Abstract
BACKGROUND
In the Beta-Blocker Evaluation of Survival Trial (BEST) trial, systolic blood pressure (SBP) ≤120 mm Hg was an independent predictor of poor prognosis in ambulatory patients with chronic systolic heart failure (HF). Because SBP is an important predictor of response to beta-blocker therapy, the BEST protocol had pre-specified a post hoc analysis to determine if the effect of bucindolol varied by baseline SBP.
METHODS
In the BEST, 2706 patients with chronic systolic (left ventricular ejection fraction <35%) HF and New York Heart Association class III (92%) or IV (8%) symptoms and receiving standard background therapy were randomized to receive either bucindolol (n=1354) or placebo (n=1354). Of these, 1751 had SBP ≤120 mm Hg and 955 had SBP >120 mm Hg at baseline.
RESULTS
Among patients with SBP >120 mm Hg, all-cause mortality occurred in 28% and 22% of patients receiving placebo and bucindolol, respectively (hazard ratio when bucindolol was compared with placebo, 0.77; 95% confidence interval, 0.59–0.99; P=0.039). In contrast, among those with SBP ≤120 mm Hg, 36% and 35% of patients in the placebo and bucindolol groups died, respectively (hazard ratio, 0.95; 95% confidence interval, 0.81–1.12; P=0.541). Hazard ratios (95% confidence intervals) for HF hospitalization associated with bucindolol use were 0.70 (0.56–0.89; P=0.003) and 0.82 (0.71–0.95; P=0.008) for patients with SBP >120 and ≤120 mm Hg, respectively.
CONCLUSION
Bucindolol, a nonselective beta-blocker with weak alpha-blocking properties, significantly reduced HF hospitalization in systolic HF patients regardless of baseline SBP. However, bucindolol reduced mortality only in those with SBP >120 mm Hg.
Keywords: Bucindolol, systolic blood pressure, outcomes, heart failure
In the Beta-Blocker Evaluation of Survival Trial (BEST) trial, the presence of baseline systolic blood pressure (SBP) ≤120 mm Hg was an independent predictor of poor prognosis in ambulatory patients with chronic systolic heart failure (HF).1 Because SBP is an important predictor of response to beta-blocker therapy, the BEST protocol had pre-specified a post hoc analysis to determine if the effect of bucindolol varied by baseline SBP.2 However, to the best of our knowledge, that important post hoc analysis has never been conducted or reported. The objective of this study was to examine if the effect of bucindolol on outcomes varied by baseline SBP.
METHODS
Study design and participants
The BEST was a multicenter randomized placebo-controlled clinical trial of bucindolol, a non-selective beta-blocker, in patients with HF, the details of which have been previously described.2, 3 Briefly, 2708 HF patients with New York Heart Association (NYHA) class III-IV symptoms and left ventricular ejection fraction (LVEF) <35% were enrolled from 90 different sites across the United States and Canada between May 1995 and December 1998, and were randomized to receive bucindolol or placebo. Over 90% of all patients were receiving angiotensin-converting enzyme (ACE) inhibitors, diuretics, and digitalis. Patients were followed up for a mean duration of 2 years. BEST was sponsored by the US Department of Veterans Affairs and the US National Heart, Lung and Blood Institute. The latter provided a public-use copy of the BEST dataset used for the current analysis. The public-use copy of the data is similar to the original BEST data, and all but one patient consented to be included in the de-identified public-use copy of the data.
SBP measurements
Data on SBP and other characteristics were measured and documented by study investigators. Data on baseline SBP were available on 2706 participants, of which 1751 (65%) had SBP ≤120 (median, 108; range, 70–120) mm Hg and 955 had SBP >120 (median, 134; range 121–192) mm Hg. Because SBP 120 mm Hg has been recommended as target SBP for HF patients,4 and because BEST participants with SBP <120 mm Hg had poor outcomes,1 we used 120 mm Hg as the cutoff for SBP in our study.
