Abstract
The Mycobacterium tuberculosis (M. tb) infection is largely spread in world’s population. Most infected individuals develop latent tuberculosis infection (LTBI). Tuberculin skin test (TST) and interferon-gamma release assays (IGRAs) are the available tests to detect the infection. It has been reported that some individuals take a longer period of time to develop the infection than others with the same exposure level. It is suggested that the innate immunity, in which neutrophils have an important protective role, is responsible for this. Many hematologic abnormalities have been described as common findings during severe disease. To investigate if these changes are related to LTBI development and if they interfere in TST and IFN-γ production, we recruited 88 household contacts of tuberculosis (TB) pulmonary patients and compared blood cell counts with these tests’ results. There were no statistically significant changes in hemoglobin, hematocrit, platelets, global leukocyte, neutrophils, basophils, eosinophils, typical lymphocytes, atypical lymphocytes, and monocytes counts between infected and noninfected individuals. Also, there was no correlation between TST or IGRA and blood cell counts. These results suggest that blood cell counts are not LTBI markers and do not interfere in TST results or IFN-γ levels obtained by IGRA.
1. Introduction
Tuberculosis (TB) continues to be a public health problem worldwide. The disease is one of the most important causes of death from infectious diseases, and the infection with Mycobacterium tuberculosis (M. tb) is also disseminated throughout world’s population [1]. Brazil has notified the highest number of TB cases in America for the last 10 years. In 2010, there were estimated 85,000 new cases of TB, and approximately 6,700 of them occurred in Bahia state [1, 2].
The transmission occurs by inhalation of droplets containing the bacilli from pulmonary TB individuals, mainly for their household contacts (HHCs) who are the most exposed to the infection. Only 5%–10%of infected individuals develop active TB, and the remaining individuals will develop latent tuberculosis infection (LTBI). The latency represents a balance between intracellular bacilli survival and host defense [3]. However, considering that the reactivation of latent infection is related to a high percentage of cases and significantly increases the transmission, diagnosing and treating people with LTBI is crucial to control the disease worldwide [4].
The most common test to detect LTBI is the tuberculin skin test (TST) that basically consists in measuring the delayed-type hypersensitivity reaction to purified protein derivate (PPD). However, this test has a low specificity due to the possibility of cross-reactivity to Bacillus Calmette-Guérin (BCG) or exposure to nontuberculous mycobacterial strains from the environment [5–7]. Detection of surrogate markers that allow the infection diagnosis has been pursued. Recently, a new sensitive and more specific test that has been developed to diagnose the infection is known as interferon-gamma release assays (IGRAs). This blood-based method evaluates the T-cell response to bacilli antigens, including ESAT-6, CFP-10, and TB7.7 [6, 8].
It is well documented that HIV-positive individuals have an increased risk of developing active TB, and HIV infection may have some influence on IGRA’s performance [9, 10]. On the other hand, it has been suspected that some exposed individuals take longer to be infected by M. tb compared to others despite similar exposure levels. It is probably associated to the possibility that the innate immunity may clear the infection before the induction of the acquired T-cell response [11]. Also, many hematological abnormalities, such as leucocytosis, monocytosis, or anemia, have been observed in TB patients, especially in severe disease, and may be valuable markers to diagnosis [12–14]. However, there is still a lack of available information about the relation between the hematological status and the infection occurrence after certain exposure to pulmonary TB patients (index cases). In addition, the new IGRA test is based on the IFN-γ release by lymphocytes, which depends on a complex cell interaction. Thus, this current study aim is to study possible hematological changes in household contacts that may be related to LTBI development and their influence upon IFN-γ levels.
