Abstract
The objectives of this review are to describe the clinical manifestations of the growing spectrum of monogenic autoinflammatory diseases including recently described syndromes. The autoinflammatory diseases can be grouped based on clinical findings: 1. the three classic hereditary “periodic fever syndromes”, familial Mediterranean Fever (FMF); TNF receptor associated periodic syndrome (TRAPS); and mevalonate kinase deficiency/hyperimmunoglobulinemia D and periodic fever syndrome (HIDS); 2. the cryopyrin associated periodic syndromes (CAPS), comprising familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and neonatal-onset multisystem inflammatory disease (NOMID) or CINCA, and; 3. pediatric granulomatous arthritis (PGA); 4. disorders presenting with skin pustules, including deficiency of interleukin 1 receptor antagonist (DIRA); Majeed syndrome; pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome; deficiency of interleukin 36 receptor antagonist (DITRA); CARD14 mediated psoriasis (CAMPS), and early-onset inflammatory bowel diseases (EO-IBD); 5. inflammatory disorders caused by mutations in proteasome components, the proteasome associated autoinflammatory syndromes (PRAAS) 6. very rare conditions presenting with autoinflammation and immunodeficiency.
Keywords: autoinflammatory diseases, CAPS, FMF, TRAPS, HIDS, DIRA
1. Introduction
The history of “autoinflammatory diseases” dates back to the identification of the genetic causes of the most prevalent monogenic autoinflammatory disease worldwide, the autosomal recessive disease, familial Mediterranean fever (FMF), in 1997, and the discovery of TNF receptor mutations in the autosomal dominant disorder, TNF receptor associated periodic syndrome (TRAPS) in 1999 (1-3). These discoveries have marked the beginning of an exciting journey that led not only to the molecular understanding of previously clinically defined entities, but also to the characterization of novel diseases that were not previously clinically described.
The discovery that gain of function mutations in NLRP3, a component of an IL-1β processing complex, the NLRP3 inflammasome, can cause the spectrum of clinical disorders now called cryopyrinopathies, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and neonatal-onset multisystem inflammatory disease (NOMID) also called chronic infantile neurological and articular syndrome (CINCA) led to the hypothesis that the proinflammatory cytokine IL-1β may play an important role in the pathogenesis of these disorders (4, 5). Early proof of concept studies with the IL-1 blocking agent anakinra (Kineret®) surprisingly showed impressive clinical responses in the patients treated. These studies not only validated the role of IL-1 blockade in these disorders, but also led to the FDA approval of now three IL-1 blocking agents for the treatment of these disorders, the long acting IL-1 blocking agents, rilonacept (Arcalyst®) and canakinumab (Ilaris®) approved in 2008 and 2009, respectively, and the short acting IL-1 inhibitor anakinra (Kineret®) in 2012.
The wider use of IL-1 blocking therapies in other autoinflammatory phenotypes with similarities to the cryopyrinopathies has led to the identification of disorders that respond and those that do not respond to IL-1 blocking therapies and has fueled research in exploring other inflammatory pathways that can lead to autoinflammatory phenotypes (4).
The concept of autoinflammatory diseases was initially proposed to distinguish the then known autoimmune diseases, including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), disorders that were thought to be caused by adaptive immune dysregulation, from the two hereditary fever syndromes, FMF and TRAPS that lack features of adaptive immune dysregulation, specific autoantibodies and auto-reactive lymphocytes, and were thus suggested to be caused by innate immune defects (5, 6). This concept has been tremendously successful in promoting the recognition of innate immune pathway dysregulation in the pathogenesis of the expanding spectrum of noninfectious fever syndromes (6). However, features of innate and adaptive immune dysregulation are present together in a number of disorders, most prevalent in the proteasome-associated disorders that often also present with specific autoantibodies. Moreover, the discovery that SLE, a “classic autoimmune” disease, has immune regulatory defects in the innate immune system, illustrates the overlapping immunological features of some autoinflammatory diseases with the classical autoimmune diseases and vice versa (4, 7).
Any classification of autoinflammatory diseases should be regarded as preliminary, and the complex consequences of the genetic defect associated with the respective diseases are not fully recognized and understood. In a number of diseases, the immune dysregulation is not limited to one cytokine pathway and may vary in different cells.
In this review, we grouped the monogenic autoinflammatory diseases based on easily identifiable clinical criteria that can be elicited from a patient's history and physical exam, taking into account fever patterns, characteristic skin lesions and associated clinical findings (Table 1).
Table 1. Clinical grouping of autoinflammatory diseases by fever and skin manifestations.
| 1. NON-SPECIFIC MACULO-PAPULAR RASHES WITH RECURRENT EPISODIC FEVER AND ABDOMINAL PAIN (THE CLASSICAL “PERIODIC FEVER SYNDROMES”) |
|
|
| Recurrent fever attacks of short duration (typically < 7 days) |
|
| Recurrent fever attacks of longer duration (typically > 7 days) |
| TRAPS: TNF receptor associated periodic syndrome |
|
|
| 2. NEUTROPHILIC URTICARIA (THE CRYOPYRINOPATHIES) |
|
|
| Recurrent fever attacks of short duration (typically < 24 hours) |
|
| Continuous low grade fever |
|
|
|
| 3. GRANULOMATOUS SKIN LESIONS AND MINIMAL OR LOW GRADE FEVER ATTACKS |
|
|
|
|
|
| 4. PUSTULAR SKIN RASHES AND EPISODIC FEVER |
|
|
| With inflammatory bone disease |
|
| With pyogenic arthritis |
|
| With inflammatory bowel disease |
|
| Without other organ involvement |
|
|
|
| 5. ATYPICAL NEUTROPHILIC DERMATOSIS WITH HISTIOCYTIC-LIKE INFILTRATE |
|
|
|
|
|
| 6. SYNDROMES WITH AUTOINFLAMMATION AND IMMUNODEFICIENCY |
|
|
|
2. The classic “periodic fever syndromes” presenting with recurrent episodic fevers, non-specific maculopapular rashes and abdominal pain
Among the three disorders, FMF and MVK deficiency/HIDS present with recurrent fever attacks of short duration (<7 days), while TRAPS presents with fever attacks of longer that 7-day duration that can last up to weeks.
2.1 Familial Mediterranean fever (FMF)
Epidemiology and Genetics
FMF (OMIM#249100) is the most prevalent monogenic autoinflammatory disease, affecting more than 100,000 subjects worldwide (8, 9). The first FMF patients were reported by Janeway and Mosenthal in 1908 (10) and a case series by Siegel in 1945 (11). FMF is most prevalent in eastern Mediterranean populations including Sephardic Jews, Armenian, Turkish and Arabian descendants, with a disease prevalence of up to 1:248 in Libyan Jewish populations (12-14) and a carrier rate of 1:3-1:7 in North African Jews, Iraq Jews, Armenians and Turks (15)
FMF is caused by autosomal recessive, mostly missense mutations in the MEFV gene (1, 2). In the last 15 years, over 50 disease-causing MEFV variants have been reported (16). Almost half of these mutations are in exon 10, encoding the B30.2 (SPRY) domain, a regulatory protein-protein domain found in nearly 100 human proteins (17). The most common missense mutations detected in FMF patients are: M694V, M680I, M694I and E726A (1, 14). Genetic variants found in exons 2 and 3 are often associated with nonspecific inflammatory manifestations and are of uncertain clinical significance. Although the amino acid change E148Q encoded by a missense mutation in exon 2 is commonly found in MEFV, it has an allele frequency of 0.5–5% in Caucasians and up to 23% in Japanese and has been suggested to be a benign polymorphism (18, 19).
Clinical Presentation and Diagnosis
Most of the FMF patients develop symptoms before the third decade of life (20). The disease typically presents with recurrent episodes of fever, associated with acute abdominal pain and large joint arthritis that last 1 to 3 days (20). Flares recur periodically between once a week and once a year with symptom free intervals in between flares (8, 17, 21). The fever attacks occur suddenly with high-grade fever (38.5-40°C) and severe asthenia (22). Moderate to severe abdominal pain occurs in 95% of the patients and is secondary to an acute generalized peritonitis (23). On physical examination patients often have abdominal distension and intense rebound pain, mimicking an acute abdomen, which has been estimated to lead to abdominal surgery (appendectomy or cholecystectomy) in 30 to 40% of FMF patients (23). Other gastrointestinal symptoms such as constipation and diarrhea may occur (13).
Articular involvement is present in up to three quarters of FMF patients, typically presenting as acute monoarthritis of large lower extremities joints (20, 24). However, chronic arthritis of hips, knees or ankles may occur in about 5% of the patients (25). During the flares, myalgia is observed in 10% of the patients, typically in lower limbs and triggered by physical exercise (20, 26). Refractory febrile myalgia can rarely be observed and is thought to be due to vasculitis. It typically presents with severe bilateral lower limb pain with normal CK levels, and with fever and abdominal pain that can last up to 6 weeks (26, 27).
Besides peritonitis, other areas of serositis include pleuritis and pericarditis in about 39% and 2% of the patients, respectively (28-30). Scrotal pain due to tunica vaginalis, a remnant of the peritoneum, involvement is found in 9% of the subjects (31).
Overall skin involvement is more variable and often absent. The most common cutaneous manifestation in FMF is erysipelas-like erythema, reported in 7 to 40% of patients in some cohorts of patients and characterized by lower limb erythematous and rash that are frequently misdiagnosed as cellulitis (20, 21).
The diagnosis of FMF has been based on clinical criteria that include frequent symptoms such as abdominal and thoracic pain, family history and response to treatment with colchicine (32). An original set of diagnostic criteria from 1997 had a high sensitivity (99%) and low specificity (55%) and a newer set of criteria requiring the presence of at least two of the following five criteria: fever > 38oC, abdominal pain, chest pain, arthritis and family history of FMF had an improved specificity to 94% with a sensitivity of 86% for FMF diagnosis in the Turkish population, and was created in a study that included 170 affected children and 141 healthy controls. (33). Nevertheless, the finding of mutations in MEFV gene is mandatory for a definitive FMF diagnosis (16).
During FMF flares, laboratory exams typically indicate leukocytosis and increased acute phase reactants, such as ESR and CRP (20). In most patients, the inflammatory markers normalize in between the attacks. Type AA secondary amyloidosis is the most frequent complication that varies between counties (34). In a multicenter study the country of recruitment was the most important risk factor for the occurrence of renal amyloidosis and, from the 260 patients with amyloidosis evaluated, 74% of them were recruited in Armenia (28.1%), Israel (24.2%) or Turkey (21.5%) (34). The prevalence of FMF secondary amyloidosis has not been reported, except by in Turkish patients where is reported to be 13% (35). Kidneys are the most affected organs and these patients present with progressive proteinuria, nephrotic syndrome leading to chronic renal failure (35). Secondary AA amyloidosis is caused by the tissue deposition of persistently elevated serum amyloid A (SAA) levels. The development of AA amyloidosis is unlikely with low serum concentrations of this protein (<4mg/L) (36).
Treatment
Colchicine remains the first choice treatment for FMF, it in many cases induces a complete remission or diminishes the frequency, length or severity of the flares (37). Additionally, colchicine use can prevent, delay or revert renal amyloidosis and is considered safe even during pregnancy (38). Side effects include: diarrhea, abdominal pain, skin rash, leukopenia, thrombocytopenia, neuropathy, myopathy and liver damage (37, 39). For patients that are unresponsive or do not tolerate colchicine, depending on the center, IL-1 inhibition is an evolving second choice (40, 41). A randomized placebo-controlled trial has recently suggested that the long acting IL-1 inhibitor rilonacept, is a treatment option for FMF patients that are refractory or intolerant to colchicine (41). Other treatment regimes that have been reported include treatment with interferon-alpha (42, 43), thalidomide (44) and TNF inhibiting drugs such as etanercept (45, 46) and infliximab (47, 48).
2.2 Mevalonate kinase deficiency (MVK) / Hyperimmunoglobulinemia D with periodic fever syndrome (HIDS)
Epidemiology and Genetics
HIDS (OMIM#260920), an autosomal recessive disease, is caused by mutations in MVK (mevalonate kinase gene) (49). Of the more than 100 variants in MVK have been described only about one third are thought to be disease causing (16)(50). Although the V377I variant is found in about 50% of HIDS patients, it has been suggested that the presence of this mutation in homozygosity is associated with mild or asymptomatic HIDS clinical phenotypes (51). Mutations in MVK can also cause a more severe and rare phenotype called mevalonic aciduria (MA) (52, 53); the severity of the disease phenotype is correlated with the residual enzymatic function of the mutated protein (54). Whereas in HIDS MVK activity is reduced to 1 to 10% of normal, in MA this activity is below 1% (55). MA is clinically characterized by periodic fever, severe neurological impairment, severe growth retardation and early death (52, 53).
Clinical Presentation and Diagnosis
HIDS fever episodes last 3 to 7 days and typically recur every 4 to 6 weeks (54, 56). Most HIDS patients present with their first HIDS attack in early childhood, 78% have the first fever attack before 12 months of age and 94% before the age of 4 years (57). Fever episodes may recur for many years and are more frequent during childhood and adolescence (58). The flares are generally triggered by immunizations, trauma, surgery or stress and are characterized by high-grade fever with chills (21, 56). Bilateral, tender, cervical lymphadenopathy is a common feature in HIDS (94%) (58). Abdominal pain is also frequent (72%) and can be associated with diarrhea (82%) or vomiting (56%) (58, 59). Other clinical findings can include headache, splenomegaly and hepatomegaly (56). Polyarthralgia is present in 80% of the patients and 68% of them have non-erosive arthritis of large joints (60). Over 80% of the patients present with a variety of skin lesions, such as papular, urticarial, nodular or purpuric rash (58, 60). Rarely observed features are serositis, myalgia, and oral and genital ulcers (58).