Outcomes
Primary outcomes for the current analysis were all-cause mortality and HF hospitalization during 4.1 years of follow-up (mean, 2 years; range, 10 days to 4.14 years). Secondary outcomes were sudden cardiac death, HF mortality and cardiovascular mortality, and all-cause hospitalization.
Statistical analysis
Baseline characteristics between patients in the placebo and bucindolol groups were examined separately among patients in the two SBP groups, and were tested using chi-square and student’s t-tests as appropriate. Kaplan-Meier survival analyses and Cox regression analyses were used to determine the effect of bucindolol on outcomes separately in the two SBP groups. Log-minus-log scale survival plots were used to check proportional hazards assumptions. All statistical tests were two-tailed with a P-value <0.05 considered significant. All data analyses were performed using SPSS for Windows version 15 (SPSS Inc., Chicago, IL).
RESULTS
Baseline characteristics
As presented in the original report, BEST participants had a mean (±standard deviation) age of 60 (±12) years, 22% were women and 23% were African American.3 Also, as presented before, patients with SBP ≤120 mm Hg were younger than those with SBP >120 mm Hg.1 All key baseline characteristics were balanced between patients receiving placebo and bucindolol in both SBP groups (Table 1).
Table 1.
Baseline patient characteristics, by placebo and bucindolol, and by systolic blood pressure (SBP)
| n (%) or mean (±SD) | SBP>120 mm Hg (n=955) | SBP ≤120 mm Hg (n=1751) | ||||
|---|---|---|---|---|---|---|
| Placebo (n=481) |
Bucindolol (n=474) |
P value |
Placebo (n=871) |
Bucindolol (n=880) |
P value |
|
| Age, years | 62.1 (±11.4) | 61.1 (±11.5) | 0.193 | 59.4 (±12.5) | 59.7 (±13) | 0.607 |
| Female | 112 (23.3%) | 110 (23.2%) | 0.977 | 194 (22%) | 176 (20%) | 0.244 |
| African American | 123 (26%) | 116 (24%) | 0.695 | 182 (21%) | 206 (23%) | 0.205 |
| Smoker | 74 (15%) | 88 (19%) | 0.190 | 138 (16%) | 174 (20%) | 0.032 |
| Body mass index, kg/m2 | 37.4 (±8.6) | 38.0 (±8.5) | 0.278 | 36.2 (±8.3) | 35.8 (±8.3) | 0.298 |
| NYHA class III | 452 (94%) | 453 (96%) | 0.267 | 788 (90%) | 787 (89%) | 0.470 |
| Medical history | ||||||
| Heart failure duration, months | 48.6 (±51.1) | 46 (±45.9) | 0.415 | 52 (±49) | 49 (±48) | 0.177 |