2. Materials and Methods
2.1. Survey Population
Eighty-eight TB household contacts were identified from thirty-eight patients with pulmonary tuberculosis (index cases). TB patients were recruited from Hospital Especializado Octávio Mangabeira (HEOM), Salvador, Bahia, Brazil, from March to August of 2007. All participants signed a formal written consent, approved by the Ethics Committee for Human Research, CEP-FIOCRUZ number 92/2006. All TB index cases (ICs) and household contacts (HHCs) were evaluated at baseline with standardized questionnaires, and HHCs were submitted to TST and chest radiograph. Blood samples for hematologic tests, IFN-γ assays, and HIV screening were obtained from all donors at study entry. Household contacts were defined as individuals who (1) had lived with the index case for at least 15 days, (2) had apparent clinical health, and (3) had normal chest radiograph. Exclusion criteria were active TB in the past and HIV seropositivity. According to the TST results, they were classified into two groups: TST-negative and TST-positive. Individuals with induration ≥10 mm were considered infected (TST-positive).
2.2. Tuberculin Skin Test (TST)
After all blood samples were collected for the hematological tests and IGRA, a TST was carried out by the Mantoux procedure with 2TU of RT23 purified protein derivate (RT23 PPD) (Staten Serum Institute, Copenhagen, Denmark). Reading was performed after 72 hours using the ball-point method [15].
2.3. Hematological Tests
Peripheral venous blood samples were collected in tubes containing ethylenediamine tetra acetic acid (EDTA). Hematological tests were carried out using commercially available kits and done in the lab of HEOM using routine laboratory process.
2.4. Cell Culture and IFN-γ Levels
The IGRA used was a whole blood assay, the QuantiFERON-TB Gold In-Tube kit (QuantiFERON-TB Gold In-Tube, Cellestis Ltd., Carnegie, Australia), performed according to the manufacturer’s recommendations. Cells were cultured in vitro with specific antigens: ESAT-6, CFP-10, and TB7.7. Samples were then incubated for 16–24 hours, centrifuged, and its supernatant were collected and stored in −80°C until use. IFN-γ levels in culture supernatants were assessed by ELISA. A positive test was defined as IFN-γ blood concentration ≥0.35 international units (IUs)/mL [16].
2.5. Statistical Analysis
Correlations were established by Mann Whitney U test. Statistical differences were considered significant when p < 0.05. All statistical tests and graphs were performed by GraphPad Prism 5.0 (GraphPad software, San Diego, CA).
3. Results
3.1. Household Contacts’ Characteristics
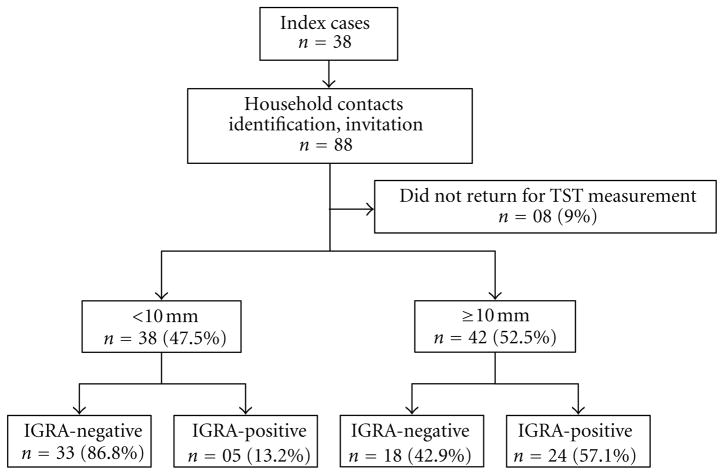
Out of 38 TB pulmonary index cases, 88 household contacts were identified, in an average of 2.31 contacts per index case. Thirty-eight HHCs were considered uninfected (TST-negative), while 42 were considered infected (TST-positive) with the TST conversion. Eight HHCs were excluded from analysis because they did not return for TST measurement. Agreement between the TST and IGRA was 57.1%. Out of the 42 TST-positive individuals, 24 had also a positive IGRA test (Figure 1).
Figure 1.
Flow chart showing the distribution of TST and IGRA result. TST: tuberculin skin test; IGRA: interferon-gamma release assay.
