. duration of rapid eye movement sleep) and did not produce any decrements in tasks of attention and verbal memory [27]. The decrements in performance, or lack of effects of HRT, may be due, in part, to older post-menopausal women having low baseline levels of P4 for a prolonged period [21]. A consideration of these dosing effects is relevant for studies in young women. Progesterone administration to normally cycling, healthy women during the early follicular phase increases amygdala activity as measured by fMRI when subjects are presented emotional material compared to neutral images [36], but impairs recall of negative images among normally cycling, healthy women [37]. A P4 dosing regimen that results in much higher levels than typically occur during the luteal phase of women impairs cognitive performance in tasks assessing information processing and verbal memory function [8]. However, P4 treatment to healthy adult women enhances response inhibition and sustained attention, response speed, and visuomotor coordination [31]. A question is the extent to which some of these effects may be due to actions of allopregnanolone. In a study of young, healthy men and women, intramuscular injections of progesterone (200 mg) or placebo similarly increased circulating levels of P4 and allopregnanolone, self-reported ratings of fatigue, and impaired smooth eye pursuit [30]. Thus, the nature of P4’s effects on cognitive function may be influenced by task, age-related differences in duration in a P4-deficient state and/or the current levels of P4 and allopregnanolone produced by exogenous administration.
The type of progestin administered (e.g. P4 vs. medroxyprogesterone acetate, MPA) may influence the nature of the effects on cognitive performance. The Women's Health Initiative reported little or no benefit of HRT containing estrogen and MPA on global cognitive function in post-menopausal women [26]. Although MPA binds progestin receptors (PRs) as does P4, MPA differs from P4 in other ways that make it difficult to generalize findings obtained with MPA to other synthetic progestins or P4. For example, P4 is normally converted by 5α-reductase and 3α-hydroxysteroid dehydrogenase (3α-HSD) to form allopregnanolone, which can have actions at neurotransmitter targets, such as GABAA receptors [20], and is involved in reproductive functions, reward, stress response, and neuroprotection [10]. In one study, MPA administration to the dentate gyrus inhibited 3α-HSD, and prevented the oxidation of allopregnanolone to its precursor, dihydroprogesterone, which had an overall effect to increase GABAergic inhibitory tone [2]. Another study showed that GABA α 4subunit mRNA was decreased by P4, but not MPA, to rats [23]. MPA administration to ovariectomized mice and rats and aged, acyclic female rats do not produce the same levels of allopregnanolone, nor does it have beneficial effects for cognitive processes or neuroprotection as P4 [6,11,12]. Indeed, one of these reports indicates that a lack of induction of a neurotrophic factor, brain-derived neurotrophic factor (BDNF), in explants of the cerebral cortex may be related to a lack of neuroprotection by MPA [16]. Thus, the progestin administered may influence the nature of effects in the central nervous system.
Although P4 may enhance cognitive performance, these effects may be influenced by many variables, such as sex, age, prior steroid exposure, P4 dosing, and progestin regimen. Using animal models, it is possible to control such factors to elucidate individual differences in response to progestogens. We hypothesize that P4’s conversion to allopregnanolone may be essential to mediate some mnemonic effects of P4. To address this, post-training administration of P4, allopregnanolone, or MPA were compared to placebo for effects on performance in the object recognition and object placement task among mice that have the capacity to metabolize P4 to allopregnanolone (wildtype; WT), or not (mice lacking 5α-reductase gene). We, and others, have demonstrated that there is no beneficial effect of MPA for cognitive performance [6,11,12]; however, the extent to which this is due to reliance on progestogen metabolism or induction of growth factors is unclear. The role of BDNF was of interest in this study because there are few reports in the literature regarding the impact of ovarian hormones' on BDNF, and those related to cognitive and neuroprotective effects are inconclusive. Female mice have higher BDNF levels in the hippocampus compared to males [7]. Ovariectomy leads to a reduction in BDNF [28] and estradiol elicits an increase in numerous models [13]. P4 co-administration with estradiol mitigates the BDNF increasing properties of estradiol [3] but can reduce stress-induced reduction in BDNF in the hippocampus [7]. Given previous work showing the involvement of progestogens in the prefrontal cortex and hippocampus for performance in the object recognition placement tasks [11,22], BDNF levels in the prefrontal cortex and hippocampus were determined to ascertain the possible involvement of this trophic factor here.