| Coronary artery disease | 290 (60%) | 260 (55%) | 0.089 | 503 (58%) | 539 (61%) | 0.136 |
| Angina pectoris | 271 (56%) | 240 (51%) | 0.077 | 431 (50%) | 457 (52%) | 0.306 |
| Hypertension | 363 (76%) | 344 (73%) | 0.308 | 433 (50%) | 455 (52%) | 0.405 |
| Diabetes mellitus | 188 (39%) | 194 (41%) | 0.561 | 276 (32%) | 305 (35%) | 0.187 |
| Hyperlipidemia | 217 (45%) | 212 (45%) | 0.904 | 357 (41%) | 383 (44%) | 0.283 |
| Thromboembolic disease | 83 (17%) | 76 (16%) | 0.612 | 173 (20%) | 155 (18%) | 0.228 |
| Chronic kidney disease | 177 (37%) | 174 (38%) | 0.977 | 313 (40%) | 342 (39%) | 0.206 |
| Atrial fibrillation | 109 (23%) | 125 (26%) | 0.183 | 213 (25%) | 206 (23%) | 0.608 |
| Peripheral vascular disease | 95 (20%) | 86 (18%) | 0.526 | 128 (15%) | 132 (15%) | 0.858 |
| Medications | ||||||
| ACE inhibitors /ARB | 464 (97%) | 449 (95%) | 0.190 | 840 (96%) | 854 (97%) | 0.476 |
| Digitalis | 439 (91%) | 434 (92%) | 0.872 | 805 (92%) | 816 (93%) | 0.808 |
| Diuretics | 439 (91%) | 430 (91%) | 0.766 | 820 (94%) | 834 (95%) | 0.566 |
| Vasodilators | 218 (45%) | 198 (42%) | 0.269 | 387 (44%) | 380 (43%) | 0.598 |
| Anti–coagulants | 271 (56%) | 251 (53%) | 0.293 | 521 (60%) | 526 (60%) | 0.985 |
| Physical examination | ||||||
| Systolic blood pressure, mm Hg | 137 (±12) | 137 (±12) | 0.747 | 107 (±9.5) | 106 (±10) | 0.279 |
| Diastolic blood pressure, mm Hg | 78 (±11) | 78 (±11) | 0.174 | 67 (±9.2) | 67 (±9.3) | 0.531 |
| Pulse, per minute | 80 (±13) | 80 (±13) | 0.523 | 82 (±13) | 83(±14) | 0.486 |
| Jugular venous distension | 206 (43%) | 193 (41%) | 0.509 | 405 (47%) | 431 (49%) | 0.299 |
| S3 gallop | 174 (36%) | 181 (38%) | 0.520 | 394 (45%) | 429 (49%) | 0.141 |
| Pulmonary râles | 63 (13%) | 61 (13%) | 0.916 | 120 (14%) | 115 (13%) | 0.663 |
| Lower extremity edema | 140 (29%) | 123 (26%) | 0.275 | 220 (25%) | 247 (28%) | 0.184 |
| Laboratory data | ||||||
| Serum creatinine, mg/dL | 1.2 (±0.41) | 1.2 (±0.43) | 0.924 | 1.23 (±0.40) | 1.27 (±0.40) | 0.042 |
| Serum potassium, mEq/L | 4.3 (±0.5) | 4.3 (±0.5) | 0.268 | 4.3 (±0.47) | 4.3 (±0.48) | 0.633 |
| Serum glucose, mg/dl | 144 (±81.2) | 145 (±78.7) | 0.873 | 127 (±69.6) | 130 (±72.2) | 0.476 |
| Left bundle branch block | 106 (22%) | 110 (23%) | 0.666 | 237 (27%) | 226 (26%) | 0.468 |
| Pulmonary edema by chest x-ray | 49 (10%) | 55 (12%) | 0.482 | 96 (11%) | 108 (12%) | 0.415 |