During flares, laboratory findings include increased acute phase reactants (ESR and CRP), leukocytosis and neutrophilia. Elevated urinary mevalonic acid levels during the crisis have been used in the past to corroborate a HIDS diagnosis (21). IgD serum levels are persistently elevated (≥ 100 U/mL) in more than 90% of HIDS patients, and 80% of them present with concomitant increased IgA (≥ 260 mg/dL) (61). However, IgD concentrations may be normal, especially in children under 3 years of age (62). Furthermore, increased IgD levels are not a HIDS specific finding and can be found in other autoinflammatory conditions including FMF and TRAPS, although in these syndromes, IgD concentrations usually are below 100 U/mL (61, 62). Serum IgD levels are not specific for a diagnosis of HIDS (62). A definitive diagnosis is only established by mutations in MVK gene (16).
Nevertheless, in order to avoid unnecessary genetic tests clinical criteria have been proposed for the HIDS investigation in patients with recurrent fevers (63), Genetic screening for HIDS is suggested for children with recurrent fever who are below the age of 5 years, or in children presenting with arthralgia and fever lasting less than 14 days (63).
In most instances, HIDS has a benign evolution and the frequency of flares progressively diminishes during adulthood (21, 57). Amyloidosis is rarely observed and there have only been five patients reported (64-67). Interestingly, seven cases of secondary macrophage activation syndrome have been reported (58).
In a multicenter study including 18 countries, the frequency of HIDS crises decreased with age, and amyloidosis was found in 3 out of the 103 (2.9%) patients evaluated (57). The mortality in this study was 2.9% with pneumococcal sepsis being the cause of death in one of the 3 patients (57) The other two deaths did not seem to be associated with HIDS complications (suicide and cerebral hemorrhage) (57).
In a French survey of disease outcomes in 50 HIDS patients, 3 patients deceased and the causes of death were macrophage activation syndrome secondary to staphylococcal sepsis in one patient and multiple organ failure secondary to undefined cause in two patients (58). From the 31 surviving symptomatic patients that were followed up for more than 5 years, 14 patients became asymptomatic or had the disease activity decreased over time and 17 remained with active disease (58). The mean age at disease onset was similar between these two groups of patients (8.2 vs. 12.2 months, p>0.05) (58). Strikingly, recurrent infections were observed in 27% of the patients, including severe pneumococcal infections, suggesting that HIDS might also present with an immunodeficiency leading to an increase in occurrence of infections (58).
Treatment
Most of the usual therapies, such as NSAIDs, corticosteroids, colchicine and thalidomide, are not efficacious in controlling HIDS symptoms (58). Because mevalonate kinase plays a role in cholesterol and other isoprenoids synthesis pathways, simvastatin has been used for HIDS treatment (68). However, in the 50-patient French survey, 8 out of 8 patients treated with simvastatin did not respond (58), and in the International HIDS Study Group multicenter study comprising 103 patients, 12 of 18 simvastatin treated patients did not respond to this therapy (57). Etanercept (57, 69-72) and anakinra (57, 73, 74) have also been used in individual cases with satisfactory responses. Recently, the efficacy of anti-IL-1 drugs (anakinra and canakinumab) was assessed in a study that included 11 HIDS patients. Complete and partial remission was observed in 4 and 7 patients, respectively (74).
2.3 TNF receptor associated periodic syndrome (TRAPS)
Epidemiology and Genetics
TRAPS (OMIM#142680) is the second most common autoinflammatory disease (75). It is caused by autosomal dominant mutations in the TNFRSF1A gene, which encodes the p55 TNF receptor (TNFR1) (3). Over 100 mutations have been found to cause TRAPS (16). Variants affecting cysteine residues are associated with a more severe disease phenotype and a higher risk of the development of amyloidosis (3, 75, 76). The prevalence of TRAPS has not been well established, however, the incidence of this disease among children in Germany was estimated as 5.6 per 106 person-years (77).
Clinical Presentation and Diagnosis
The clinical manifestations of TRAPS start more commonly during childhood and adolescence (mean age 10 years-old), but the age at disease onset can vary from 1 to 63 years old (21). The mean length of the fever episodes in a TRAPS attack is around 14 days with a maximum duration of many weeks (78). Abdominal pain is frequently present (77%) with the fever episodes (79). Similarly to FMF patients, the sudden onset and severe abdominal pain is often mistaken for an acute abdomen and about one third of patients with abdominal pain undergo abdominal surgery (75, 76, 78).
Myalgia is a frequent musculoskeletal symptom in TRAPS (64%) and, in an instance when biopsied, was associated with monocytic fasciitis that can also be seen on magnetic nuclear resonance imaging (80). The pain is typically migratory and is associated with an overlying tender erythematous skin rash (78). The rash can be macular, edematous or urticarial (81) but can also be reticular or serpiginous (78). Half of the patients have ocular manifestations presenting with recurrent conjunctivitis or anterior uveitis (76, 81). Periorbital edema is a feature that may suggest the diagnosis of TRAPS when consistent with the other clinical findings (81).
Other common symptoms in TRAPS are arthralgia or arthritis (51%) and pleuritis (32%) (78). Neurological manifestations including headache (68%), aseptic meningitis, optic neuritis and behavioral alterations are also seen in TRAPS (82, 83). Other rarely reported features are scrotal pain, pericarditis, pharyngitis and cervical lymphadenopathy (78). In three published cases, genetically confirmed TRAPS patients denied a fever history and the presenting feature was renal amyloidosis in one patient, recurrent migratory myalgia, arthritis and exanthema in one patient, and recurrent localized myalgia and erythema in another patient (84, 85).
During the febrile episodes, laboratory exams demonstrate increased acute phase reactants (ESR, CRP and SAA), leukocytosis and thrombocytosis (3). According to the severity of the disease, inflammatory markers may remain increased in the symptoms-free intervals and chronic normocytic normochromic anemia is also common in the severe cases, who do not normalize the inflammatory markers between attacks (3, 81). The presence of a mutation in TNFRSF1A is mandatory to make the diagnosis of TRAPS (3).
Amyloidosis is a feared complication of TRAPS and may occur in up to 24% of the patients with mutations in cysteine residues and in 2% of patients with non-cysteine mutations, who are not appropriately treated (86). In a US cohort, 14% of TRAPS presented with systemic amyloidosis and 93% of them had cysteine residues mutations (86).
Treatment
The genetic defect leading to the expression of mutant TNFR1 receptor (87) suggested that patients with TRAPS may benefit from TNF inhibition. In a prospective study that enrolled 15 TRAPS patients, treatment with the recombinant soluble TNF receptor etanercept, that targets TNF, reduced but not completely normalized the frequency and severity of the inflammatory episodes and the laboratory markers (88). In other studies, etanercept efficacy often diminishes after prolonged use (89) and paradoxical development of an inflammatory flare after administration of the chimeric anti-TNF antibody infliximab has been seen in some TRAPS patients (90). In some centers, anti- IL-1 therapy with either the recombinant IL1Ra anakinra or the longer acting agent canakinumab have been used with satisfactory responses (91-93), and have become the treatment of choice. However, failure to treatment with anakinra has also been reported in individual cases (94). In refractory cases, combinations of IL-1 and TNF inhibitions are used with extreme caution (Dr. Daniel Kastner personal communication).
3. Syndromes presenting with neutrophilic urticaria (The cryopyrinopathies)
The cryopyrinopathies or cryopyrin associated periodic syndromes (CAPS) comprise a clinical continuum of three previously defined diseases: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and neonatal-onset multisystem inflammatory disease (NOMID) also called chronic infantile, neurological, cutaneous and articular (CINCA) syndrome (95-97). Although clinical similarities between these disorders exist, the discovery that these diseases are all caused by mutations in NLRP3/CIAS1 forged the concept of a common underlying pathogenic mechanism. While the inheritance pattern in FCAS and MWS is usually familial (98), the disease is sporadic in patients with the clinically severe phenotype of NOMID/CINCA (95). The sporadic inheritance pattern in NOMID/CINCA is likely due to the severe phenotype of untreated patients, which results in the inability to reproduce (95). The NLRP3 gene encodes the protein cryopyrin, a component of an IL-1β activating complex, the NLRP3 inflammasome, which led to the hypothesis that IL-1β may play a major role in the inflammatory disease manifestations of CAPS.
Epidemiology and Genetics
CAPS is caused by autosomal dominant gain of function mutations in NLRP3/CIAS1 and over 130 mutations have been reported, of which 90% are located in exon 3 (16, 98). The diagnosis of NOMID/CINCA is made clinically, based on the presence of characteristic features, and about 40% of patients with “clinical NOMID” are mutation negative by Sanger sequencing. Recently, 70% of these Sanger sequencing “mutation negative” NOMID patients were found to have somatic mosaicism in NLRP3. In the majority of cases, the disease severity seems to be milder than in patients with germline mutations (99-102).
Clinical Presentation and Diagnosis
The spectrum of characteristic overlapping features that are associated with the entire spectrum of CAPS include fever, urticarial rash, conjunctivitis, articular involvement and a marked increase of acute phase reactants (21, 103). The urticaria-like rash can start as a maculopapular lesion that rapidly evolves into wheels, it is migratory and, in contrast to the pruritic rash seen in allergic urticaria, patients describe the rash typically as burning or stinging (Figure 1, panel A1) (104). Histologically, skin biopsy shows a dermal polymorphonuclear perivascular infiltrate, which is distinct from the lymphocytic and eosinophilic infiltrate found in classical allergic urticaria (104). The fever pattern in these disorders varies with the disease severity. While in FCAS the inflammatory attacks are induced by cold exposure and subside within several hours often to a state without residual inflammation, NOMID patients have continuous often low-grade fever with disease exacerbations (105).
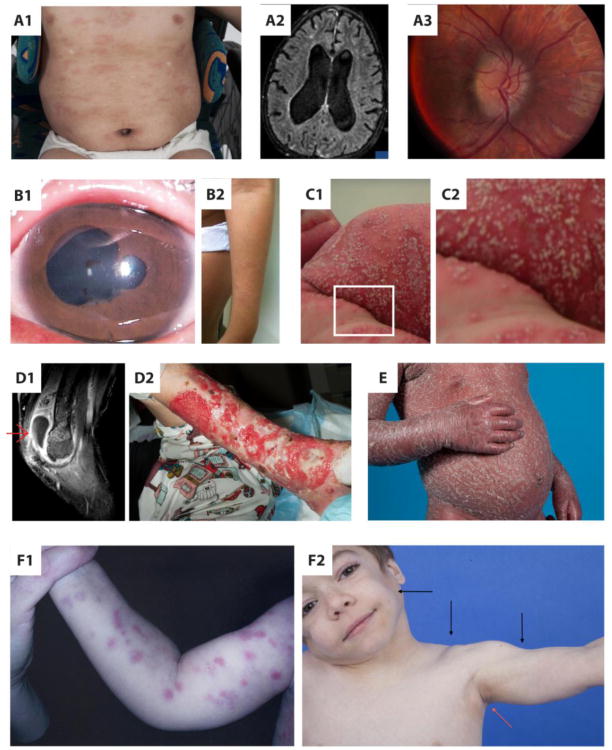
Figure 1.
A1 – Urticarial rash in neonatal-onset multisystem inflammatory disease, (NOMID), A2 – Severe hydrocephalus in NOMID syndrome, A3 – Papilledema in NOMID syndrome, B1 – Anterior synechiae in Blau syndrome chronic uveitis, B2 – Left arm ichthyosis-like exanthema in Blau syndrome, C1 – Generalized pustulosis in deficiency of interleukin-1 receptor antagonist (DIRA), C2 – Amplified image of DIRA skin rash, D1 – Sagittal image in fat suppressed T1 sequence showing synovial thickening and enhancement with moderate fluid in the right elbow joint in pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome, D2 - Extense left lower leg pyoderma gangrenosum lesion in (PAPA) syndrome, E – Generalized psoriasis in CARD14 mediated psoriasis (CAMPS), F1 – Erythematous, annular and nodular rash in chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome, F2 - Lipodystrophy (black arrows) and acanthosis nigricans (red arrow) in CANDLE syndrome
3.1 Familial cold autoinflammatory syndrome (FCAS)
FCAS (OMIM#120100) is at the milder end of the spectrum of CAPS (96). FCAS clinically presents with recurrent flares of low-grade fever triggered by cold exposure (93%), polyarthralgia (96%) and urticarial rash (100%) that start 1 to 2 hours after exposure to cold and last 12 to 48 hours (21, 106). Other symptoms in FCAS patients are as follows: conjunctivitis (84%), profuse sweating (78%), dizziness (67%), headache (58%), nausea (51%) and excessive thirst (53%) (106). Rarely, FCAS patients present with recurrent fever as the only manifestation, mild arthralgia, inflammatory myocardiopathy, nephropathy and thyroiditis (107). A definitive diagnosis is typically established through the finding of genetic mutations in NLRP3(16). Secondary amyloidosis is not frequent and can occur in 2% of the cases (26).
3.2 Muckle-Wells syndrome (MWS)
In 1962, Muckle & Wells described a familial syndrome with urticaria, hearing loss and amyloidosis in 9 subjects (108). Patients with MWS (OMIM#191100) usually present in childhood with urticarial rash, low-grade fever and arthralgia (109). Recurrent arthritis and conjunctivitis episodes frequently occur (109, 110). During severe attacks, patients often complain of headache and aseptic meningitis and some MWS patients present with papilledema. Sensorineural hearing loss is the most typical clinical feature in MWS and is due to chronic inflammation of the inner ear, likely leading to damage of the Corti organ (110, 111). Abnormal laboratory findings include marked leukocytosis with neutrophilia and thrombocytosis, anemia and increased acute phase reactants, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) (110). In contrast to patients with FCAS, secondary amyloidosis is frequently observed in patients with MWS living in Europe and affects 25 to 33% of the untreated patients (112). Although the majority of patients with MWS carry germline mutations in NLRP3, detected by Sanger sequencing, the diagnosis of MWS can often be made on clinical grounds. The diagnosis of MWS should particularly be considered in patients presenting with classical neutrophilic urticarial and familial disease. As discussed, CAPS can be caused by somatic mosaicism that can currently only be assessed in research settings. Given the significant benefit from IL-1 blocking therapies, a treatment challenge might help to confirm the clinical suspicion of CAPS.