Materials and Methods
Methods using animal subjects were approved by The Institutional Animal Care and Use Committee at The University at Albany and carried out in accordance with the Guidelines of The National Institute of Health and all regulatory agencies.
Mouse Strain and Genotyping
Female wildtype (WT; n=60), or homozygous (n=60) 5α-reductase knockout (5αRKO) mice were derived from heterozygous breeder pairs. These mice, originally characterized by Mahendroo’s lab [18,19], are genetically deficient in 5α-reductase type 1 enzyme and they have low levels of allopregnanolone in the brain during proestrous and after P4 administration [9,17].
Ovariectomy
Mice were ovariectomized (ovx) under sodium pentobarbital anesthesia (80 mg/kg, or to effect; intraperitoneal injection) at 8 weeks of age, with post-operative analgesic. Although mice were not screened for normative cycling condition prior to or following ovx, inspection of ovaries and fallopian tubes during surgery and uterus following euthanasia did not suggest abnormalities or incomplete ovx. We have no indication of abnormal cycling [17]. Mice were screened for normative motor and species-typical behaviors, sensory responses, and visually-inspected for good general health. Mice were started in the testing protocol one week after surgery, which is our standard procedure to ensure that ovarian steroids have declined before initiation in the study. Previous studies have demonstrated that mice ovx in this timeframe have poorer object recognition and placement performance compared to proestrous mice [34,35].
Hormone conditions
Immediately after training in the object recognition and placement tasks (described below), mice were subcutaneously administered their randomly-assigned hormone treatments: vehicle (vegetable oil, 20% DMSO) or P4 (4 mg/kg; Steraloids, Newport, RI), allopregnanolone (4 mg/kg; Sigma, St. Louis, MO) and/or MPA (10 mg/kg; Sigma).
Behavioral Testing
In the object recognition and object placement tasks, mice were trained with two identical objects (plastic toy block or bottle) that were placed in an open field by free exploration for three minutes. The duration spent within 5 cm of the object, directly in contact, investigating and/or orienting towards the objects was automatically-recorded using a tracking program (Any-Maze; Stoelting Co., Wood Lawn, IL). During testing four hours later, there was a novel object (object recognition task), or displaced object (object placement task). The percentage of time the mice spent exploring the novel object or the displaced object was recorded [11]. There were no significant differences between genotypes for time spent investigating objects during training (WT: 9.8 secs ± 1.1 SEM; 5α-RKO: 8.3 secs ± 1.1 SEM) in the object recognition task, and there was a non-significant reduction in time investigating the objects during object placement task training among 5αRKO (7.8 secs ± 1.4 SEM) compared to WT (13.1 secs ± 1.9 SEM) mice.
BDNF Levels
The prefrontal cortex and hippocampus were dissected out from some mice in each experimental condition (n=3–7), and homogenized in distilled water and cell lysis buffer (Qiagen). Fifty microliters of homogenates were diluted in 4 volumes of Dulbecco's Phosphate-Buffered Saline. Diluted samples were acid-treated by adding 1 microliter of 1N HCl, incubating for 15minutes, and then neutralizing the samples by adding 1 microliter of 1N NaOH. Protein concentrations in each sample were measured using a Nanodrop Spectrometer. Analyses of BDNF were per standard methods of the Emax Immunoassay system (Promega).
Statistical Analyses
Two-way analyses of variances (ANOVAs) were used to examine effects of hormone condition and genotype on behavioral and BDNF levels. When the α level for statistical significance was reached p ≤ 0.05, or a trend was observed p < 0.10, Fisher’s post hoc tests were used to examine group differences.