| Cardiothoracic ratio by chest x-ray | 55 (±7.1) | 54 (±6.9) | 1.000 | 56 (±7.1) | 56 (±7.2) | 0.772 |
| LV ejection fraction, % | 25 (±7) | 26 (±7) | 0.719 | 22 (±7) | 21 (±7) | 0.085 |
| RV ejection fraction, % | 37 (±12) | 37 (±11) | 0.984 | 34 (±12) | 33 (±12) | 0.229 |
ACE=angiotensin converting enzyme; ARB=angiotensin receptor blocker; LV=left ventricular; NYHA=New York Heart Association; RV=right ventricular
Bucindolol and all-cause mortality
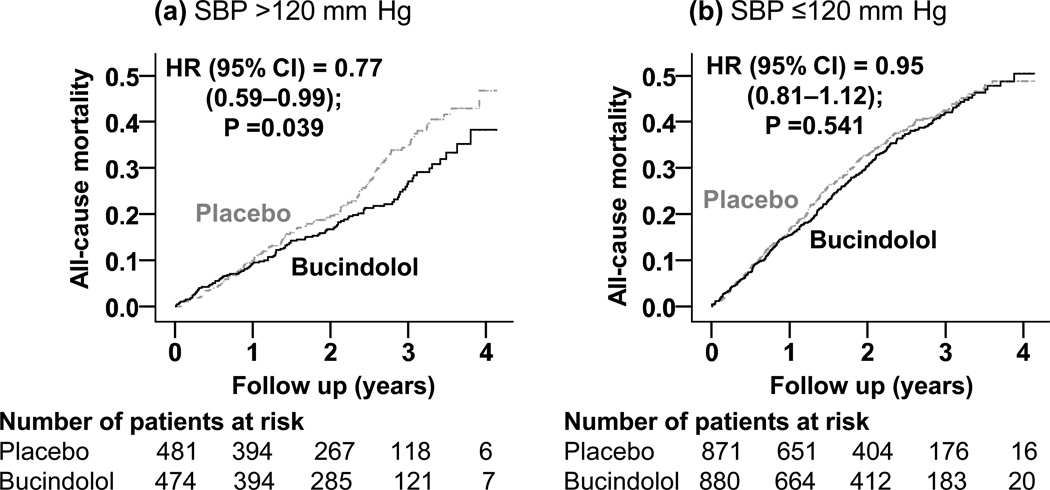
Among the 955 patients with baseline SBP >120 mm Hg, all-cause mortality occurred in 28% and 22% of those receiving placebo and bucindolol respectively (hazard ratio [HR] when bucindolol was compared with placebo, 0.77; 95% confidence interval [CI], 0.59–0.99; P=0.039; Table 2 and Figure 1). Among the 1751 patients with baseline SBP ≤120 mm Hg, all-cause mortality occurred in 36% and 35% of those receiving placebo and bucindolol respectively (HR when bucindolol was compared with placebo, 0.95; 95% CI, 0.81–1.12; P=0.541; Table 2 and Figure 1). When we examined mortality rate by deciles of baseline SBP, we noted that except for the 101–107 decile, in all subgroups with SBP ≤120 mm Hg, bucindolol had no effect on mortality. This difference in the effect of bucindolol on mortality between the two SBP groups was not statistically significant (P for interaction, 0.156).
Table 2.
Effects of bucindolol on all-cause mortality and heart failure hospitalization, by systolic blood pressure (SBP)
| SBP >120 mm Hg (n=955) | |||||
| Outcomes | Events (%) | Absolute risk decrease * |
Hazard ratio | P value | |
| Placebo (n=481) |
Bucindolol (n=474) |
(95% confidence interval) |
|||
| All-cause mortality | 135 (28) | 108 (22) | − 6% | 0.77 (0.59–0.99) | 0.039 |
| Heart failure hospitalization | 177 (36) | 130 (27) | − 9% | 0.70 (0.56–0.89) | 0.003 |
| SBP ≤120 mm Hg (n=1751) | |||||
| Outcomes | Events (%) | Absolute risk decrease * |
Hazard ratio | P value | |
| Placebo (n=871) |
Bucindolol (n=880) |
(95% confidence interval) |
|||
| All-cause mortality | 313 (36) | 305 (35) | − 1% | 0.95 (0.81–1.12) | 0.541 |
| Heart failure hospitalization | 396 (46) | 346 (39) | − 6% | 0.82 (0.71–0.95) | 0.008 |
Absolute rate decrease was calculated by subtracting the rates of events in the placebo group from those in bucindolol group
Figure 1.