Among all contacts, 56 (63.64%) were women. The mean ± SD age was 2.8 ± 1.8years for 0–5 years old individuals, 11.4 ± 3.3 for 6–15 years old ones, and 36.1 ± 13.9 for those who were older than 15 years. Most HHCs were mulatto (34.1%) or black (50%). BCG scar was present in 62 HHCs (70.4%). In relation to the number of people/home, 39 (44.3%) HHCs lived in a house with one to three people, 28 (31.8%) with four to five people, and 21 (23.9%) lived with six or more people. Seventy-six HHCs (86.4%) had no previous contact when traced, and the higher mean exposure time was found in 12 HHCs with exposure time ≥7 months. Most HHCs were first-degree relatives of the index case (68.9%) (Table 1).
Table 1.
Demographic characteristics of household contacts of pulmonary tuberculosis patients included in the study (n = 88).
| Characteristic | n (%) | Mean ± SD |
|---|---|---|
| Age | — | 28.3 ± 16.9 |
| Gender | ||
| Male | 32 (36.4) | — |
| Female | 56 (63.6) | — |
| Self-definition | ||
| White | 11 (12.5) | — |
| Mulatto | 30 (34.1) | — |
| Black | 44 (50.0) | — |
| Refused to classify | 03 (3.4) | — |
| BCG scar presence | ||
| Yes | 62 (70.4) | — |
| No | 26 (29.6) | — |
| Index case first-degree relative | ||
| Yes | 60 (68.2) | — |
| No | 28 (31.8) | — |
| Previous contact tracing | ||
| Yes | 12 (13.6) | — |
| No | 76 (86.4) | — |
| Exposure time | ||
| 0–1 month | — | 0.85 ± 0.34 |
| 2–6 months | — | 3.65 ± 1.56 |
| ≥7 months | — | 12 |
| Household per home | ||
| 1–3 | 39 (44.3) | — |
| 4–5 | 28 (31.8) | — |
| ≥6 | 21 (23.9) | — |
BCG: Bacille Calmette-Guérin; SD: Standard deviation.
3.2. Hematological Profile
Comparisons of hematology tests’ values are shown in Table 2 for both TST-negative and TST-positive and in Table 3 for both IGRA-negative and IGRA-positive individuals, respectively. Comparing the hematology results of 80 TST-negative and TST-positive individuals, the median values were similar for hemoglobin, hematocrit, and platelets. Global leukocyte, neutrophils, basophils, eosinophils, typical lymphocytes, atypical lymphocytes, and monocytes counts were also similar for TST-negative and TST-positive (Table 2).
Table 2.
Hematological tests and TST result. Median and range of the values for hematological tests and TST result of household contacts (n = 80).
| Variable | TST < 10 mm | TST ≥ 10 mm | P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Hemoglobin (g/dL) | 3.80 | 10–19.8 | 13.70 | 11.6–15.8 | 0.686 |
| Hematocrit (%) | 39.70 | 33.5–59.9 | 40.60 | 34.5–87.4 | 0.992 |
| Platelets (mm3) | 289.000 | 169.000–532.000 | 305.500 | 172.000–441.000 | 0.458 |
| Global (mm3) | 6.950 | 2.200–13.600 | 6.450 | 4.100–15.900 | 0.372 |
| Neutrophils (mm3) | 3.743 | 1.204–8.094 | 3.558 | 1.377–7.204 | 0.689 |
| Basophils (mm3) | 26.35 | 0–228 | 22.20 | 0–206 | 0.693 |
| Eosinophils (mm3) | 192.30 | 0–1.406 | 290.50 | 0–1.919 | 0.221 |
| Typical lymphocytes (mm3) | 2.424 | 0.240–5.572 | 2.147 | 0.806–13.106 | 0.058 |
| Atypical lymphocytes (mm3) | 0 | 0–17.50 | 0 | 0–59.85 | 0.630 |
| Monocytes (mm3) | 536.20 | 65–2.033 | 522.50 | 153–1.113 | 0.859 |
Mann Whitney U test. TST: tuberculin skin test.
Table 3.