3.3 Neonatal-onset multisystem inflammatory disease (NOMID)/ Chronic infantile neurologic cutaneous and articular syndrome (CINCA)
NOMID (OMIM#607115) is at the most severe end of the CAPS spectrum and was first described in 1981 (113). NOMID is diagnosed clinically and is characterized by continuous often low-grade fever, cutaneous rash, aseptic meningitis and arthropathy, starting in the first weeks of life (114). Neutrophilic urticarial skin lesions are found in almost all patients (104). Neurological involvement is a diagnostic feature of NOMID and is characterized by chronic aseptic neutrophilic meningitis that causes chronic irritability, headache, seizures, transitory hemiplegia and rarely lower limb spasticity (114, 115).
If untreated, NOMID patients develop permanent organ damage as a consequence of persistent inflammation in the affected organs (116). Ongoing cochlear inflammation leads to sensorineural hearing loss. Chronic aseptic meningitis leads to the development of increased intracranial pressures and hydrocephalus, brain atrophy, and chronic papilledema that leads to optic nerve atrophy and progressive vision loss (Figure 1, panels A2 and A3). Patients with hydrocephalus often present with a “typical facies” with frontal bossing, large cephalic perimeter and the appearance of a “saddleback nose” (114, 115). The most common inflammatory eye manifestation is conjunctivitis. Anterior and rarely posterior uveitis, can contribute to the progressive vision loss that is primarily caused by optic nerve atrophy (114). Cognitive delay is multifactorial and due to perinatal insult, and the degree of CNS inflammation and brain atrophy.
Thirty to forty percent of NOMID patients present with a deforming arthropathy that results in abnormal epiphyseal calcification, cartilage overgrowth and joint deformities (117). Premature patellar ossification and patellar overgrowth is a typical finding in NOMID (115).
Laboratory findings are not specific but include a marked leukocytosis with mainly mature neutrophils, normocytic normochromic anemia, thrombocytosis and increased ESR and CRP (21, 114).
The diagnosis of NOMID is based on the clinical characteristics including neutrophilic urticaria, neutrophilic aseptic meningitis and/or characteristic bone deformities and variable symptoms described above. The presence of CIAS1/NLRP3 mutations is confirmatory but not necessary to make the diagnosis and initiate appropriate therapy as “mutation positive and negative patients” equally respond to IL-1 blocking therapy (114). Somatic mosaicism is the cause NOMID in about 70% of Sanger sequencing “mutation negative” NOMID patients. However, subcloning or deep sequencing are required to establish a genetic diagnosis. Thus making a genetic diagnosis is currently not clinically established and would unnecessarily hold up a therapeutic intervention (99-102).
Complications observed in NOMID are described above and are related to physical disability due to joint deformities, cognitive impairment, hearing and vision loss and secondary renal amyloidosis, that is mainly observed in Europe. To prevent development of many of these complications, therapeutic intervention should not be delayed. (111, 114, 116).
Treatment of CAPS
IL-1 inhibition is the standard treatment for patients with CAPS (111, 114, 118). While treatment of some patients with FCAS is geared to improve the cold induced symptoms which can be achieved by preventing cold exposure, optimal treatment with the IL-1 blocking agents (anakinra, rilonacept or canakinumab) leads to complete resolution of symptoms in most cases (92, 119). In patients with MWS and NOMID who develop organ damage from untreated disease, optimal IL-1 inhibiting therapy needs to be initiated early to prevent the development and progression of organ damage (116). The rapid responses of CAPS to IL-1 blocking agents have led to the approval of three agents for CAPS: the long acting IL-1 inhibitors rilonacept (120, 121) and canakinumab (122), and the short acting IL-1 receptor antagonist anakinra (116). Recombinant IL1Ra (anakinra) has been used for NOMID treatment for over 10 years (114). All patients show a rapid response to anakinra with dramatic improvement of inflammatory symptoms, fever, skin rash, and acute phase reactants (114). However dose escalation to control organ inflammation, particulary CNS and inner ear inflammation, is required to achieve appropriate disease control. Doses of IL-1 blocking agents needed to treat FCAS patients are lower than those needed to treat NOMID patients.
Responses to IL-1 blocking therapies are sustained, follow up on anakinra up to 5 years (111, 116, 123), and up to 2 years on rilonacept (121, 124) and canakinumab (125) have recently been published.
4. Syndromes presenting with granulomatous skin lesions and minimal or low-grade fever attacks
4.1 Blau syndrome / early onset sarcoidosis (pediatric granulomatous arthritis)
Epidemiology and Genetics
Pediatric granulomatous arthritis (PGA) (OMIM#186580) is caused by autosomal dominant gain of function mutations in the NACHT domain (exon 4) of NOD2/CARD15(16, 126). PGA can be inherited in an autosomal dominant pattern and familial cases have traditionally been called Blau syndrome. However mutations can occur sporadically and in these instances the disease has been referred to as early-onset sarcoidosis (127, 128). The identification of the same mutations led to the recognition that these two disorders are the same disease. So far 12 disease causing mutations in NOD2 gene, have been reported to cause PGA (16). PGA symptoms occur due to a granulomatous inflammation of eyes, joints and skin leading to an early-onset (before 4 years of age) classical triad of disease manifestations comprising chronic uveitis, arthritis and dermatitis (129).
Clinical Presentation and Diagnosis
Chronic arthritis is seen in 100% of patients, who mainly present with polyarthritis (96%) (129). A symmetric hypertrophic tenosynovitis is observed in approximately 40% of the patients (129). Ocular involvement is very frequent (84%) and is usually chronic and persistent (129, 130). Uveitis presents more commonly as panuveitis (50%), but 25% of the subjects may present with anterior uveitis (Figure 1, panel B1) (129, 130). Most of the subjects have bilateral eye involvement, and cataract and glaucoma occur in 50% and 30% of them, respectively (128, 129). Up to 40% of the untreated patients with PGA uveitis develop irreversible blindness (130). Typical PGA exanthema is described as ichthyosis-like and occurs in 88% of the patients (Figure 1, panel B2) (127, 128). Less commonly observed findings include fever, camptodactyly and central neuropathy (127).
Laboratory exams demonstrate persistent leukocytosis, thrombocytosis and increased ESR and CRP (129). Synovial, skin and liver biopsies may show non-caseating granulomata, even though a definitive diagnosis is only achieved with DNA sequencing showing NOD2/CARD15 mutations (16, 130).
Treatment
Optimal therapy for PGA has not been established. NSAIDs can be used for mild clinical manifestations, whereas severe symptoms are treated with systemic corticosteroids (131). Immunosupressant (methotrexate and cyclosporine) and biologics targeting TNF and IL-1 (etanercept, infliximab and anakinra) have been reported to result in clinical benefit, especially in patients with refractory uveitis (128, 130).
5. Syndromes presenting with pustular skin rashes and episodic or continuous fever
This group of disorders presents the most pathogenically heterogeneous group. Although all patients present with pustular skin lesions, the underlying pathogenic mechanism and the other clinical organ manifestations differ between the entities. Treatment is also different among the diseases. While the DIRA and Majeed syndromes present with sterile osteomyelitis that is IL-1 mediated, PAPA syndrome presents with pyogenic arthritis and pustular lesions that rapidly develop into pyoderma gangrenosum lesions. Bloody diarrhea and abdominal symptoms associated with inflammatory bowel disease are the characteristics of disorders caused by absent IL-10 signaling; and, predominantly skin lesions without other organ manifestations are seen in disorders presenting with mutations in the IL-36 pathway and with mutations in CARD14, a regulator of the NFκB pathway.
5.1 With inflammatory bone disease
5.1.1 Deficiency of interleukin 1 receptor antagonist (DIRA)
Epidemiology and Genetics
DIRA (OMIM#612852) was first described in 2009 and it is caused by autosomal recessive loss of function mutations in IL1RN gene, which encodes the IL-1 receptor antagonist (IL-1Ra) (132, 133). Nine disease-causing mutations have been reported in DIRA, three missense mutations, three non-sense mutations, two small deletions (2 and 15bp) and one large genomic deletion (175Kb) (16, 134).
Clinical Presentation and Diagnosis
DIRA patients present with early-onset pustular dermatitis and multifocal osteomyelitis and periosteitis, associated with marked elevations of acute phase reactants (132, 133). Pustular skin lesions may be sparse, occur in crops or develop into a severe generalized pustular dermatitis that at times can be ichthyosis-like (Figure 1, panel C) (132, 135). Histological findings on the skin biopsy include corneal pustules, acanthosis and hyperkeratosis (132, 135). Nail changes presenting with onychomadesis, are seen in very young untreated children (132). All DIRA patients identified so far present with osteomyelitis, which is clinically characterized by pain on manipulation and periarticular swelling. TypicaI radiographic manifestations show widened ribs and clavicles, and multifocal osteolytic lesions are mainly seen in long bones (132, 133, 135-137). Heterotopic ossification or periosteal cloaking around the proximal femoral metaphysis are seen in very young children.
Fever is often low grade or can be absent in DIRA and can be related to secondary skin infections in the untreated patients (132, 136). Less frequently observed manifestations in DIRA include interstitial lung disease, atlantoaxial subluxation due to odontoid destruction (135) and, rarely, CNS vasculitis.
Treatment
Recombinant IL-1Ra (anakinra) has been used in DIRA with impressive results (132, 133, 135-138). Treatment with anakinra leads to rapid resolution of symptoms and normalization of the acute phase reactants and prevents the recurrence of both skin and bone manifestations (132, 133, 135-138).
5.1.2 Majeed syndrome
Epidemiology and Genetics
Majeed syndrome (OMIM#146462) is a pyogenic autoinflammatory disease caused by autosomal recessive loss of function mutations in LPIN2 gene (16, 139, 140). Twelve mutations have been so far described in LPIN2(16).
Clinical Presentation and Diagnosis
Majeed syndrome is clinically characterized by neonatal-onset recurrent multifocal osteomyelitis, neutrophilic dermatosis and congenital dyserythropoietic anemia (141). Pustular dermatitis is the most common skin manifestation, although psoriasis-like lesions have already been reported (141). The most frequent sites of osteomyelitis are clavicles, sternum and long bones, whereas mandible and vertebral bodies are rarely affected (139, 142). Bone biopsy studies usually show a neutrophilic infiltrate (140, 142).
Treatment
Majeed syndrome is unresponsive to treatment with antibiotics and patients may respond to NSAIDs, corticosteroids, interferon gamma, bisphosphonates, and anti-TNF drugs (139-141). Recently, the efficacy of IL-1 inhibition with either anakinra or canakinumab was shown in two brothers with Majeed syndrome who were refractory to anti-TNF therapy (143), suggesting that the pathogenesis of Majeed syndrome may be IL-1β mediated.
5.2 With pyogenic arthritis
5.2.1 Pyogenic sterile arthritis, pyoderma gangrenosum and acne (PAPA) syndrome
Epidemiology and Genetics
PAPA syndrome (OMIM#604416) is a pyogenic autoinflammatory disease caused by autosomal dominant mutations in the PSTPIP1 gene (144). Seven disease-causing variants have been described in PSTPIP1, so far (16).
Clinical Presentation and Diagnosis
Fever is rarely observed in PAPA patients, who present with early-onset flares of painful sterile and deforming arthritis (Figure1, panel D1), cutaneous ulcers (pyoderma gangrenosum) (Figure1, panel D2) and pathergy, cystic acne or skin abscesses at the injections sites (54, 144, 145). Laboratory findings are non-specific and include leukocytosis and increased ESR and CRP during flares (144, 145). PAPA syndrome symptoms usually persist into adulthood and significant joint destruction with an impaired quality of life related to physical disability is observed (146). In a recent report of five PAPA syndrome patients, three patients had A230T, one had E250Q and one had a novel E250K mutation. All patients presented with sterile arthritis and pyoderma gangrenosum, three patients had sterile skin abscesses and two had cystic acne. Other clinical findings were one osteomyelitis episode in one patient, recurrent otitis in two patients and lymphadenopathy, splenomegaly, thrombocytopenia, hemolytic anemia, pharyngeal papillomatosis and T cell large granular lymphocytosis in one patient (146).
Treatment
Treatment of PAPA syndrome is challenging and corticosteroids, thalidomide, cyclosporine, dapsone, tacrolimus and IVIG have been used with variable individual responses (146, 147). Regarding biologics, two case reports showed that etanercept was efficacious in PAPA syndrome treatment (147, 148). Responses to infliximab were observed in one patient, to adalimumab in two patients and to anakinra in one case (146). On the other hand, two patients did not respond to anakinra, one did not respond to infliximab and another did not respond to etanercept therapy (146). In general, monoclonal anti-TNF antibodies (infliximab and adalimumab) were considered more effective in treating skin manifestations of PAPA syndrome (146).
5.3 With inflammatory bowel disease
5.3.1 Early onset inflammatory bowel disease (EO-IBD)
Epidemiology and Genetics
Infantile IBD is caused by autosomal recessive mutations in IL-10R (OMIM#613148) (OMIM#612567) and IL-10 (OMIM#612381) encoding genes (149, 150). Glocker et al. first reported that autosomal recessive mutations in IL10RA and/or IL10RB in 4 out of 9 patients with severe IBD presenting in the first year of life (149). In 2010, the same group found an autosomal recessive mutation in IL10 gene in two unrelated patients with refractory early-onset IBD (150). In total, six disease causing mutations in each of the IL-10 receptor genes (IL10RA and IL10RB) and two mutations in the IL-10 gene have been described. (16).