Kaplan-Meier plots for all-cause mortality in systolic heart failure patients randomized to receive bucindolol versus placebo, by baseline systolic blood pressure (SBP) (a) >120 mm Hg and (b) ≤120 mm Hg (HR=hazard ratio; CI=confidence interval)
Bucindolol and HF hospitalization
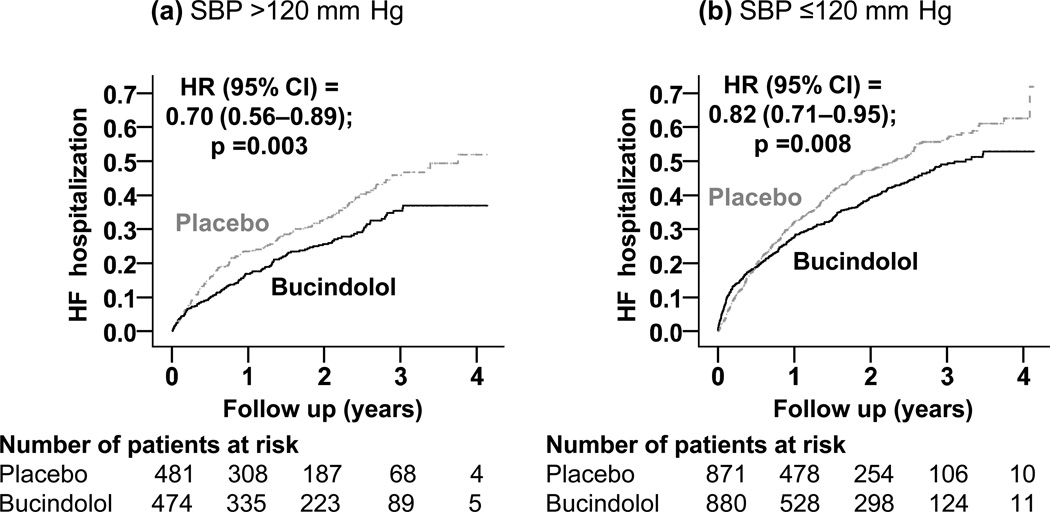
Among the 955 patients with baseline SBP >120 mm Hg, HF hospitalization occurred in 36% and 27% of those receiving placebo and bucindolol respectively (HR, 0.70; 95% CI, 0.56–0.89; P=0.003; Table 2 and Figure 2). Among the 1751 patients with baseline SBP ≤120 mm Hg, HF hospitalization occurred in 46% and 39% of those receiving placebo and bucindolol respectively (HR, 0.82; 95% CI, 0.71–0.95; P=0.008; Table 2 and Figure 2). There was no statistically significant difference in the effect of bucindolol on HF hospitalization between the two SBP groups (P for interaction, 0.269). Associations of bucindolol with other outcomes in the two SBP groups are displayed in Table 3.
Figure 2.
Kaplan-Meier plots for heart failure (HF) hospitalization in systolic HF patients randomized to receive bucindolol versus placebo, by baseline systolic blood pressure (SBP) (a) >120 mm Hg and (b) ≤120 mm Hg (HR=hazard ratio; CI=confidence interval)
Table 3.
Effects of bucindolol on other outcomes, by systolic blood pressure (SBP)
| SBP >120 mm Hg (n=955) | |||||
| Outcomes | Events (%) | Absolute risk decrease * |
Hazard ratio | P value | |
| Placebo (n=481) |
Bucindolol (n=474) |
(95% confidence interval) |
|||
| Cardiovascular mortality | 113 (24) | 88 (19) | − 5% | 0.76 (0.57–1.00) | 0.052 |
| Heart failure mortality | 31 (6) | 17 (4) | − 2% | 0.53 (0.29–0.96) | 0.035 |
| Sudden cardiac death | 68 (14) | 62 (13) | − 1% | 0.89 (0.63–1.26) | 0.516 |
| All-cause hospitalization | 297 (62) | 262 (55) | − 7% | 0.85 (0.72–1.00) | 0.058 |
| SBP ≤120 mm Hg (n=1751) | |||||
| Outcomes | Events (%) | Absolute risk decrease * |
Hazard ratio | P value | |
| Placebo (n=871) |
Bucindolol (n=880) |
(95% confidence interval) |
|||
| Cardiovascular mortality | 275 (32) | 254 (30) | − 2% | 0.90 (0.76–1.07) | 0.240 |
| Heart failure mortality | 109 (13) | 105 (12) | − 1% | 0.94 (0.72–1.23) | 0.649 |
| Sudden cardiac death | 134 (15) | 120 (14) | − 1% | 0.88 (0.69–1.12) | 0.297 |
| All-cause hospitalization | 576 (66) | 567 (64) | − 2% | 0.97 (0.86–1.09) | 0.593 |
Absolute rate decrease was calculated by subtracting the rates of events in the placebo group from those in bucindolol group
DISCUSSION
Findings from the current study demonstrate that nearly two thirds of the patients with advanced chronic systolic HF enrolled in the BEST trial had baseline SBP ≤120 mm Hg, and that patients randomized to bucindolol in this SBP group has similar all-cause mortality as in patients randomized to placebo. On the other hand, despite a much smaller sample size of the group with SBP >120 mm Hg, those randomized to receive bucindolol had a significantly lower risk of death. Bucindolol, however, reduced HF hospitalization in both SBP groups. Although bucindolol is not an approved beta-blocker for use in HF, the BEST trial was terminated early as the effect of bucindolol on outcomes in HF was similar to other beta-blockers, thus the findings of the current analysis provide important insights as to how baseline SBP may modify the effect of beta-blockers.