Hematological tests and IGRA result. Median and range of the values for hematological tests and IGRA result among household contacts of patients recently diagnosed as pulmonary TB (n = 88).
| Variable | IGRA-negative | IGRA-positive | P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Hemoglobin (g/dL) | 13.55 | 10.5–19.8 | 13.85 | 10–15.6 | 0.301 |
| Hematocrit (%) | 39.45 | 31.1–87.4 | 40.75 | 34.5–45.9 | 0.184 |
| Platelets (mm3) | 293.500 | 169.000–532.000 | 302.500 | 172.000–441.000 | 0.878 |
| Global (mm3) | 6.950 | 2.200–13.600 | 6.650 | 4.500–15.900 | 0.467 |
| Neutrophils (mm3) | 3.882 | 1.204–8.100 | 3.562 | 1.472–5.928 | 0.570 |
| Basophils (mm3) | 26.95 | 0–228 | 17.90 | 0–126 | 0.217 |
| Eosinophils (mm3) | 206.9 | 0–1.704 | 306.9 | 0–1.919 | 0.178 |
| Typical lymphocytes (mm3) | 2.403 | 868–9.520 | 2.191 | 1.093–3.672 | 0.190 |
| Atypical lymphocytes (mm3) | 0 | 0–50 | 0 | 0–18 | 0.989 |
| Monocytes (mm3) | 542.0 | 65–2.033 | 505.70 | 153–1.113 | 0.280 |
Mann Whitney U test. IGRA: interferon-gamma release assay.
Similar to TST-negative and TST-positive comparisons, there were no significant differences in the median values for hemoglobin, hematocrit, platelets, global leukocyte, neutrophils, basophils, eosinophils, typical lymphocytes, atypical lymphocytes, and monocytes counts between IGRA-negative and IGRA-positive individuals (Table 3).
3.3. Neutrophil-Lymphocyte and Monocyte-Lymphocyte Ratios
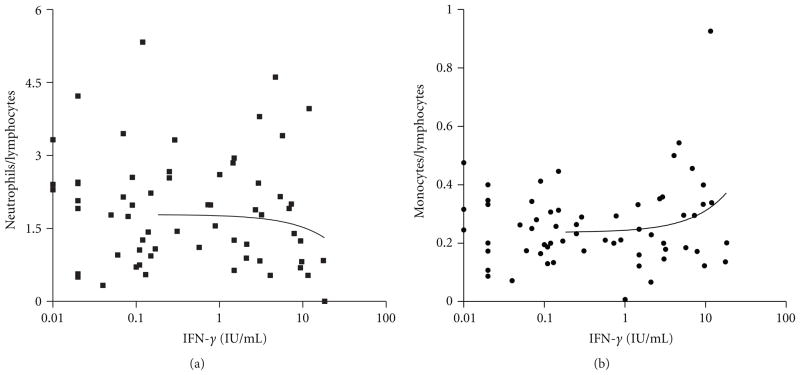
Comparing neutrophil-lymphocyte (N/L) and monocyte-lymphocyte (M/L) ratios, which are considered sensitive detectors for tuberculosis infection [17], to IFN-γ production levels, there were no linear correlations between IFN-γ levels and these ratios (Figures 2(a) and 2(b)). Apparently, for higher numbers of neutrophils in relation to lymphocytes, there is a slight reduction of IFN-γ levels detected by IGRA. On the other hand, IFN-γ levels increased, but not significantly, when M/L ratio is higher.
Figure 2.
Correlation between IFN-γ production (IGRA test) and ratios. (a) Neutrophils/lymphocytes ratio; r2 =0 .009; P = 0.3 (ns). (b) Monocytes/lymphocytes ratio, r2 = 0.04; P = 0.06 (ns). IGRA: interferon-gamma release assay.