Clinical Presentation and Diagnosis
Patients with EO-IBD present with severe enterocolitis, characterized by bloody diarrhea, colonic abscesses, perianal fistula and oral ulcers (149, 151). Symptoms usually start in the first year of life, before 3 months of age (151). Additionally, they present with impaired weight and height development and recurrent fever (151). Musculoskeletal manifestations include acute recurrent arthritis of large joints, especially affecting knees (151). Remarkably, recurrent folliculitis has been observed in 75% of the patients (151). Recurrent infections, such as otitis media, bronchitis, pneumonia, septic arthritis and renal abscesses may indicate a defect in the immune responses in patients with EO-IBD (149, 151).
Treatment
Because EO-IBD is a severe disease and frequently refractory to standard immunosuppressants, hematopoietic stem cell transplantation (HSCT) has been proposed as a curative treatment (152). HSCT was performed in 9 out of the 29 patients with IL-10 deficiency described and complete clinical remission was achieved in all but one of the patients (151-153).
5.4 Without other organ manifestation
5.4.1 Deficiency of interleukin 36 receptor antagonist (DITRA)
Epidemiology and Genetics
Recently 16 family members of nine Tunisian families with generalized pustular psoriasis were found to be homozygous for a mutation in IL36RN gene, L27P, indicating a founder mutation in Tunisia leading to this disease (16, 154). Most of the patients described (75%) developed the disease during childhood (7 days to 11 years of age) (154). Additionally, three unrelated English patients with a similar phenotype presented with mutations in IL36RN, two patients were homozygous for the S113L mutation and one patient was a compound heterozygous for S113L and R48W mutations (155).
Clinical Presentation and Diagnosis
Clinically, these patients presented with recurrent and sudden onset flares of generalized erythematous and pustular skin rash, associated with fever (40-42°C), asthenia and increased CRP and white blood cell counts (154, 155). Secondary skin infections and sepsis may also occur (154). In all patients, the disease flares are thought to be triggered by viral or bacterial infections (14/16 patients), but withdrawal of retinoid therapy (n=7), menstruation in (n=6), and pregnancy in (n=4) were also triggers (154, 155). Skin biopsy demonstrates spongiform pustules, acanthosis and parakeratosis in the stratum corneum, and infiltration of the skin by CD8 and CD3 T cells and macrophages (154). Because the IL36RN mutation described results in a decrease or absence of the IL36 receptor antagonist, the resulting disease was proposed to be called deficiency of the IL-36 receptor antagonist (DITRA - OMIM#614204) (16, 154).
Treatment
Definitive treatment for this disease has not been established yet. Acitretin was used as treatment in 12 of the 19 patients described so far (154, 155). Other therapeutic regimens tried with variable individual responses were oral steroids in 4 patients, methotrexate in 3 patients, cyclosporin in 3 patients, adalimumab in 2 patients, infliximab in 2 and etanercept in one subject (154, 155). Topical steroids were used in 3 patients (154).
5.4.2 CARD14 mediated psoriasis (CAMPS)
Epidemiology and Genetics
Autosomal dominant gain of function mutations in CARD14 gene cause plaque psoriasis in two families and severe generalized pustular psoriasis in an unrelated child (156). Mutations in CARD14 were also shown to cause familial pityriasis rubra pilaris in 15 family members of 3 families (157). In addition to the 3 mutations reported to cause familial disease, additional variants were found in a cohort of patients with psoriasis. The contribution of those variants to psoriasis is not fully understood, but some of these mutations are NFkB activating and likely constitute rare variants with a large effect size, most likely leading to the sporadic development of psoriasis. (156, 158).
Clinical Presentation and Diagnosis
Patients with CARD14 mutations present with typical plaque psoriasis with variable severities and one 3 year-old female patient presented with generalized pustular psoriasis (Figure 1, panel E) (156-158). Fever and other systemic manifestations are generally not present but can occur with superinfections of the skin in patients with CAMPS (OMIM#602723) (156, 158).
Treatment
The therapeutic approach in CAMPS includes drugs used for the treatment of moderate-to-severe psoriasis, such as, methotrexate, cyclosporine and anti-TNF agents, and more recently including biologics targeting IL17 and IL23 (159-161). Familial pityriasis rubra pilaris is considered refractory to the standard therapies and a partial response has been described with retinoids, cyclosporine and etanercept (157, 162).
6. Syndromes with atypical neutrophilic dermatosis with histiocytic-like infiltrate Proteasome associated autoinflammatory syndromes (PRAAS)
Epidemiology and Genetics
In 2010, four patients with early-onset recurrent fever, violaceous cutaneous rash, periorbital edema and erythema, lipodystrophy and increased acute phase reactants were reported and the syndrome was proposed to be called chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) (163). In 2011, several studies showed that a number of disorders referred to as joint contractures, muscle atrophy, microcytic anemia and panniculitis-induced lipodystrophy (JMP) syndrome (164), Nakajo-Nishimura syndrome (165), Japanese autoinflammatory syndrome with lipodystrophy (JASL) (166), and CANDLE (167) (OMIM#256040) are caused by mutations in proteasome subunit β type 8 (PSMB8) gene, indicating that these disorders are disease phenotypes along the same disease spectrum (163, 167, 168). Currently, three mutations have been described in PSMB8 (T75M, C135X and G201V).
Clinical Presentation and Diagnosis
All patients reported presented with recurrent fever, skin rash characterized by diffuse annular plaques (Figure 1, panel F1), violaceous eyelids, lipodystrophy (Figure 1, panel F2), arthritis or arthralgia, and increased acute phase reactants (ESR and CRP) (167). Other common clinical findings were finger swelling, hepatomegaly, lymphadenopathy, prominent abdomen and low weight and height, in 7 out of 9 patients (167). Muscle atrophy was observed in 4/9 and splenomegaly in 3/9 patients (167). Other laboratory findings included hypochromic anemia and increased liver function tests (LFT) in 8 out of 9 patients (167). Less frequent clinical and laboratory findings observed were perioral swelling, parotitis, conjunctivitis or episcleritis, acanthosis nigricans (Figure 1, panel F2), hypertrichosis, ear and nose chondritis, lymphocytic aseptic meningitis, basal ganglia calcifications, recurrent otitis or sinusitis, epididymitis, thrombocytosis, hypertriglyceridemia, neutropenia and thrombocytopenia (167). Long term sequela from untreated patients who survive into adulthood include the development of muscle atrophy, cardiac arrhythmias and dilated cardiomyopathy (165, 166).
Treatment
Considering the 9 patients previously mentioned, most clinical symptoms partially responded to high doses of steroids (1–2 mg/kg/day)(167). NSAIDs, colchicine, dapsone, methotrexate, tacrolimus and azathioprine were not effective in most of the patients (167). A variable response was observed to anti-TNF, anti-IL-1 and anti-IL-6 agents, but complete disease remission was not achieved with any of the therapies described (167). Additionally, lipodystrophy progressed in all patients despite immunosuppressive and cytokine targeted therapy (167). Increased STAT-1 phosphorylation was seen as well as a strong interferon response signature, which led to a compassionate use protocol with the JAK1/JAK2 inhibitor baricitinib (clinicaltrials.gov/NCT01683409). Preliminary observations showed a down-regulation of IFN-induced genes.
7. Syndromes presenting with autoinflammation and with immunodeficiency
The description of additional monogenic diseases has led to the identification of conditions that present with autoinflammatory phenotypes but also with features of immunodeficiencies. The “mixed phenotype” is determined by the impact the mutation can have in different immune cells. Immunodeficiencies have also been suggested to be present in patients with HIDS and EO-IBD. Two recently described conditions caused by autosomal dominant mutations in PLCG2, PLCγ2-associated antibody deficiency and immune dysregulation (PLAID) and autoinflammation and PLCγ2-associated antibody deficiency and immune dysregulation (APLAID) were described (169, 170). Another condition is caused by autosomal recessive mutations in HOIL, a component of the linear ubiquitination chain assembly complex (LUBAC)(171). These mutations result in impairment of LUBAC stability leading to reduced NF-κB activation in response to interleukin 1β (IL-1β) in fibroblasts but hyper-responsiveness to IL-1 in mononuclear leukocytes, particularly monocytes (171). While PLAID leads to cold induced urticaria, autoimmune manifestations and susceptibility to infections, APLAID leads to early-onset recurrent erythematous plaques and vesicopustular skin lesions associated with arthralgia, corneal erosions and interstitial pneumonia (170). The two patients identified with APLAID so far develop recurrent sinopulmonary infections presumed due to a lack of class-switched memory B cells on lymphocyte immunophenotyping (170). Both patients described, were partially responsive to anakinra and to high-dose corticosteroids (170).
Of the 3 patients with HOIL deficiency who have been described, two of them were siblings born to non-consanguineous parents (171). All 3 patients presented with early-onset recurrent fever, hepatosplenomegaly and increased acute phase reactants (171). Two patients presented with chronic bloody diarrhea and two had eczema and diffuse erythroderma. These patients showed severe recurrent pyogenic, fungal and viral infections and all three patients had a fatal outcome (171). Additionally, all three patients had cardiomyopathy, and two patients had amylopectinosis in cardiomyocytes (171). The autoinflammatory manifestations in two of the three patients were partially responsive to corticosteroids but refractory to colchicine, anti-TNF and anti-IL-1 agents. The third patient had an allogeneic HSCT performed at the age of 13 months but deceased 3 years after the transplant (171).
Table 2 shows the clinical features and Table 3 emphasizes distinctive characteristics and clues for diagnosis of the monogenic autoinflammatory diseases described herein.
Table 2. Disease Manifestations of Autoinflammatory Diseases.
| Disease | Gene | Pathogenesis | Clinical manifestations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cutaneous | Ocular | Musculoskelet al | Abdominal | Neurologic al | Specific characteristics | Treatment | ||||
| GROUP 1 | FMF | MEFV | Predominant ly IL-1 mediated + unknown pathway | Erysipellas-like erythema | Uncommon | Large joints episodic arthritis | Aseptic peritonitis | Uncommon | Pleuritis, pericarditis, scrotal pain | Colchicine |
| HIDS | MVK | Partially IL-1 mediated + unknown pathway | Maculopapular or purpuric exanthema | Uncommon | Arthralgia, non-erosive acute polyarthritis | Abdominal pain, vomiting, diarrhea, hepatosplenomegaly | Uncommon | Painful cervical adenopathy, increased IgD serum levels and urinary mevalonate, flares triggered by immunizations | NSAIDs, CS, simvastatin, anti-IL1 agents (anakinra, canakinumab) | |
| TRAPS | TNFRSF1A | Partially IL-1 mediated + TNF + ROS | Erysipellas-like erythema | Periorbital edema, conjunctivitis | Migratory myalgia, arthralgia, non-erosive arthritis | Abdominal pain, peritonitis, diarrhea, constipation | Headache | Rash on myalgia area, periorbital edema, long lasting periodic fever (>7days) | Etanercept, anti-IL1 agents (anakinra) | |
| GROUP 2 | CAPS-FCAS | NLRP3 | IL-1β mediated | Urticarial exanthema | Conjunctivitis | Myalgia, arthralgia | Nausea | Headache | Cold induced urticaria | Anti-IL1 agents (anakinra, canakinumab, rilonacept) |
| CAPS-MWS | NLRP3 | IL-1β mediated | Urticarial exanthema | Conjunctivitis, episcleritis, optic disk edema | Myalgia, arthralgia, oligoarticular arthritis | Abdominal pain | Sensorineur al hearing loss | Sensorineural hearing loss | Anti-IL1 agents (anakinra, canakinumab, rilonacept) | |
| CAPS-NOMID | NLRP3 | IL-1β mediated | Urticarial exanthema | Conjunctivitis, uveitis, papilledema, progressive amaurosis | Epiphyseal and patella enlargement, periostitis, chronic arthropathy | Hepatosplenomeg aly during exacerbations | Delayed mental development, chronic aseptic meningitis, headache, sensorineural hearing loss | Continuous symptoms with exacerbation periods, typical facies (frontal bossing and saddleback nose) | Anti-IL1 agents (anakinra, canakinumab) | |
| GROUP 3 | PGA | NOD2/CARD 15 | NF-kB activation + unknown pathway | Ichthyosis-like exanthema | Chronic uveitis, cataract, glaucoma, amaurosis | Polyarthritis, hypertrophic tenosynovitis | Uncommon | Uncommon | Arthritis, uveitis and exanthema triad | CS, methotrexate, azathioprine, cyclosporine, anti-TNF agents |
| GROUP 4 | DIRA | IL1RN | IL- 1 mediated | Pustular dermatitis | Uncommon | Recurrent multifocal aseptic osteomyelitis | Uncommon | Uncommon | Psoriasis-like pustulosis, rapid response to anakinra | Anti-IL1 agents (anakinra) |
| Majeed | LPIN2 | IL-1 mediated (?) | Pustular dermatitis | Uncommon | Recurrent multifocal aseptic osteomyelitis | Uncommon | Uncommon | Dyserythropoietic anemia | CS, anti-TNF agents | |
| PAPA | PSTPIP1 | Arthritis: IL-1 mediated | ||||||||
| Skin features: unknown pathway | Pyoderma gangrenosu m, pathergy, skin abscesses, cystic acne | Uncommon | Deforming aseptic pyogenic arthritis | Uncommon | Uncommon | Aseptic pyogenic arthritis, pyoderma gangrenosum and puogenic skin lesions triad | CS, anti-TNF agents, anti-IL1 agents (anakinra) | |||
| EO-IBD | IL10, IL10RA and IL10RB | IL-10 deficiency + TNF + unknown pathway | Folliculitis | Uncommon | Arthritis | Severe colitis: bloody diarrhea, abscesses, perianal fistula, oral aphtous lesions | Uncommon | Early-onset severe colitis, failure to thrive, recurrent fever and infections | CS, CsA, AZA, mesalamine, mercaptopurine, MTX, TAC, anti-TNF agents, allogenic HSCT | |
| DITRA | IL36RN | IL-36 + IL-17/IL-23 (?) | Generalized pustular psoriasis | Uncommon | Uncommon | Uncommon | Uncommon | Generalized pustular psoriasis with high-grade fever (40-42oC) | CS, cyclosporine, retinoids, anti-TNF agents | |
| CAMPS | CARD14 | IL-17/IL-23 + unknown pathway | Plaque or pustular psoriasis | Uncommon | Arthritis | Uncommon | Uncommon | Plaque or pustular psoriasis | Anti-IL-12/IL-23 agents (ustekinumab) | |
| GROUP 5 | PRAAS | PSMB8 | IFN-mediated | Nodular exanthema, panniculitis, lipodystrophy | Eyelids edema and erythema | Myositis, arthralgia, arthritis | Increased abdominal volume and hepatosplenomegaly | Basal ganglia calcification s | Panniculitis, lipodystrophy | JAK inhibitors (baricitinib) |
| GROUP 6 | PLAID | PLCG2 | Unknown (?) | Cold-induced urticaria | Uncommon | Uncommon | Uncommon | Uncommon | Cold-induced urticarial, recurrent sinopulmonary infection, antibody deficiency, autoimmune manifestation, positive ANA, atopy | IVIG |
| APLAID | PLCG2 | Partially IL-1 mediated + unknown pathway | Erythematous plaques and vesicopustula r lesions, cellulitis | Corneal erosions, ulcerations, intraocular hypertension, cataracts | Arthralgia | Abdominal pain and bloody diarrhea | Uncommon | Nonspecific interstitial pneumonitis, mild immunodeficiency, skin lesions worsened by heat and sun exposure | CS and anti-IL-1 agents (partial response) | |
| HOIL-1 DEFICIENCY | HOIL1 | Partially IL-1 mediated + NF-κB activation + unknown pathway | Eczema, diffuse erythroderma | Uncommon | Uncommon | Bloody diarrhea | Uncommon | Periodic fever, hepatoesplenomega ly, lymphadenitis, severe immunodeficiency with invasive bacterial pyogenic, fungal and viral infections, cardiomyopathy with amylopectinosis | CS, anti-TNF, anti-IL-1, allogenic HSCT |
CAPS: cryopyrin asscoiated periodic syndrome; NOMID: neonatal-onset multisystem inflammatory disease; MWS: Muckle-Wells syndrome; FCAS: familial cold autoinflammatory syndrome; FMF: familial Mediterranean fever; HIDS: hyperimmunoglobulinemia D syndrome with periodic fever; TRAPS: TNF receptor associated periodic syndrome; PGA: pediatric granulomatous arthritis; DIRA: deficiency of interleukin 1 receptor antagonist; PAPA: pyogenic arthritis, pyoderma gangrenosum and acne syndrome; EO-IBD: early-onset inflammatory bowel disease; DITRA: deficiency of interleukin 36 receptor antagonist; CAMPS: CARD14 mediated psoriasis; PRAAS: proteasome associated autoinflammatory syndromes; PLAID: PLCγ2-associated antibody deficiency and immune dysregulation; APLAID: autoinflammation and PLCγ2-associated antibody deficiency and immune dysregulation; ANA: antinuclear antibody; IVIG: intravenous immunoglobulin; NSAIDs: non-steroidal anti-inflammatory drugs; CS: corticosteroids; CsA: cyclosporine; AZA: azathioprine; MTX: methotrexate; TAC: tacrolimus; HSCT: hematopoietic stem cell transplantation
Table 3. Distinctive characteristics and clues to the diagnosis of the autoinflammatory diseases.