The beneficial effect of therapy is often more pronounced in those with poor outcomes.5 Therefore, it was intriguing that despite higher mortality, HF patients with low SBP did not benefit from therapy with bucindolol. One potential explanation might be that systolic HF patients with low SBP had advanced HF, in whom progressive HF is a relatively more common cause of death than sudden cardiac death.6–9 We also observed that HF mortality was more common in patients with baseline SBP ≤120 mm Hg (Table 3; lower panel). Yet, bucindolol had no effect on HF mortality in this group while it significantly reduced HF mortality by 50% in those with SBP >120 mm Hg. It is possible that patients with SBP ≤120 mm Hg with lower mean baseline RVEF and LVEF (than those with SBP >120 mm Hg) had more advance disease and were more critically dependent on adrenergic drive, which may have attenuated the mortality benefit of bucindolol. Interestingly, adrenergic blockade with bucindolol did not seem to attenuate it's effect on HF hospitalization. The effect of beta-blockade with bucindolol in advanced systolic HF patients with low SBP may be complex and needs to be examined in other study cohorts.
These findings are important as with the increasing use of beta-blockers and device-based therapies, the rate of sudden cardiac death is decreasing in contemporary HF patients.10, 11 As HF patients are living longer, they are more likely to die from progressive HF, especially after hospitalization due to acute decompensation. Therefore, reducing death due to progressive HF will be an important target to reduce cause-specific deaths in patients with advanced systolic HF. Findings from the MERIT-HF trial suggest the metoprolol succinate, extended release, a beta-1 selective blocker reduced mortality regardless of baseline SBP.12 No such data was provided on the effect of bisoprolol, another beta-1 selective blocker, approved for use in HF.13 A post hoc analysis of the COPERNICUS trial suggested that patients with the lower SBP groups were at higher risk of events, and that carvedilol, the only non-selective beta-blocker approved for use in HF, have similar effect regardless of baseline SBP.7 However, nearly one-third of patients in that study had SBP <116 mm Hg and carvedilol had no significant effect on all-cause mortality or the combined end points of death or HF mortality in those patients.7
Several limitations of our study need to be acknowledged. The findings reported here are based on post hoc analysis of a trial that was prematurely stopped by the data safety monitoring board because of the “totality of evidence regarding the usefulness of beta-blocker treatment derived from BEST and other studies”.3 However, at the time of stopping, although bucindolol significantly reduced cardiovascular mortality (P=0.04), its effect on all-cause mortality did not achieve statistical significance (P=0.10).3 Although, like carvedilol, bucindolol is also a non-selective beta-blocker, unlike carvedilol, it has no alpha-blocking and anti-oxidant properties.14, 15 Further, bucindolol is also distinguished by both partial and inverse agonist activities.15 Therefore, findings of the current study based on bucindolol should be interpreted with caution when applying to HF patients receiving other approved beta-adrenergic blockers, such as carvedilol.