3.4. Correlation between IFN-γ Production and Blood Cells
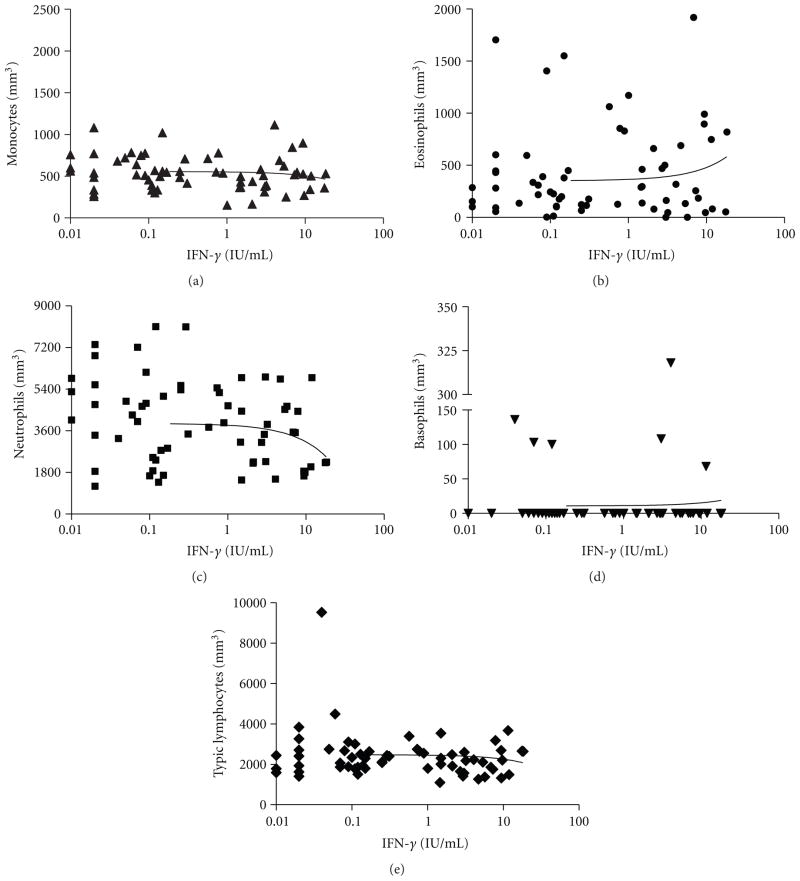
To determine the correlation between IFN-γ levels and cell counts, we individually compared each cell count to IFN-γ production levels. There were no significant differences in monocytes, eosinophils, basophils, and typical lymphocytes counts compared to different IFN-γ production (Figures 3(a), 3(b), 3(d), and 3(e)). However, at higher amounts of IFN-γ, the number of neutrophils slightly decreased. Thus, there was a negative linear correlation between IFN-γ production and neutrophils counts, but it does not reach statistical significance (Figure 3(c)).
Figure 3.
Correlation between IFN-γ production (IGRA test) and blood cell counts (mm3). (a) Monocytes, r2 = 0.005; P = 0.521 (ns). (b) Eosinophils, r2 = 0.014; P = 0.260 (ns). (c) Neutrophils, r2 = 0.03; P = 0.103; (ns). (d) Basophils, r2 = 0.001; P = 0.722 (ns). (e) Typical lymphocytes, r2=0.005; P = 0.482 (ns).
4. Discussion
This is the first study that evaluated the correlation between hematological profile, TST, and IGRA in latent tuberculosis infected individuals. Different authors have compared IGRA test to TST in LTBI diagnosis in household contacts, but no one has analyzed the influence of the hematology in these tests.
As IGRA is based on the stimulation of sensitized T-lymphocytes followed by measurement of interferon-γ produced by CD4 T-cells, we excluded HIV-positive individuals from this study. Recent studies have shown that there is a strong correlation between low CD4 T-cell count and a low mitogen response, and it has implications for LTBI diagnosis. In patients with low CD4 cell count, there was an increased proportion of both indeterminate and false negative IGRA results [10, 18, 19]. Furthermore, our objective was to identify any hematological change directly related to LTBI infection in household contacts and possible influence on IFN-γ levels.