| FMF | PAPA | ||
|
|
||
| HIDS | Early-onset IBD | ||
|
|
||
| TRAPS | DITRA | ||
|
|
||
| CAPS/FCAS | CAMPS | ||
|
|
||
| CAPS/MWS | PRAAS | ||
|
|
||
| CAPS/NOMID | PLAID | ||
|
|
||
| PGA | APLAID | ||
|
|
||
| DIRA | HOIL-1 DEFICENCY | ||
|
|
||
| Majeed syndrome | |||
|
|||
CAPS: cryopyrin asscoiated periodic syndrome; NOMID: neonatal-onset multisystem inflammatory disease; MWS: Muckle-Wells syndrome; FCAS: familial cold autoinflammatory syndrome; FMF: familial Mediterranean fever; HIDS: hyperimmunoglobulinemia D syndrome with periodic fever; TRAPS: TNF receptor associated periodic syndrome; PGA: pediatric granulomatous arthritis; DIRA: deficiency of interleukin 1 receptor antagonist; PAPA: pyogenic arthritis, pyoderma gangrenosum and acne syndrome; EO-IBD: early-onset inflammatory bowel disease; DITRA: deficiency of interleukin 36 receptor antagonist; CAMPS: CARD14 mediated psoriasis; PRAAS: proteasome associated autoinflammatory syndromes;PLAID: PLCγ2-associated antibody deficiency and immune dysregulation; APLAID: autoinflammation and PLCγ2-associated antibody deficiency and immune dysregulation;
8. Conclusion
The past 15 years were marked by the discovery of monogenic causes for a number of immune dysregulatory disorders, many presenting with fever, systemic and organ specific inflammation. Not only new insights into the genetics and the pathogenesis of these syndromes were gained, but a number of new clinical phenotypes have been described, thus growing the spectrum and heterogeneity of this group of autoinflammatory diseases/phenotypes. The discovery of novel diseases led to the recognition that some disorders are IL-1β mediated, but the unresponsiveness of others to IL-1 blockade suggested that additional signaling and cytokine pathways are affected. It is necessary that primary care physicians and those who are likely to see young infants and children be familiar with the clinical aspects of these diseases in order to recognize them and start early immunosuppressive therapy when needed, and make a prompt referral. In many of these syndromes, early treatment is mandatory to prevent physical sequela, prevent mortality and guarantee a better quality of life for these patients.
Highlights.
The spectrum of monogenic autoinflammatory diseases has grown over the years.
We grouped the autoinflammatory diseases (AID) according to clinical manifestations.
AID clinical findings should be recognized by primary care physicians.
Early referral and treatment is mandatory to improve morbi-mortality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.A candidate gene for familial Mediterranean fever. Nature genetics. 1997;17(1):25–31. doi: 10.1038/ng0997-25. Epub 1997/09/01. [DOI] [PubMed] [Google Scholar]
- 2.Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell. 1997;90(4):797–807. doi: 10.1016/s0092-8674(00)80539-5. Epub 1997/08/22. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97(1):133–44. doi: 10.1016/s0092-8674(00)80721-7. Epub 1999/04/13. [DOI] [PubMed] [Google Scholar]
- 4.Goldbach-Mansky R. Immunology in clinic review series; focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clinical and experimental immunology. 2012;167(3):391–404. doi: 10.1111/j.1365-2249.2011.04533.x. Epub 2012/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annual review of immunology. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. Epub 2009/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell. 2010;140(6):784–90. doi: 10.1016/j.cell.2010.03.002. Epub 2010/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D, Aziz A, Dickie LJ, McDermott MF. An integrated classification of pediatric inflammatory diseases, based on the concepts of autoinflammation and the immunological disease continuum. Pediatric research. 2009;65(5 Pt 2):38R–45R. doi: 10.1203/PDR.0b013e31819dbd0a. Epub 2009/02/05. [DOI] [PubMed] [Google Scholar]
- 8.Ozen S, Hoffman HM, Frenkel J, Kastner D. Familial Mediterranean fever (FMF) and beyond: a new horizon. Annals of the rheumatic diseases; Fourth International Congress on the Systemic Autoinflammatory Diseases held in Bethesda; USA. 6-10 November 2005; 2006. pp. 961–4. Epub 2006/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touitou I, Kone-Paut I. Autoinflammatory diseases. Best practice & research Clinical rheumatology. 2008;22(5):811–29. doi: 10.1016/j.berh.2008.08.009. Epub 2008/11/26. [DOI] [PubMed] [Google Scholar]
- 10.Janeway TC, Mosenthal HO. An unusual paroxysmal syndrome, probably allied to recurrent vomiting, with a study of the nitrogen metabolism. Trans Ass Am Phys. 1908;23:15. [Google Scholar]
- 11.Siegal S. Benign paroxysmal peritonitis. Annals of internal medicine. 1945;23:21. [Google Scholar]
- 12.Marek-Yagel D, Berkun Y, Padeh S, Abu A, Reznik-Wolf H, Livneh A, et al. Clinical disease among patients heterozygous for familial Mediterranean fever. Arthritis and rheumatism. 2009;60(6):1862–6. doi: 10.1002/art.24570. Epub 2009/05/30. [DOI] [PubMed] [Google Scholar]
- 13.Onen F. Familial Mediterranean fever. Rheumatology international. 2006;26(6):489–96. doi: 10.1007/s00296-005-0074-3. Epub 2005/11/12. [DOI] [PubMed] [Google Scholar]
- 14.Chae JJ, Aksentijevich I, Kastner DL. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. British journal of haematology. 2009;146(5):467–78. doi: 10.1111/j.1365-2141.2009.07733.x. Epub 2009/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoffman N, Magal N, Shohat T, Lotan R, Koman S, Oron A, et al. Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups. European journal of human genetics : EJHG. 2000;8(4):307–10. doi: 10.1038/sj.ejhg.5200446. [DOI] [PubMed] [Google Scholar]
- 16.Infevers: an online database for autoinflammatory mutations. [Accessed 08/28/2012]; Copyright. Available at http://fmf.igh.cnrs.fr/ISSAID/infevers/ database on the Internet.