In conclusion, in patients with advanced systolic HF, although bucindolol significantly reduced HF hospitalization regardless of baseline SBP, it reduced all-cause mortality only in those with SBP >120 mm Hg, not in those with SBP ≤120 mm Hg. Future studies are needed to clarify the outcomes in HF patients with lower SBP who are using approved beta-blockers.
Acknowledgement
The Beta-Blocker Evaluation of Survival Trial (BEST) is conducted and supported by the NHLBI in collaboration with the BEST Study Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the BEST or the NHLBI.
Funding sources
Dr. White holds the Carolyn and Richard J. Renaud Research Chair in Heart Failure of the Montreal Heart Institute. Dr. Ahmed is supported by grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Maryland and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Disclosures
None.
References
- 1.Desai RV, Banach M, Ahmed MI, Mujib M, Aban I, Love TE, White M, Fonarow G, Deedwania P, Aronow WS, Ahmed A. Impact of baseline systolic blood pressure on long-term outcomes in patients with advanced chronic systolic heart failure (insights from the best trial) Am J Cardiol. 2010;106:221–227. doi: 10.1016/j.amjcard.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The BEST Steering Committee. Design of the beta-blocker evaluation survival trial (BEST) Am J Cardiol. 1995;75:1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- 3.The BEST Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 4.Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, Kaplan NM, O'Connor CM, O'Gara PT, Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease: A scientific statement from the american heart association council for high blood pressure research and the councils on clinical cardiology and epidemiology and prevention. Circulation. 2007;115:2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: Importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 6.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 7.Rouleau JL, Roecker EB, Tendera M, Mohacsi P, Krum H, Katus HA, Fowler MB, Coats AJ, Castaigne A, Scherhag A, Holcslaw TL, Packer M. Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: The carvedilol prospective randomized cumulative survival (copernicus) study. J Am Coll Cardiol. 2004;43:1423–1429. doi: 10.1016/j.jacc.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 8.The MERIT-HF Investigators. Effect of metoprolol cr/xl in chronic heart failure: Metoprolol cr/xl randomised intervention trial in congestive heart failure (merit-hf) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 9.Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L. Acute coronary findings at autopsy in heart failure patients with sudden death: Results from the assessment of treatment with lisinopril and survival (atlas) trial. Circulation. 2000;102:611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 10.Howlett JG, McKelvie RS, Costigan J, Ducharme A, Estrella-Holder E, Ezekowitz JA, Giannetti N, Haddad H, Heckman GA, Herd AM, Isaac D, Kouz S, Leblanc K, Liu P, Mann E, Moe GW, O'eara E, Rajda M, Siu S, Stolee P, Swiggum E, Zeiroth S. The 2010 canadian cardiovascular society guidelines for the diagnosis and management of heart failure update: Heart failure in ethnic minority populations, heart failure and pregnancy, disease management, and quality improvement/assurance programs. Can J Cardiol. 2010;26:185–202. doi: 10.1016/s0828-282x(10)70367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouleau JL. New and emerging drugs and device therapies for chronic heart failure in patients with systolic ventricular dysfunction. Can J Cardiol. 2011;27:296–301. doi: 10.1016/j.cjca.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The metoprolol cr/xl randomized intervention trial in congestive heart failure (merit-hf). Merit-hf study group. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 13.The CIBIS Investigators. The cardiac insufficiency bisoprolol study ii (cibis-ii): A randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 14.Bristow MR, Larrabee P, Muller-Beckmann B, Minobe W, Roden R, Skerl L, Klein J, Handwerger D, Port JD. Effects of carvedilol on adrenergic receptor pharmacology in human ventricular myocardium and lymphocytes. Clin Investig. 1992;70(Suppl 1):S105–S113. doi: 10.1007/BF00207620. [DOI] [PubMed] [Google Scholar]
- 15.Hershberger RE, Wynn JR, Sundberg L, Bristow MR. Mechanism of action of bucindolol in human ventricular myocardium. J Cardiovasc Pharmacol. 1990;15:959–967. doi: 10.1097/00005344-199006000-00014. [DOI] [PubMed] [Google Scholar]