Results
Object recognition
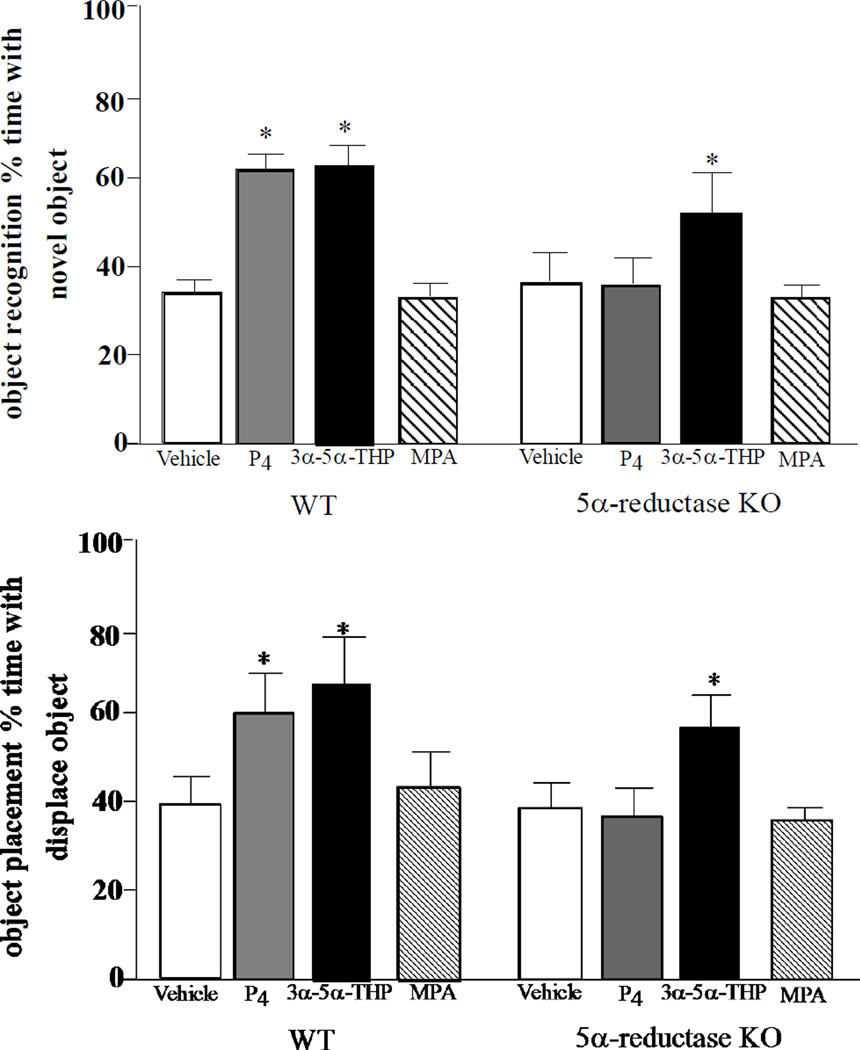
There were significant main effects of genotype [F (1, 110) = 5.15, p = 0.02] and hormone condition [F (3, 110) = 4.51, p < 0.01] and a trend for there to be interaction of genotype and hormone condition [F (3, 110) = 2.49, p = 0.06] for object recognition performance (Figure 1, top). WT, but not 5α-RKO, mice administered P4, compared to vehicle, spent more time investigating the novel object. Both WT and 5αRKO mice administered allopregnanolone spent more time investigating the novel object compared to vehicle-administered mice. There was no difference in performance of mice administered vehicle or MPA.
Figure 1.
Time (mean secs ±SEM) spent with novel object in the object recognition task (TOP) and with a displaced object in the object placement task (BOTTOM) of mice administered vehicle (Veh), progesterone (P4), allopregnanolone (3α,5α,THP), or medroxyprogesterone acetate (MPA). * indicates enhanced cognitive performance compared to vehicle (P<0.05).