It was expected to find no great differences in peripheral blood. At a very early phase of infection, there is a formation of just a few bacilli and granuloma. So, there are no specific circulating blood cells. Some changes were observed in circulating blood cells during disease. It has been demonstrated that hematological abnormalities are detected in severe pulmonary and in disseminated TB. Anemia, monocytosis, basophilia, leukocytosis, leucopenia, and “leukemoid reactions” are some of these abnormalities found [12–14, 20]. Otherwise, a recent study demonstrated that many patients with TB presented normal blood inflammatory markers [21]. So, it is possible to consider that in a very initial phase of M. tb infection, the blood cells profile may not reflect the characteristical changes that appear during the infection progress to disease [17]. Our study is in agreement with this idea. There were no significant differences between hematology data by comparing either TST-negative versus TST-positive or IGRA-negative versus IGRA-positive individuals. This result suggests that the hematological profile has no interference in both TB infection diagnostic tests.
Some studies have shown that the N/L and M/L ratios are reliable detectors of tuberculosis infection [17, 22]. We did not find any relation between these ratios and IFN-γ levels obtained by IGRA, despite a slightly negative influence of neutrophils in IFN-γ amounts. Apparently, IFN-γ increased in individuals with high M/L ratios.
As neutrophils showed a negative effect on IFN-γ release, they may be important in TB infection control. These cells are persistently recruited and accumulated in the sites of infection, with a T-cell-independent peak on the first day and a T-cell-dependent peak after 8–15 days and lasting until the end of the infection [23]. However, neutrophil protective role in host response to mycobacteria remains unclear. Multiple functional roles have been suggested, like alternative antigen presentation for T-cells, release of chemokines, and induction of granuloma formation [24–28]. There is no consensus about neutrophil antimicrobial activity. Some in vitro studies have demonstrated that neutrophils may be activated by soluble mycobacterial antigens and are capable of controlling mycobacteria growth in cultures [29, 30]. Other studies have evidenced a cell-cell cooperation of neutrophils and macrophages. It was shown that neutrophil components increased in vitro antimycobacterial activity of peritoneal macrophages [31]. Recently, it has been demonstrated that apoptotic neutrophils may be phagocytized by macrophages infected with mycobacteria, and thereby these macrophages acquire neutrophil antimicrobial peptides [32]. In contrast, the inability of human neutrophils to kill M. tb independently of cytokine activation was reported and also that the M. tb bacilli were not found in association with neutrophils [23, 33].
A recent work demonstrated an inverse relation between M. tb infection risk and peripheral neutrophil count. African TB contacts were more susceptible to develop the infection because they had lower neutrophil counts and lower serum concentrations of HNP1-3 and lipocalin 2 than South Asian and white individuals [11]. In our study, we did not observe significant differences in neutrophil counts related to TST and IGRA results, and it was not related to ethnicity. It is known that both IFN-γ and IL-17 producing T-cells are induced in mycobacterial infection and that IL-17 is an active recruiter of neutrophils to infectious foci. It has been shown that IFN-γ controls the induction and proliferation of IL-17 producing cells [34]. Maybe IL-17 levels and neutrophil counts are increased in the acute phase of infection and that an increased IFN-γ production may limit the expansion and production of IL-17 producing cells in periphery and thereby decrease neutrophils counts in a latter phase.
Although there was no correlation between TST or IGRA and blood cell counts, these results should be interpreted carefully. As there is no gold standard for the diagnosis of LTBI, the TST is used as a standard diagnostic tool to assess TB infection in Brazil. TST may have a low specificity among BCG-vaccinated persons [35] as in this study population. However, we previously demonstrated in another study using the same population that prior BCG vaccination did not influence the outcome of TST results [36]. Furthermore, both tests do not discriminate recent from past TB infection. Thus, TB infection is not uncommon among people who live in high-transmission setting such as Salvador, Brazil. It is possible that the same study participants were exposed to M. tb in the past and that these results may not reflect a recent infection, contributing to the lack of correlation between TST or IGRA and blood cells count.