- 17.Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):9982–7. doi: 10.1073/pnas.0602081103. Epub 2006/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marek-Yagel D, Bar-Joseph I, Pras E, Berkun Y. Is E148Q a benign polymorphism or a disease-causing mutation? The Journal of rheumatology. 2009;36(10):2372. doi: 10.3899/jrheum.090250. [DOI] [PubMed] [Google Scholar]
- 19.Touitou I. The spectrum of Familial Mediterranean Fever (FMF) mutations. European journal of human genetics : EJHG. 2001;9(7):473–83. doi: 10.1038/sj.ejhg.5200658. [DOI] [PubMed] [Google Scholar]
- 20.Fonnesu C, Cerquaglia C, Giovinale M, Curigliano V, Verrecchia E, de Socio G, et al. Familial Mediterranean Fever: a review for clinical management. Joint, bone, spine : revue du rhumatisme. 2009;76(3):227–33. doi: 10.1016/j.jbspin.2008.08.004. Epub 2008/12/19. [DOI] [PubMed] [Google Scholar]
- 21.Glaser RL, Goldbach-Mansky R. The spectrum of monogenic autoinflammatory syndromes: understanding disease mechanisms and use of targeted therapies. Current allergy and asthma reports. 2008;8(4):288–98. doi: 10.1007/s11882-008-0047-1. Epub 2008/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padeh S, Livneh A, Pras E, Shinar Y, Lidar M, Feld O, et al. Familial Mediterranean fever in children presenting with attacks of fever alone. The Journal of rheumatology. 2010;37(4):865–9. doi: 10.3899/jrheum.090687. Epub 2010/03/03. [DOI] [PubMed] [Google Scholar]
- 23.Simon A, van der Meer JW, Drenth JP. Familial Mediterranean fever--a not so unusual cause of abdominal pain. Best practice & research Clinical gastroenterology. 2005;19(2):199–213. doi: 10.1016/j.bpg.2004.11.009. Epub 2005/04/19. [DOI] [PubMed] [Google Scholar]
- 24.Younes M, Kahn MF, Meyer O. Hip involvement in patients with familial Mediterranean fever. A review of ten cases. Joint, bone, spine : revue du rhumatisme. 2002;69(6):560–5. doi: 10.1016/s1297-319x(02)00452-9. Epub 2003/01/23. [DOI] [PubMed] [Google Scholar]
- 25.Bilgen SA, Kilic L, Akdogan A, Kiraz S, Kalyoncu U, Karadag O, et al. Effects of anti-tumor necrosis factor agents for familial mediterranean fever patients with chronic arthritis and/or sacroiliitis who were resistant to colchicine treatment. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2011;17(7):358–62. doi: 10.1097/RHU.0b013e31823682f5. Epub 2011/09/29. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman HM, Simon A. Recurrent febrile syndromes: what a rheumatologist needs to know. Nature reviews Rheumatology. 2009;5(5):249–56. doi: 10.1038/nrrheum.2009.40. Epub 2009/05/05. [DOI] [PubMed] [Google Scholar]
- 27.Langevitz P, Zemer D, Livneh A, Shemer J, Pras M. Protracted febrile myalgia in patients with familial Mediterranean fever. The Journal of rheumatology. 1994;21(9):1708–9. Epub 1994/09/01. [PubMed] [Google Scholar]
- 28.Kees S, Langevitz P, Zemer D, Padeh S, Pras M, Livneh A. Attacks of pericarditis as a manifestation of familial Mediterranean fever (FMF) QJM : monthly journal of the Association of Physicians. 1997;90(10):643–7. doi: 10.1093/qjmed/90.10.643. Epub 1998/01/24. [DOI] [PubMed] [Google Scholar]
- 29.Barski L, Shalev L, Zektser M, Malada-Mazri H, Abramov D, Rafaely Y. Large hemorrhagic pericardial effusion. The Israel Medical Association journal : IMAJ. 2012;14(6):367–71. Epub 2012/08/16. [PubMed] [Google Scholar]
- 30.Padeh S. Periodic fever syndromes. Pediatric clinics of North America. 2005;52(2):577–609. vii. doi: 10.1016/j.pcl.2005.01.005. Epub 2005/04/12. [DOI] [PubMed] [Google Scholar]
- 31.Majeed HA, Ghandour K, Shahin HM. The acute scrotum in Arab children with familial Mediterranean fever. Pediatric surgery international. 2000;16(1-2):72–4. doi: 10.1007/s003830050019. Epub 2000/02/09. [DOI] [PubMed] [Google Scholar]
- 32.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis and rheumatism. 1997;40(10):1879–85. doi: 10.1002/art.1780401023. Epub 1997/10/23. [DOI] [PubMed] [Google Scholar]
- 33.Yalcinkaya F, Ozen S, Ozcakar ZB, Aktay N, Cakar N, Duzova A, et al. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology (Oxford) 2009;48(4):395–8. doi: 10.1093/rheumatology/ken509. Epub 2009/02/06. [DOI] [PubMed] [Google Scholar]
- 34.Touitou I, Sarkisian T, Medlej-Hashim M, Tunca M, Livneh A, Cattan D, et al. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis and rheumatism. 2007;56(5):1706–12. doi: 10.1002/art.22507. Epub 2007/05/01. [DOI] [PubMed] [Google Scholar]
- 35.Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine. 2005;84(1):1–11. doi: 10.1097/01.md.0000152370.84628.0c. Epub 2005/01/12. [DOI] [PubMed] [Google Scholar]
- 36.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, et al. Natural history and outcome in systemic AA amyloidosis. The New England journal of medicine. 2007;356(23):2361–71. doi: 10.1056/NEJMoa070265. Epub 2007/06/08. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Chetrit E, Levy M. Colchicine: 1998 update. Seminars in arthritis and rheumatism. 1998;28(1):48–59. doi: 10.1016/s0049-0172(98)80028-0. Epub 1998/09/03. [DOI] [PubMed] [Google Scholar]
- 38.Ozcakar ZB, Yalcinkaya F, Yuksel S, Ekim M. The expanded clinical spectrum of familial Mediterranean fever. Clinical rheumatology. 2007;26(9):1557–60. doi: 10.1007/s10067-006-0447-3. Epub 2006/10/25. [DOI] [PubMed] [Google Scholar]
- 39.Lidar M, Scherrmann JM, Shinar Y, Chetrit A, Niel E, Gershoni-Baruch R, et al. Colchicine nonresponsiveness in familial Mediterranean fever: clinical, genetic, pharmacokinetic, and socioeconomic characterization. Seminars in arthritis and rheumatism. 2004;33(4):273–82. doi: 10.1053/s0049-0172(03)00137-9. Epub 2004/02/24. [DOI] [PubMed] [Google Scholar]
- 40.Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, et al. Successful treatment of familial Mediterranean fever with Anakinra and outcome after renal transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association- European Renal Association. 2009;24(2):676–8. doi: 10.1093/ndt/gfn646. Epub 2008/11/27. [DOI] [PubMed] [Google Scholar]
- 41.Hashkes PJ, Spalding SJ, Giannini EH, Huang B, Johnson A, Park G, et al. Rilonacept for colchicine-resistant or -intolerant familial Mediterranean fever: a randomized trial. Annals of internal medicine. 2012;157(8):533–41. doi: 10.7326/0003-4819-157-8-201210160-00003. Epub 2012/10/17. [DOI] [PubMed] [Google Scholar]
- 42.Tweezer-Zaks N, Rabinovich E, Lidar M, Livneh A. Interferon-alpha as a treatment modality for colchicine- resistant familial Mediterranean fever. The Journal of rheumatology. 2008;35(7):1362–5. Epub 2008/06/06. [PubMed] [Google Scholar]
- 43.Calguneri M, Apras S, Ozbalkan Z, Ozturk MA, Ertenli I, Kiraz S. The efficacy of continuous interferon alpha administration as an adjunctive agent to colchicine-resistant familial Mediterranean fever patients. Clinical and experimental rheumatology. 2004;22(4 Suppl 34):S41–4. Epub 2004/11/02. [PubMed] [Google Scholar]
- 44.Seyahi E, Ozdogan H, Masatlioglu S, Yazici H. Successful treatment of familial Mediterranean fever attacks with thalidomide in a colchicine resistant patient. Clinical and experimental rheumatology. 2002;20(4 Suppl 26):S43–4. Epub 2002/10/10. [PubMed] [Google Scholar]
- 45.Seyahi E, Ozdogan H, Celik S, Ugurlu S, Yazici H. Treatment options in colchicine resistant familial Mediterranean fever patients: thalidomide and etanercept as adjunctive agents. Clinical and experimental rheumatology. 2006;24(5 Suppl 42):S99–103. Epub 2006/10/28. [PubMed] [Google Scholar]
- 46.Mor A, Pillinger MH, Kishimoto M, Abeles AM, Livneh A. Familial Mediterranean fever successfully treated with etanercept. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2007;13(1):38–40. doi: 10.1097/01.rhu.0000255772.25658.7c. Epub 2007/02/07. [DOI] [PubMed] [Google Scholar]
- 47.Ozcakar ZB, Yuksel S, Ekim M, Yalcinkaya F. Infliximab therapy for familial Mediterranean fever-related amyloidosis: case series with long term follow-up. Clinical rheumatology. 2012;31(8):1267–71. doi: 10.1007/s10067-012-2009-1. Epub 2012/06/08. [DOI] [PubMed] [Google Scholar]
- 48.Yuksel S, Yalcinkaya F, Acar B, Ozcakar ZB, Ozturk B, Ekim M. Clinical improvement with infliximab in a child with amyloidosis secondary to familial Mediterranean fever. Rheumatology (Oxford) 2006;45(10):1307–8. doi: 10.1093/rheumatology/kel250. Epub 2006/08/02. [DOI] [PubMed] [Google Scholar]
- 49.Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerkhof A, Romeijn GJ, et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nature genetics. 1999;22(2):175–7. doi: 10.1038/9691. Epub 1999/06/16. [DOI] [PubMed] [Google Scholar]
- 50.van der Hilst JC, Bodar EJ, Barron KS, Frenkel J, Drenth JP, van der Meer JW, et al. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine. 2008;87(6):301–10. doi: 10.1097/MD.0b013e318190cfb7. Epub 2008/11/18. [DOI] [PubMed] [Google Scholar]
- 51.Houten SM, van Woerden CS, Wijburg FA, Wanders RJ, Waterham HR. Carrier frequency of the V377I (1129G>A) MVK mutation, associated with Hyper-IgD and periodic fever syndrome, in the Netherlands. European journal of human genetics : EJHG. 2003;11(2):196–200. doi: 10.1038/sj.ejhg.5200933. Epub 2003/03/14. [DOI] [PubMed] [Google Scholar]
- 52.Haas D, Hoffmann GF. Mevalonate kinase deficiencies: from mevalonic aciduria to hyperimmunoglobulinemia D syndrome. Orphanet journal of rare diseases. 2006:1–13. doi: 10.1186/1750-1172-1-13. Epub 2006/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruiz Gomez A, Couce ML, Garcia-Villoria J, Torres A, Bana Souto A, Yague J, et al. Clinical, genetic, and therapeutic diversity in 2 patients with severe mevalonate kinase deficiency. Pediatrics. 2012;129(2):e535–9. doi: 10.1542/peds.2010-2192. Epub 2012/01/25. [DOI] [PubMed] [Google Scholar]
- 54.Simon A, van der Meer JW. Pathogenesis of familial periodic fever syndromes or hereditary autoinflammatory syndromes. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292(1):R86–98. doi: 10.1152/ajpregu.00504.2006. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 55.Cuisset L, Drenth JP, Simon A, Vincent MF, van der Velde Visser S, van der Meer JW, et al. Molecular analysis of MVK mutations and enzymatic activity in hyper-IgD and periodic fever syndrome. European journal of human genetics : EJHG. 2001;9(4):260–6. doi: 10.1038/sj.ejhg.5200614. [DOI] [PubMed] [Google Scholar]
- 56.Drenth JP, Haagsma CJ, van der Meer JW. Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-Ig D Study Group. Medicine. 1994;73(3):133–44. Epub 1994/05/01. [PubMed] [Google Scholar]
- 57.van der Hilst JC, Bodar EJ, Barron KS, Frenkel J, Drenth JP, van der Meer JW, et al. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine. 2008;87(6):301–10. doi: 10.1097/MD.0b013e318190cfb7. [DOI] [PubMed] [Google Scholar]
- 58.Bader-Meunier B, Florkin B, Sibilia J, Acquaviva C, Hachulla E, Grateau G, et al. Mevalonate kinase deficiency: a survey of 50 patients. Pediatrics. 2011;128(1):e152–9. doi: 10.1542/peds.2010-3639. Epub 2011/06/29. [DOI] [PubMed] [Google Scholar]
- 59.Gattorno M, Sormani MP, D'Osualdo A, Pelagatti MA, Caroli F, Federici S, et al. A diagnostic score for molecular analysis of hereditary autoinflammatory syndromes with periodic fever in children. Arthritis and rheumatism. 2008;58(6):1823–32. doi: 10.1002/art.23474. Epub 2008/06/03. [DOI] [PubMed] [Google Scholar]
- 60.Drenth JP, Boom BW, Toonstra J, Van der Meer JW. Cutaneous manifestations and histologic findings in the hyperimmunoglobulinemia D syndrome. International Hyper IgD Study Group. Archives of dermatology. 1994;130(1):59–65. Epub 1994/01/01. [PubMed] [Google Scholar]
- 61.Saulsbury FT. Hyperimmunoglobulinemia D and periodic fever syndrome (HIDS) in a child with normal serum IgD, but increased serum IgA concentration. The Journal of pediatrics. 2003;143(1):127–9. doi: 10.1016/S0022-3476(03)00212-9. Epub 2003/08/14. [DOI] [PubMed] [Google Scholar]
- 62.Ammouri W, Cuisset L, Rouaghe S, Rolland MO, Delpech M, Grateau G, et al. Diagnostic value of serum immunoglobulinaemia D level in patients with a clinical suspicion of hyper IgD syndrome. Rheumatology (Oxford) 2007;46(10):1597–600. doi: 10.1093/rheumatology/kem200. Epub 2007/09/07. [DOI] [PubMed] [Google Scholar]
- 63.Steichen O, van der Hilst J, Simon A, Cuisset L, Grateau G. A clinical criterion to exclude the hyperimmunoglobulin D syndrome (mild mevalonate kinase deficiency) in patients with recurrent fever. The Journal of rheumatology. 2009;36(8):1677–81. doi: 10.3899/jrheum.081313. Epub 2009/06/18. [DOI] [PubMed] [Google Scholar]
- 64.Lachmann HJ, Goodman HJ, Andrews PA, Gallagher H, Marsh J, Breuer S, et al. AA amyloidosis complicating hyperimmunoglobulinemia D with periodic fever syndrome: a report of two cases. Arthritis and rheumatism. 2006;54(6):2010–4. doi: 10.1002/art.21901. Epub 2006/05/30. [DOI] [PubMed] [Google Scholar]
- 65.Obici L, Manno C, Muda AO, Picco P, D'Osualdo A, Palladini G, et al. First report of systemic reactive (AA) amyloidosis in a patient with the hyperimmunoglobulinemia D with periodic fever syndrome. Arthritis and rheumatism. 2004;50(9):2966–9. doi: 10.1002/art.20490. Epub 2004/10/01. [DOI] [PubMed] [Google Scholar]
- 66.Siewert R, Ferber J, Horstmann RD, Specker C, Heering PJ, Timmann C. Hereditary periodic fever with systemic amyloidosis: is hyper-IgD syndrome really a benign disease? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;48(3):e41–5. doi: 10.1053/j.ajkd.2006.05.027. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 67.Li Cavoli G, Passantino D, Tortorici C, Bono L, Ferrantelli A, Giammarresi C, et al. Renal amyloidosis due to hyper-IgD syndrome. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2012;32(6):865–6. doi: 10.3265/Nefrologia.pre2012.Aug.11660. Epub 2012/11/22. [DOI] [PubMed] [Google Scholar]