Object placement
There was a significant effect of genotype [F (1, 110) = 3.77, p < 0.05] and hormone condition [F (3, 110) = 3.80, p < 0.01], but no significant interaction between these variables, for object placement performance (Figure 1, bottom). WT mice outperformed 5α-RKO mice by spending more time investigating the displaced object. P4 and allopregnanolone significantly increased time spent investigating the displaced object compared to vehicle; albeit, the effect of P4 to enhance performance of 5α-RKO mice was not apparent. Effects of MPA administration were not different from vehicle.
BDNF levels
There was a trend for an effect of hormone condition [F (1, 110) = 2.29, p = 0.09] on levels of BDNF in the prefrontal cortex. Mice administered MPA had lower levels of BDNF than did mice administered vehicle (Table 1). There was a trend for an interaction between genotype and hormone condition [F (3, 110) = 2.60, p = 0.07] for levels of BDNF in the hippocampus, such that 5αRKO, but not WT, mice administered MPA had lower levels of BDNF than vehicle-administered mice (Table 1).
Table 1.
Brain derived neurotrophic factor (BDNF) levels (mean pg/mg ± SEM) in the prefrontal cortex and hippocampus of WT and 5αRKO mice.
| Genotype | Wildtype | 5α–reductase knockout | ||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Veh | P4 | 3α,5α,THP | MPA | Veh | P4 | 3α,5α,THP | MPA |
| Prefrontal Cortex | 61.1±4.9 | 56.2±8.7 | 61.1±6.3 | 44.4#±4.0 | 74.3±15.2 | 60.2±12.5 | 62.2±13.5 | 44.4#±12.6 |
| Hippocampus | 81.9±6.5 | 58.6±8.3 | 56.5±7.5 | 81.7±4.8 | 69.2±13.0 | 66.3±16.2 | 73.6±13.7 | 48.8^±5.0 |
indicates a trend for MPA to decrease BDNF levels in the prefrontal cortex
indicates a trend for BDNF levels to be decreased in the hippocampus with administration of MPA to 5α -reductase knockout, but not wildtype, mice.
Discussion
Findings from the present study that P4 and/or allopregnanolone enhanced object recognition and placement memory among ovx mice supported our hypothesis and confirm and extend prior reports. Here we see that P4’s and allopregnanolone’s effects on cognitive performance were genotype specific: WT mice showed improvement with P4 and allopregnanolone, whereas 5αRKO mice only responded to allopregnanolone. Consistent with these findings are results of prior reports in rats and mice with different capacities to form allopregnanolone. Middle-aged rats with greater capacity to metabolize P4 to allopregnanolone had better cognitive performance on the prefrontal cortex- and hippocampus-mediated tasks (object recognition, Y-maze, and water maze) compared to rats of the same age that had reduced capacity to form allopregnanolone [12,22]. As well, P4 and allopregnanolone, but neither MPA, nor P4 in conjunction with MPA, enhanced performance in hippocampal (object placement, conditioned fear, water maze) and prefrontal tasks (object recognition, T maze) [11]. P4, but not MPA, exerts neuroprotection against kainic acid-induced seizures of rats coincident with increasing allopregnanolone levels [6]. MPA dosing was associated with lower BDNF levels in this study and lower allopregnanolone levels in previous work [11]. One interpretation of these results could be that lower BDNF levels in the prefrontal cortex and hippocampus with MPA treatment may underlie the cognitive decrements that were also observed with this treatment in the present study. Thus, progestin regimen is an important consideration.