Finally, the effect of LTBI on peripheral blood cells turnover, especially lymphocytes and monocytes, has been hampered by the lack of data in the literature. The large variation in T-cell turnover was reported in HIV infection. In this study, it was postulated that decreased production and peripheral turnover cells cause T-helper cell depletion [37]. The clinical consequences of increased cell turnover could be measured by anemia and hypermetabolism leading to fatigue, weakness, weight loss, and anorexia. No clinical signs were reported in this LTBI population. Thus, we believe that the blood cells turnover rate can be restricted to the site of infection and might be relevant in progression to TB disease; however, this cannot be detected in peripheral blood of infected individuals.
5. Conclusion
In summary, there was no significant interference of hemoglobin, hematocrit, and platelets values, as well as monocyte, eosinophil, basophil, lymphocyte, and neutrophil counts on TST or IFN-γ production. Neutrophil-lymphocyte and monocyte-lymphocyte ratios were not related to IFN-γ levels. It is not known yet if neutrophils have direct bactericidal or other immunological functions in mycobacterium infection. The negative linear correlation between INF-γ levels and neutrophil counts supports that IFN-γ increasing levels are related to neutrophil decreasing counts. In conclusion, blood cell counts are not LTBI markers and do not influence TST or IFN-γ production in LTBI.
Acknowledgments
The authors acknowledge Raildes Maria do Espírito Santo from HEOM laboratory for her technical assistance with hematological tests. They also thank EBMSP students for their contribution with medical questionnaire interviews. Financial support was provided by NIH Fogarty International Center 5U2RTW 006885 and the Bahia State Research Support Foundation (FAPESB).
Footnotes
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Conflict of Interests
None of the authors of this paper have a conflict of interests.
References
- 1.WHO. Global Tuberculosis Control: WHO Report 2011. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 2.Ministério da Saúde, Secretaria de Políticas de Saúde Brazil. Ministério da Saúde; Brasilia, Brazil: DATASUS. http://http://dtr2004.saude.gov.br/sinanweb/tabnet/dh?sinannet/tuberculose/bases/tubercbrnet.def. [Google Scholar]
- 3.Kaufmann SHE. Tuberculosis: back on the Immunologists’ Agenda. Immunity. 2006;24(4):351–357. doi: 10.1016/j.immuni.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Arend SM, Engelhard ACF, Groot G, et al. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clinical and Diagnostic Laboratory Immunology. 2001;8(6):1089–1096. doi: 10.1128/CDLI.8.6.1089-1096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso FLL, Antas PRZ, Milagres AS, et al. T-cell responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 in Brazilian tuberculosis patients. Infection and Immunity. 2002;70(12):6707–6714. doi: 10.1128/IAI.70.12.6707-6714.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunst H. Diagnosis of latent tuberculosis infection: the potential role of new technologies. Respiratory Medicine. 2006;100(12):2098–2106. doi: 10.1016/j.rmed.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Pais TF, Silva RA, Smedegaard B, Appelberg R, Andersen P. Analysis of T cells recruited during delayed-type hypersensitivity to purified protein derivative (PPD) versus challenge with tuberculosis infection. Immunology. 1998;95(1):69–75. doi: 10.1046/j.1365-2567.1998.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection: an interferon-γ-based assay using new antigens. American Journal of Respiratory and Critical Care Medicine. 2004;170(1):59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 9.Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. The New England Journal of Medicine. 2004;350(20):2060–2115. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 10.Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon-γ test. Respiratory Research. 2006;7:article 56. doi: 10.1186/1465-9921-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. Journal of Clinical Investigation. 2007;117(7):1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glasser RM, Walker RI, Herion JC. The significance of hematologic abnormalities in patients with tuberculosis. Archives of Internal Medicine. 1970;125(4):691–695. [PubMed] [Google Scholar]
- 13.Morris CDW, Bird AR, Nell H. The haematological and biochemical changes in severe pulmonary tuberculosis. Quarterly Journal of Medicine. 1989;73(272):1151–1159. [PubMed] [Google Scholar]