- 68.Simon A, Drewe E, van der Meer JW, Powell RJ, Kelley RI, Stalenhoef AF, et al. Simvastatin treatment for inflammatory attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Clinical pharmacology and therapeutics. 2004;75(5):476–83. doi: 10.1016/j.clpt.2004.01.012. Epub 2004/04/30. [DOI] [PubMed] [Google Scholar]
- 69.Demirkaya E, Caglar MK, Waterham HR, Topaloglu R, Ozen S. A patient with hyper-IgD syndrome responding to anti-TNF treatment. Clinical rheumatology. 2007;26(10):1757–9. doi: 10.1007/s10067-006-0501-1. Epub 2006/12/16. [DOI] [PubMed] [Google Scholar]
- 70.Nevyjel M, Pontillo A, Calligaris L, Tommasini A, D'Osualdo A, Waterham HR, et al. Diagnostics and therapeutic insights in a severe case of mevalonate kinase deficiency. Pediatrics. 2007;119(2):e523–7. doi: 10.1542/peds.2006-2015. Epub 2007/01/31. [DOI] [PubMed] [Google Scholar]
- 71.Takada K, Aksentijevich I, Mahadevan V, Dean JA, Kelley RI, Kastner DL. Favorable preliminary experience with etanercept in two patients with the hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis and rheumatism. 2003;48(9):2645–51. doi: 10.1002/art.11218. Epub 2003/09/18. [DOI] [PubMed] [Google Scholar]
- 72.Topaloglu R, Ayaz NA, Waterham HR, Yuce A, Gumruk F, Sanal O. Hyperimmunoglobulinemia D and periodic fever syndrome; treatment with etanercept and follow-up. Clinical rheumatology. 2008;27(10):1317–20. doi: 10.1007/s10067-008-0911-3. Epub 2008/05/29. [DOI] [PubMed] [Google Scholar]
- 73.Rigante D, Ansuini V, Bertoni B, Pugliese AL, Avallone L, Federico G, et al. Treatment with anakinra in the hyperimmunoglobulinemia D/periodic fever syndrome. Rheumatology international. 2006;27(1):97–100. doi: 10.1007/s00296-006-0164-x. Epub 2006/07/28. [DOI] [PubMed] [Google Scholar]
- 74.Galeotti C, Meinzer U, Quartier P, Rossi-Semerano L, Bader-Meunier B, Pillet P, et al. Efficacy of interleukin-1-targeting drugs in mevalonate kinase deficiency. Rheumatology (Oxford) 2012;51(10):1855–9. doi: 10.1093/rheumatology/kes097. Epub 2012/06/29. [DOI] [PubMed] [Google Scholar]
- 75.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Current opinion in rheumatology. 2005;17(5):586–99. doi: 10.1097/bor.0000174210.78449.6b. Epub 2005/08/12. [DOI] [PubMed] [Google Scholar]
- 76.Haas SL, Lohse P, Schmitt WH, Hildenbrand R, Karaorman M, Singer MV, et al. Severe TNF receptor-associated periodic syndrome due to 2 TNFRSF1A mutations including a new F60V substitution. Gastroenterology. 2006;130(1):172–8. doi: 10.1053/j.gastro.2005.09.014. Epub 2006/01/13. [DOI] [PubMed] [Google Scholar]
- 77.Lainka E, Neudorf U, Lohse P, Timmann C, Stojanov S, Huss K, et al. Incidence of TNFRSF1A mutations in German children: epidemiological, clinical and genetic characteristics. Rheumatology (Oxford) 2009;48(8):987–91. doi: 10.1093/rheumatology/kep140. Epub 2009/06/23. [DOI] [PubMed] [Google Scholar]
- 78.Stojanov S, McDermott MF. The tumour necrosis factor receptor-associated periodic syndrome: current concepts. Expert reviews in molecular medicine. 2005;7(22):1–18. doi: 10.1017/S1462399405009749. Epub 2005/10/12. [DOI] [PubMed] [Google Scholar]
- 79.Hull KM, Drewe E, Aksentijevich I, Singh HK, Wong K, McDermott EM, et al. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine. 2002;81(5):349–68. doi: 10.1097/00005792-200209000-00002. Epub 2002/09/28. [DOI] [PubMed] [Google Scholar]
- 80.Hull KM, Wong K, Wood GM, Chu WS, Kastner DL. Monocytic fasciitis: a newly recognized clinical feature of tumor necrosis factor receptor dysfunction. Arthritis and rheumatism. 2002;46(8):2189–94. doi: 10.1002/art.10448. [DOI] [PubMed] [Google Scholar]
- 81.Jesus AA, Oliveira JB, Aksentijevich I, Fujihira E, Carneiro-Sampaio MM, Duarte AJ, et al. TNF receptor-associated periodic syndrome (TRAPS): description of a novel TNFRSF1A mutation and response to etanercept. European journal of pediatrics. 2008;167(12):1421–5. doi: 10.1007/s00431-008-0685-2. Epub 2008/04/15. [DOI] [PubMed] [Google Scholar]
- 82.Minden K, Aganna E, McDermott MF, Zink A. Tumour necrosis factor receptor associated periodic syndrome (TRAPS) with central nervous system involvement. Annals of the rheumatic diseases. 2004;63(10):1356–7. doi: 10.1136/ard.2003.016006. Epub 2004/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen-Wolff A, Kreth HW, Hofmann S, Hohne K, Heubner G, Mobius D, et al. Periodic fever (TRAPS) caused by mutations in the TNFalpha receptor 1 (TNFRSF1A) gene of three German patients. European journal of haematology. 2001;67(2):105–9. Epub 2001/11/28. [PubMed] [Google Scholar]
- 84.Kallinich T, Haffner D, Rudolph B, Schindler R, Canaan-Kuhl S, Keitzer R, et al. “Periodic fever” without fever: two cases of non-febrile TRAPS with mutations in the TNFRSF1A gene presenting with episodes of inflammation or monosymptomatic amyloidosis. Annals of the rheumatic diseases. 2006;65(7):958–60. doi: 10.1136/ard.2005.043570. Epub 2005/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morbach H, Richl P, Stojanov S, Lohse P, Girschick HJ. Tumor necrosis factor receptor 1-associated periodic syndrome without fever: cytokine profile before and during etanercept treatment. Rheumatology international. 2009;30(2):207–12. doi: 10.1007/s00296-009-0937-0. Epub 2009/04/22. [DOI] [PubMed] [Google Scholar]
- 86.Aksentijevich I, Galon J, Soares M, Mansfield E, Hull K, Oh HH, et al. The tumor-necrosis-factor receptor-associated periodic syndrome: new mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further genetic heterogeneity of periodic fevers. American journal of human genetics. 2001;69(2):301–14. doi: 10.1086/321976. Epub 2001/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lobito AA, Kimberley FC, Muppidi JR, Komarow H, Jackson AJ, Hull KM, et al. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS) Blood. 2006;108(4):1320–7. doi: 10.1182/blood-2005-11-006783. Epub 2006/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bulua AC, Mogul DB, Aksentijevich I, Singh H, He DY, Muenz LR, et al. Efficacy of etanercept in the tumor necrosis factor receptor-associated periodic syndrome: a prospective, open-label, dose-escalation study. Arthritis and rheumatism. 2012;64(3):908–13. doi: 10.1002/art.33416. Epub 2011/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobelli S, Andre M, Alexandra JF, Dode C, Papo T. Failure of anti-TNF therapy in TNF Receptor 1-Associated Periodic Syndrome (TRAPS) Rheumatology (Oxford) 2007;46(7):1211–2. doi: 10.1093/rheumatology/kel298. Epub 2006/08/29. [DOI] [PubMed] [Google Scholar]
- 90.Nedjai B, Hitman GA, Quillinan N, Coughlan RJ, Church L, McDermott MF, et al. Proinflammatory action of the antiinflammatory drug infliximab in tumor necrosis factor receptor-associated periodic syndrome. Arthritis and rheumatism. 2009;60(2):619–25. doi: 10.1002/art.24294. Epub 2009/01/31. [DOI] [PubMed] [Google Scholar]
- 91.Gattorno M, Pelagatti MA, Meini A, Obici L, Barcellona R, Federici S, et al. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis and rheumatism. 2008;58(5):1516–20. doi: 10.1002/art.23475. Epub 2008/04/29. [DOI] [PubMed] [Google Scholar]
- 92.Goldbach-Mansky R. Blocking interleukin-1 in rheumatic diseases. Annals of the New York Academy of Sciences. 2009;1182:111–23. doi: 10.1111/j.1749-6632.2009.05159.x. Epub 2010/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sacre K, Brihaye B, Lidove O, Papo T, Pocidalo MA, Cuisset L, et al. Dramatic improvement following interleukin 1beta blockade in tumor necrosis factor receptor-1-associated syndrome (TRAPS) resistant to anti-TNF-alpha therapy. The Journal of rheumatology. 2008;35(2):357–8. Epub 2008/02/09. [PubMed] [Google Scholar]
- 94.Quillinan N, Mannion G, Mohammad A, Coughlan R, Dickie LJ, McDermott MF, et al. Failure of sustained response to etanercept and refractoriness to anakinra in patients with T50M TNF-receptor-associated periodic syndrome. Annals of the rheumatic diseases. 2011;70(9):1692–3. doi: 10.1136/ard.2010.144279. Epub 2011/03/08. [DOI] [PubMed] [Google Scholar]
- 95.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis and rheumatism. 2002;46(12):3340–8. doi: 10.1002/art.10688. Epub 2002/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aksentijevich I, DP C, Remmers EF, Mueller JL, Le J, Kolodner RD, et al. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis and rheumatism. 2007;56(4):1273–85. doi: 10.1002/art.22491. Epub 2007/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neven B, Callebaut I, Prieur AM, Feldmann J, Bodemer C, Lepore L, et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood. 2004;103(7):2809–15. doi: 10.1182/blood-2003-07-2531. [DOI] [PubMed] [Google Scholar]
- 98.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nature genetics. 2001;29(3):301–5. doi: 10.1038/ng756. Epub 2001/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saito M, Fujisawa A, Nishikomori R, Kambe N, Nakata-Hizume M, Yoshimoto M, et al. Somatic mosaicism of CIAS1 in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis and rheumatism. 2005;52(11):3579–85. doi: 10.1002/art.21404. Epub 2005/10/29. [DOI] [PubMed] [Google Scholar]
- 100.Saito M, Nishikomori R, Kambe N, Fujisawa A, Tanizaki H, Takeichi K, et al. Disease-associated CIAS1 mutations induce monocyte death, revealing low-level mosaicism in mutation-negative cryopyrin-associated periodic syndrome patients. Blood. 2008;111(4):2132–41. doi: 10.1182/blood-2007-06-094201. Epub 2007/12/08. [DOI] [PubMed] [Google Scholar]
- 101.Arostegui JI, Lopez Saldana MD, Pascal M, Clemente D, Aymerich M, Balaguer F, et al. A somatic NLRP3 mutation as a cause of a sporadic case of chronic infantile neurologic, cutaneous, articular syndrome/neonatal-onset multisystem inflammatory disease: Novel evidence of the role of low-level mosaicism as the pathophysiologic mechanism underlying mendelian inherited diseases. Arthritis and rheumatism. 2010;62(4):1158–66. doi: 10.1002/art.27342. Epub 2010/02/05. [DOI] [PubMed] [Google Scholar]
- 102.Tanaka N, Izawa K, Saito MK, Sakuma M, Oshima K, Ohara O, et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis and rheumatism. 2011;63(11):3625–32. doi: 10.1002/art.30512. Epub 2011/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arostegui JI, Aldea A, Modesto C, Rua MJ, Arguelles F, Gonzalez-Ensenat MA, et al. Clinical and genetic heterogeneity among Spanish patients with recurrent autoinflammatory syndromes associated with the CIAS1/PYPAF1/NALP3 gene. Arthritis and rheumatism. 2004;50(12):4045–50. doi: 10.1002/art.20633. Epub 2004/12/14. [DOI] [PubMed] [Google Scholar]
- 104.Aubert P, Suarez-Farinas M, Mitsui H, Johnson-Huang LM, Harden JL, Pierson KC, et al. Homeostatic Tissue Responses in Skin Biopsies from NOMID Patients with Constitutive Overproduction of IL-1beta. PloS one. 2012;7(11):e49408. doi: 10.1371/journal.pone.0049408. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364(9447):1779–85. doi: 10.1016/S0140-6736(04)17401-1. Epub 2004/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. The Journal of allergy and clinical immunology. 2001;108(4):615–20. doi: 10.1067/mai.2001.118790. Epub 2001/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stych B, Dobrovolny D. Familial cold auto-inflammatory syndrome (FCAS): characterization of symptomatology and impact on patients' lives. Current medical research and opinion. 2008;24(6):1577–82. doi: 10.1185/03007990802081543. Epub 2008/04/22. [DOI] [PubMed] [Google Scholar]
- 108.Muckle TJ, Wellsm Urticaria, deafness, and amyloidosis: a new heredo-familial syndrome. The Quarterly journal of medicine. 1962;31:235–48. Epub 1962/04/01. [PubMed] [Google Scholar]
- 109.Dode C, Le Du N, Cuisset L, Letourneur F, Berthelot JM, Vaudour G, et al. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. American journal of human genetics. 2002;70(6):1498–506. doi: 10.1086/340786. Epub 2002/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis and rheumatism. 2004;50(2):607–12. doi: 10.1002/art.20033. Epub 2004/02/12. [DOI] [PubMed] [Google Scholar]
- 111.Neven B, Marvillet I, Terrada C, Ferster A, Boddaert N, Couloignier V, et al. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis and rheumatism. 2010;62(1):258–67. doi: 10.1002/art.25057. Epub 2009/12/30. [DOI] [PubMed] [Google Scholar]
- 112.Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis and rheumatism. 2002;46(9):2445–52. doi: 10.1002/art.10509. Epub 2002/10/02. [DOI] [PubMed] [Google Scholar]
- 113.Prieur AM, Griscelli C. Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. The Journal of pediatrics. 1981;99(1):79–83. doi: 10.1016/s0022-3476(81)80961-4. Epub 1981/07/01. [DOI] [PubMed] [Google Scholar]
- 114.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. The New England journal of medicine. 2006;355(6):581–92. doi: 10.1056/NEJMoa055137. Epub 2006/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neven B, Prieur AM, Quartier dit Maire P. Cryopyrinopathies: update on pathogenesis and treatment. Nature clinical practice Rheumatology. 2008;4(9):481–9. doi: 10.1038/ncprheum0874. Epub 2008/07/31. [DOI] [PubMed] [Google Scholar]
- 116.Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis and rheumatism. 2012;64(7):2375–86. doi: 10.1002/art.34409. Epub 2012/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hill SC, Namde M, Dwyer A, Poznanski A, Canna S, Goldbach-Mansky R. Arthropathy of neonatal onset multisystem inflammatory disease (NOMID/CINCA) Pediatric radiology. 2007;37(2):145–52. doi: 10.1007/s00247-006-0358-0. Epub 2006/12/01. [DOI] [PubMed] [Google Scholar]