Of interest are the mechanisms for the observed effects. In the brain, P4, but not allopregnanolone, binds to intracellular progestin receptors [4]. Progesterone and/or allopregnanolone bind neurotransmitter receptors, and some of progestogens’ effects on reward, stress responding, cognition and/or neuroprotection may involve actions at these targets [10], as well as growth factors. The present study investigated the role of BDNF in the prefrontal cortex and hippocampus of mice with different capacities to form allopregnanolone due to treatment received or their genotype. Although the results did not indicate that P4 and allopregnanolone increased BDNF coincident with enhancements in performance, a reduction in this growth factor with these treatments was not observed, as had been seen with MPA coincident with poor performance in the tasks assessed. As with the literature on mechanisms of progestogens and cognitive/neuroprotective effects, there is limited in vivo literature on BDNF and progestogens, as compared with estrogens. Given that opposite effects of estradiol and P4 have been reported for BDNF [3], but that progestogens have a capacity to enhance cognitive and produce neuroprotection [29], it is important to reconcile how positive effects of progestogens for cognition and neuroprotection are related to actions of growth factors and where in the brain these effects occur. In the present study, unlike others where a reduction in BDNF is shown with P4, mice were not estradiol-primed [3]. The present study focused on the grossly-dissected out prefrontal cortex and hippocampus of mice. Region-specific effects of estradiol and P4 treatment in the CA3 versus dentate gyrus of stressed rats have been reported [7]. It has been suggested that some of the differences in the effects of progestogens involving BDNF may be related to the type of progestogen and its mechanism of action (e.g. P4 acting at nuclear or membrane PRs, allopregnanolone acting at GABA receptors), the pro- versus mature form of BDNF, and/or BDNF synthesis or release [29]. Although it was not tenable to address all of these factors in the present study, the current work adds to the growing literature on in vivo effects of progestogens and growth/plasticity.
Some limitations of the present study are as follows. Differences have been reported between WT and 5α-RKO mice, such as greater anxiety-responding [17]. In this study, testing mice that are ovx, which reduces cognitive functioning, may have engendered greater opportunity for progestogens to improve cognitive performance. In a related study comparing effects of progestins to C57BL/6 mice no differences were noted based upon hormone treatment in the object placement task, in comparison to what we observed in the present study. As such, having this higher baseline response in the mice in that study may have obscured some of the potential effects of progestogens in that task. Moreover, we examined only one dosage of progestins. P4 dosage influences effects on cognitive performance. Healthy young women in the early follicular phase administered 200, but not 400 mg, dosage of P4 had enhanced cognitive performance [31]. Because progestogens can have sedative effects, we chose a regimen that produce moderate physiological, non-sedative concentrations. However, it is possible that progestogens influence cognitive performance in a non-linear/dose-dependent fashion. As such, the present mnemonic effects observed may not have captured the entire range of effects of progestogens for cognitive performance.
Conclusion
In summary, P4 and allopregnanolone, but not MPA, enhanced object recognition and placement memory of WT mice and did not reduce BDNF in the prefrontal cortex and hippocampus, compared to vehicle. These findings suggest that allopregnanolone may underlie some of the effects of P4 to benefit cognitive performance. Currently, P4 is under investigation for the treatment of addiction, seizure disorder, stroke, and traumatic brain injury and other age-related disorders [10,33]. Therefore, it is important to elucidate the manner in which progestogens/progestins may mediate these functions and be related to neural growth.
Highlights.
Progesterone enhanced cognitive performance in mice that express 5α-reductase
MPA did not enhance cognitive performance or BDNF in the cortex or hippocampus.
Allopregnanolone formation may be essential for cognitive performance of mice
Acknowledgments
Sources of support: MH06769801; IBN03-16083.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to report.