- 14.DiBella NJ, Buchanan BD, Koontz CH. Disseminated atypical tuberculosis antedating the cliical onset of neoplasia. Cancer. 1977;40(3):1276–1279. doi: 10.1002/1097-0142(197709)40:3<1276::aid-cncr2820400342>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.ATS. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC. American Journal of Respiratory and Critical Care Medicine. 2000;161(4 part 2):S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 16.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recommendations and Reports. 2005;54(15):49–55. [PubMed] [Google Scholar]
- 17.Smithburn KC, Sabin FR, Hummel LE. Haematological studies in experimental tuberculosis: variations in the blood cells of rabbits inoculated with cultures differing in virulence. American Review of Tuberculosis. 1937;36:673–691. [Google Scholar]
- 18.Raby E, Moyo M, Devendra A, et al. The effects of HIV on the sensitivity of a whole blood IFN-γ release assay in Zambian adults with active tuberculosis. PLoS ONE. 2008;3(6):Article ID e2489. doi: 10.1371/journal.pone.0002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talati NJ, Seybold U, Humphrey B, et al. Poor concordance between interferon-γ release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infectious Diseases. 2009;9:article 15. doi: 10.1186/1471-2334-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SW, Kang YA, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. Journal of Korean Medical Science. 2006;21(6):1028–1032. doi: 10.3346/jkms.2006.21.6.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breen RAM, Leonard O, Perrin FMR, et al. How good are systemic symptoms and blood inflammatory markers at detecting individuals with tuberculosis? The International Journal of Tuberculosis and Lung Disease. 2008;12(1):44–49. [PubMed] [Google Scholar]
- 22.Doan CA, Bierbaum O. Studies with artificial fever in experimental tuberculosis. The Ohio Journal of Science. 1936;36(3):117–122. [Google Scholar]
- 23.Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infection and Immunity. 2000;68(2):577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatability complex class II expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89(11):4128–4135. [PubMed] [Google Scholar]
- 25.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interferon gamma modulates the expression of neutrophil-derived chemokines. Journal of Investigative Medicine. 1995;43(1):58–67. [PubMed] [Google Scholar]
- 26.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. Journal of Immunology. 2001;167(5):2538–2546. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 27.Riedel DD, Kaufmann SHE. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infection and Immunity. 1997;65(11):4620–4623. doi: 10.1128/iai.65.11.4620-4623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seiler P, Aichele P, Bandermann S, et al. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. European Journal of Immunology. 2003;33(10):2676–2686. doi: 10.1002/eji.200323956. [DOI] [PubMed] [Google Scholar]
- 29.Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. Journal of Infectious Diseases. 1987;156(6):985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- 30.Jones GS, Amirault HJ, Andersen BR. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. Journal of Infectious Diseases. 1990;162(3):700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- 31.Silva MT, Silva MNT, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microbial Pathogenesis. 1989;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 32.Tan BH, Meinken C, Bastian M, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. Journal of Immunology. 2006;177(3):1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 33.Denis M, Andersen BR. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. Journal of Infectious Diseases. 1991;163(4):919–920. doi: 10.1093/infdis/163.4.919. [DOI] [PubMed] [Google Scholar]
- 34.Cruz A, Khader SA, Torrado E, et al. Cutting edge: IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. Journal of Immunology. 2006;177(3):1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 35.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. The Lancet. 2000;356(9235):1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 36.Machado A, Emodi K, Takenami I, et al. Analysis of discordance between the tuberculin skin test and the interferon-gamma release assay. The International Journal of Tuberculosis and Lung Disease. 2009;13(4):446–453. [PubMed] [Google Scholar]
- 37.Sopper S, Nierwetberg D, Halbach A, et al. Impact of simian immunodeficiency virus (SIV) infection on lymphocyte numbers and T-cell turnover in different organs of rhesus monkeys. Blood. 2003;101(4):1213–1219. doi: 10.1182/blood-2002-06-1644. [DOI] [PubMed] [Google Scholar]