- 118.Kone-Paut I, Lachmann HJ, Kuemmerle-Deschner JB, Hachulla E, Leslie KS, Mouy R, et al. Sustained remission of symptoms and improved health-related quality of life in patients with cryopyrin-associated periodic syndrome treated with canakinumab: results of a double-blind placebo-controlled randomized withdrawal study. Arthritis research & therapy. 2011;13(6):R202. doi: 10.1186/ar3535. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis and rheumatism. 2008;58(8):2443–52. doi: 10.1002/art.23687. Epub 2008/08/01. [DOI] [PubMed] [Google Scholar]
- 120.Hoffman HM, Throne ML, Amar NJ, Cartwright RC, Kivitz AJ, Soo Y, et al. Long-term efficacy and safety profile of rilonacept in the treatment of cryopryin-associated periodic syndromes: results of a 72-week open-label extension study. Clinical therapeutics. 2012;34(10):2091–103. doi: 10.1016/j.clinthera.2012.09.009. Epub 2012/10/04. [DOI] [PubMed] [Google Scholar]
- 121.Goldbach-Mansky R, Shroff SD, Wilson M, Snyder C, Plehn S, Barham B, et al. A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis and rheumatism. 2008;58(8):2432–42. doi: 10.1002/art.23620. Epub 2008/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. The New England journal of medicine. 2009;360(23):2416–25. doi: 10.1056/NEJMoa0810787. Epub 2009/06/06. [DOI] [PubMed] [Google Scholar]
- 123.Lepore L, Paloni G, Caorsi R, Alessio M, Rigante D, Ruperto N, et al. Follow-up and quality of life of patients with cryopyrin-associated periodic syndromes treated with Anakinra. The Journal of pediatrics. 2010;157(2):310–5 e1. doi: 10.1016/j.jpeds.2010.02.040. Epub 2010/05/18. [DOI] [PubMed] [Google Scholar]
- 124.Hoffman HM. Rilonacept for the treatment of cryopyrin-associated periodic syndromes (CAPS) Expert opinion on biological therapy. 2009;9(4):519–31. doi: 10.1517/14712590902875518. Epub 2009/04/07. [DOI] [PubMed] [Google Scholar]
- 125.Kuemmerle-Deschner JB, Hachulla E, Cartwright R, Hawkins PN, Tran TA, Bader-Meunier B, et al. Two-year results from an open-label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin-associated periodic syndrome across different severity phenotypes. Annals of the rheumatic diseases. 2011;70(12):2095–102. doi: 10.1136/ard.2011.152728. Epub 2011/08/24. [DOI] [PubMed] [Google Scholar]
- 126.Alonso D, Elgart GW, Schachner LA. Blau syndrome: a new kindred. Journal of the American Academy of Dermatology. 2003;49(2):299–302. doi: 10.1067/s0190-9622(02)61772-4. Epub 2003/08/02. [DOI] [PubMed] [Google Scholar]
- 127.Rose CD, Arostegui JI, Martin TM, Espada G, Scalzi L, Yague J, et al. NOD2-associated pediatric granulomatous arthritis, an expanding phenotype: study of an international registry and a national cohort in Spain. Arthritis and rheumatism. 2009;60(6):1797–803. doi: 10.1002/art.24533. Epub 2009/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rose CD, Martin TM, Wouters CH. Blau syndrome revisited. Current opinion in rheumatology. 2011;23(5):411–8. doi: 10.1097/BOR.0b013e328349c430. Epub 2011/07/27. [DOI] [PubMed] [Google Scholar]
- 129.Rose CD, Wouters CH, Meiorin S, Doyle TM, Davey MP, Rosenbaum JT, et al. Pediatric granulomatous arthritis: an international registry. Arthritis and rheumatism. 2006;54(10):3337–44. doi: 10.1002/art.22122. Epub 2006/09/30. [DOI] [PubMed] [Google Scholar]
- 130.Arostegui JI, Arnal C, Merino R, Modesto C, Antonia Carballo M, Moreno P, et al. NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis and rheumatism. 2007;56(11):3805–13. doi: 10.1002/art.22966. Epub 2007/10/31. [DOI] [PubMed] [Google Scholar]
- 131.Becker ML, Rose CD. Blau syndrome and related genetic disorders causing childhood arthritis. Current rheumatology reports. 2005;7(6):427–33. doi: 10.1007/s11926-005-0046-3. Epub 2005/11/24. [DOI] [PubMed] [Google Scholar]
- 132.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. The New England journal of medicine. 2009;360(23):2426–37. doi: 10.1056/NEJMoa0807865. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. The New England journal of medicine. 2009;360(23):2438–44. doi: 10.1056/NEJMoa0809568. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Altiok E, Aksoy F, Perk Y, Taylan F, Kim PW, Ilikkan B, et al. A novel mutation in the interleukin-1 receptor antagonist associated with intrauterine disease onset. Clin Immunol. 2012;145(1):77–81. doi: 10.1016/j.clim.2012.08.003. Epub 2012/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jesus AA, Osman M, Silva CA, Kim PW, Pham TH, Gadina M, et al. A novel mutation of IL1RN in the deficiency of interleukin-1 receptor antagonist syndrome: description of two unrelated cases from Brazil. Arthritis and rheumatism. 2011;63(12):4007–17. doi: 10.1002/art.30588. Epub 2011/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stenerson M, Dufendach K, Aksentijevich I, Brady J, Austin J, Reed AM. The first reported case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1 receptor antagonist. Arthritis and rheumatism. 2011;63(12):4018–22. doi: 10.1002/art.30565. Epub 2011/07/28. [DOI] [PubMed] [Google Scholar]
- 137.Schnellbacher C, Ciocca G, Menendez R, Aksentijevich I, Goldbach-Mansky R, Duarte AM, et al. Deficiency of Interleukin-1 Receptor Antagonist Responsive to Anakinra. Pediatric dermatology. 2012 doi: 10.1111/j.1525-1470.2012.01725.x. Epub 2012/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brau-Javier CN, Gonzales-Chavez J, Toro JR. Chronic cutaneous pustulosis due to a 175-kb deletion on chromosome 2q13: excellent response to anakinra. Archives of dermatology. 2012;148(3):301–4. doi: 10.1001/archdermatol.2011.2857. Epub 2012/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ferguson PJ, El-Shanti HI. Autoinflammatory bone disorders. Current opinion in rheumatology. 2007;19(5):492–8. doi: 10.1097/BOR.0b013e32825f5492. Epub 2007/09/01. [DOI] [PubMed] [Google Scholar]
- 140.Ferguson PJ, Chen S, Tayeh MK, Ochoa L, Leal SM, Pelet A, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) Journal of medical genetics. 2005;42(7):551–7. doi: 10.1136/jmg.2005.030759. Epub 2005/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.El-Shanti HI, Ferguson PJ. Chronic recurrent multifocal osteomyelitis: a concise review and genetic update. Clinical orthopaedics and related research. 2007;462:11–9. doi: 10.1097/BLO.0b013e3180986d73. Epub 2007/05/15. [DOI] [PubMed] [Google Scholar]
- 142.Majeed HA, Al-Tarawna M, El-Shanti H, Kamel B, Al-Khalaileh F. The syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia. Report of a new family and a review. European journal of pediatrics. 2001;160(12):705–10. doi: 10.1007/s004310100799. Epub 2002/01/25. [DOI] [PubMed] [Google Scholar]
- 143.Herlin T, Fiirgaard B, Bjerre M, Kerndrup G, Hasle H, Bing X, et al. Efficacy of anti-IL-1 treatment in Majeed syndrome. Annals of the rheumatic diseases. 2012 doi: 10.1136/annrheumdis-2012-201818. Epub 2012/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Human molecular genetics. 2002;11(8):961–9. doi: 10.1093/hmg/11.8.961. Epub 2002/04/25. [DOI] [PubMed] [Google Scholar]
- 145.Smith EJ, Allantaz F, Bennett L, Zhang D, Gao X, Wood G, et al. Clinical Molecular, and Genetic Characteristics of PAPA Syndrome: A Review. Current genomics. 2010;11(7):519–27. doi: 10.2174/138920210793175921. Epub 2011/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Demidowich AP, Freeman AF, Kuhns DB, Aksentijevich I, Gallin JI, Turner ML, et al. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) Arthritis and rheumatism. 2012;64(6):2022–7. doi: 10.1002/art.34332. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tofteland ND, Shaver TS. Clinical efficacy of etanercept for treatment of PAPA syndrome. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2010;16(5):244–5. doi: 10.1097/RHU.0b013e3181e969b9. Epub 2010/07/28. [DOI] [PubMed] [Google Scholar]
- 148.Cortis E, De Benedetti F, Insalaco A, Cioschi S, Muratori F, D'Urbano LE, et al. Abnormal production of tumor necrosis factor (TNF) -- alpha and clinical efficacy of the TNF inhibitor etanercept in a patient with PAPA syndrome [corrected] The Journal of pediatrics. 2004;145(6):851–5. doi: 10.1016/j.jpeds.2004.08.001. Epub 2004/12/08. [DOI] [PubMed] [Google Scholar]
- 149.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine. 2009;361(21):2033–45. doi: 10.1056/NEJMoa0907206. Epub 2009/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, et al. Infant colitis-it's in the genes. Lancet. 2010;376(9748):1272. doi: 10.1016/S0140-6736(10)61008-2. Epub 2010/10/12. [DOI] [PubMed] [Google Scholar]
- 151.Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143(2):347–55. doi: 10.1053/j.gastro.2012.04.045. Epub 2012/05/03. [DOI] [PubMed] [Google Scholar]
- 152.Engelhardt KR, Shah N, Faizura-Yeop I, Kocacik Uygun DF, Frede N, Muise AM, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. The Journal of allergy and clinical immunology. 2012 doi: 10.1016/j.jaci.2012.09.025. Epub 2012/11/20. [DOI] [PubMed] [Google Scholar]
- 153.Glocker E, Grimbacher B. Inflammatory bowel disease: is it a primary immunodeficiency? Cellular and molecular life sciences : CMLS. 2012;69(1):41–8. doi: 10.1007/s00018-011-0837-9. Epub 2011/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. The New England journal of medicine. 2011;365(7):620–8. doi: 10.1056/NEJMoa1013068. Epub 2011/08/19. [DOI] [PubMed] [Google Scholar]
- 155.Onoufriadis A, Simpson MA, Pink AE, Di Meglio P, Smith CH, Pullabhatla V, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. American journal of human genetics. 2011;89(3):432–7. doi: 10.1016/j.ajhg.2011.07.022. Epub 2011/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, et al. PSORS2 is due to mutations in CARD14. American journal of human genetics. 2012;90(5):784–95. doi: 10.1016/j.ajhg.2012.03.012. Epub 2012/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fuchs-Telem D, Sarig O, van Steensel MA, Isakov O, Israeli S, Nousbeck J, et al. Familial pityriasis rubra pilaris is caused by mutations in CARD14. American journal of human genetics. 2012;91(1):163–70. doi: 10.1016/j.ajhg.2012.05.010. Epub 2012/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jordan CT, Cao L, Roberson ED, Duan S, Helms CA, Nair RP, et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. American journal of human genetics. 2012;90(5):796–808. doi: 10.1016/j.ajhg.2012.03.013. Epub 2012/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. The New England journal of medicine. 2010;362(2):118–28. doi: 10.1056/NEJMoa0810652. Epub 2010/01/15. [DOI] [PubMed] [Google Scholar]
- 160.Laws PM, Downs AM, Parslew R, Dever B, Smith CH, Barker JN, et al. Practical experience of ustekinumab in the treatment of psoriasis: experience from a multicentre, retrospective case cohort study across the U.K. and Ireland. The British journal of dermatology. 2012;166(1):189–95. doi: 10.1111/j.1365-2133.2011.10638.x. Epub 2011/09/21. [DOI] [PubMed] [Google Scholar]
- 161.Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from five years of follow-up. The British journal of dermatology. 2013 doi: 10.1111/bjd.12214. Epub 2013/01/11. [DOI] [PubMed] [Google Scholar]
- 162.Vasher M, Smithberger E, Lien MH, Fenske NA. Familial pityriasis rubra pilaris: report of a family and therapeutic response to etanercept. Journal of drugs in dermatology : JDD. 2010;9(7):844–50. Epub 2010/08/04. [PubMed] [Google Scholar]
- 163.Torrelo A, Patel S, Colmenero I, Gurbindo D, Lendinez F, Hernandez A, et al. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. Journal of the American Academy of Dermatology. 2010;62(3):489–95. doi: 10.1016/j.jaad.2009.04.046. Epub 2010/02/18. [DOI] [PubMed] [Google Scholar]
- 164.Agarwal AK, Xing C, DeMartino GN, Mizrachi D, Hernandez MD, Sousa AB, et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. American journal of human genetics. 2010;87(6):866–72. doi: 10.1016/j.ajhg.2010.10.031. Epub 2010/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Arima K, Kinoshita A, Mishima H, Kanazawa N, Kaneko T, Mizushima T, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14914–9. doi: 10.1073/pnas.1106015108. Epub 2011/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kitamura A, Maekawa Y, Uehara H, Izumi K, Kawachi I, Nishizawa M, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. The Journal of clinical investigation. 2011;121(10):4150–60. doi: 10.1172/JCI58414. Epub 2011/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Y, Ramot Y, Torrelo A, Paller AS, Si N, Babay S, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis and rheumatism. 2012;64(3):895–907. doi: 10.1002/art.33368. Epub 2011/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ramot Y, Czarnowicki T, Maly A, Navon-Elkan P, Zlotogorski A. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome: a case report. Pediatric dermatology. 2011;28(5):538–41. doi: 10.1111/j.1525-1470.2010.01163.x. Epub 2010/06/18. [DOI] [PubMed] [Google Scholar]
- 169.Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. The New England journal of medicine. 2012;366(4):330–8. doi: 10.1056/NEJMoa1102140. Epub 2012/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou Q, Lee GS, Brady J, Datta S, Katan M, Sheikh A, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. American journal of human genetics. 2012;91(4):713–20. doi: 10.1016/j.ajhg.2012.08.006. Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boisson B, Laplantine E, Prando C, Giliani S, Israelsson E, Xu Z, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nature immunology. 2012;13(12):1178–86. doi: 10.1038/ni.2457. Epub 2012/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]