References
- 1.Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53:1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- 4.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, et al. Progesterone receptors: form and function in brain. Front. Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen H, Leach LS, Mackinnon A. Cognition in pregnancy and motherhood: prospective cohort study. British Journal of Psychiatry. 2010;196:126–132. doi: 10.1192/bjp.bp.109.068635. [DOI] [PubMed] [Google Scholar]
- 6.Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- 7.Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and nonstressful conditions. Psychoneuroendocrinology. 2006 Jan;31(1):38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C. A placebo-controlled study of effects of oral progesterone on performance and mood. Br. J. Clin. Pharmacol. 1992;33:293–298. doi: 10.1111/j.1365-2125.1992.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 alpha-reductase. Brain Res. 2004;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol. Biochem. Behav. 2007;86:209–219. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frye CA, Koonce CJ, Walf AA. Mnemonic effects of progesterone to mice require formation of 3α,5α-THP. Neuroreport. 2010;21:590–595. doi: 10.1097/WNR.0b013e32833a7e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frye CA, Walf AA, Paris JJ. Conjugated equine estrogen, with medroxyprogesterone acetate, enhances formation of 5alpha-reduced progestogens and reduces anxiety-like behavior of middle-aged rats. Behav Pharmacol. 2010;21:530–539. doi: 10.1097/FBP.0b013e32833e0a23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- 14.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. Journal of Clinical and Experimental Neuropsychology. 2007;29:793–803. doi: 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- 15.Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database Syst. Rev. 2002;3 doi: 10.1002/14651858.CD003799. CD003799. [DOI] [PubMed] [Google Scholar]
- 16.Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on bdnf expression. Endocrinology. 2009;150:3162–3168. doi: 10.1210/en.2008-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koonce CJ, Walf AA, Frye CA. Type 1 5α-reductase may be required for estrous cycle changes in affective behaviors of female mice. Behav Brain Res. 2012;226:376–380. doi: 10.1016/j.bbr.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahendroo MS, Cala KM, Landrum DP, Russell DW. Fetal death in mice lacking 5α-reductase type 1 caused by estrogen excess. Mol Endocrinol. 1997;11:917–927. doi: 10.1210/mend.11.7.9933. [DOI] [PubMed] [Google Scholar]
- 19.Mahendroo MS, Cala KM, Russell DW. 5α-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 20.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 21.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann. N.Y. Acad. Sci. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 22.Paris JJ, Walf AA, Frye CA. Cognitive performance of middle-aged female rats is influenced by capacity to metabolize progesterone in the prefrontal cortex and hippocampus. Brain Res. 2011;1379:149–163. doi: 10.1016/j.brainres.2010.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazol K, Northcutt KV, Patisaul HB, Wallen K, Wilson ME. Progesterone and medroxyprogesterone acetate differentially regulate α4 subunit expression of gaba(a) receptors in the ca1 hippocampus of female rats. Physiol. Behav. 2009;97:58–61. doi: 10.1016/j.physbeh.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson BE, Allison CM. Determination of progesterone and some of its neuroactive ring a-reduced metabolites in human serum. J. Steroid Biochem. Mol. Biol. 2000;74:137–142. doi: 10.1016/s0960-0760(00)00098-4. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SM, Sherwin BB. Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology. 1992;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- 26.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the women’s health initiative memory study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 27.Schussler P, Kluge M, Yassouridis A, Dresler M, Held K, Zihl J, et al. Progesterone reduces wakefulness in sleep eeg and has no effect on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2008;33:1124–1131. doi: 10.1016/j.psyneuen.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 29.Singh M, Su C. Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience. 2013;239:84–91. doi: 10.1016/j.neuroscience.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Söderpalm AH, Lindsey S, Purdy RH, Hauger R, deWit H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 31.Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2010;36:123–132. doi: 10.1016/j.psyneuen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solis-Ortiz S, Corsi-Cabrera M. Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology. 2008;33:989–998. doi: 10.1016/j.psyneuen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Stein DG. Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience. 2011;191:101–106. doi: 10.1016/j.neuroscience.2011.04.013. 191. [DOI] [PubMed] [Google Scholar]
- 34.Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wingen, van Broekhoven Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Molecular Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- 37.van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar J, et al. How progesterone impairs memory for biologically salient stimuli in healthy young women. J. Neurosci. 2007;27:11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]