Abstract
Poly(anhydride-esters) were prepared from catechol, fenticlor and hexachlorophene. The molecular weights (Mw) of the polymers were typically > 10,000 Da with glass transition temperatures (Tg) ranging from 23 to 84 °C. The thermal characteristics of the polymers paralleled the melting temperatures of the chemically incorporated antiseptic molecules. The in vitro release of the chemically incorporated antiseptic molecules were monitored over a 12 week period. For comparison, the in vitro release of physically admixed antiseptic molecules were also observed. After 12 weeks, the polymers were not completely degraded with drug release ranging from less than 1 to 55 %. Sessile-drop contact angles indicated that the polymers were relatively hydrophobic, contributing to the slow polymer degradation rates.
Introduction
The use of polyanhydrides for sustained drug release in controlled drug delivery applications was first explored by Langer in the 1980’s.[1] Since then, extensive research has been conducted to yield polyanhydrides for various biomedical applications including tissue scaffolds, implantable biomaterials, and related drug delivery devices.[2–11] Polyanhydrides are biodegradable polymers that are often hydrophobic yet contain hydrolytically labile anhydride bonds such that hydrolytic degradation can be controlled by manipulation of the polymer composition.[5,8,9, 11–13] In other words, the polymer degradation rate can be controlled by using aliphatic, unsaturated, aromatic, and/or branched anhydrides to concurrently release drug molecules that are physically admixed in the polymer matrix. Polyanhydrides have excellent controlled release characteristics and degrade in vitro and in vivo to their acid counterparts as non-cytotoxic products.[1,3] The controlled, non-enzymatic degradation of polyanhydrides make them particularly advantageous for drug delivery applications.
Our laboratory previously reported the synthesis of poly(anhydride-esters) (PAE) (1) that release salicylates (2) upon hydrolytic degradation of the polymer backbone (Scheme 1).[14–16] Polymer 1 is unique in that the drug 2 is chemically incorporated into the polymer backbone, not attached as a side group nor physically admixed within the polyanhydride matrix by melting or dissolving the polymer. Thus, drug release is directly dependent on the hydrolytic cleavage of the anhydride and ester bonds. In addition, the polymeric prodrug can be directly processed into films and fibers by extrusion,[17] or microspheres by emulsion-precipitation methods.[13,18]
Scheme 1.
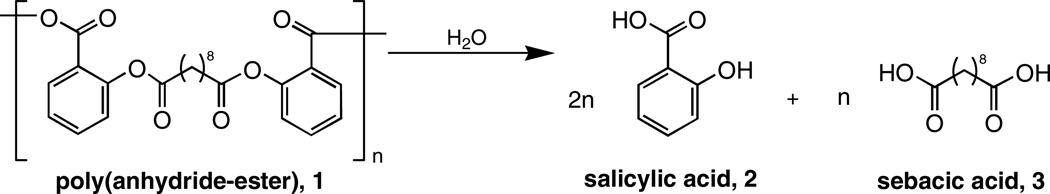
Hydrolysis of salicylate-based poly(anhydride-ester) (1).
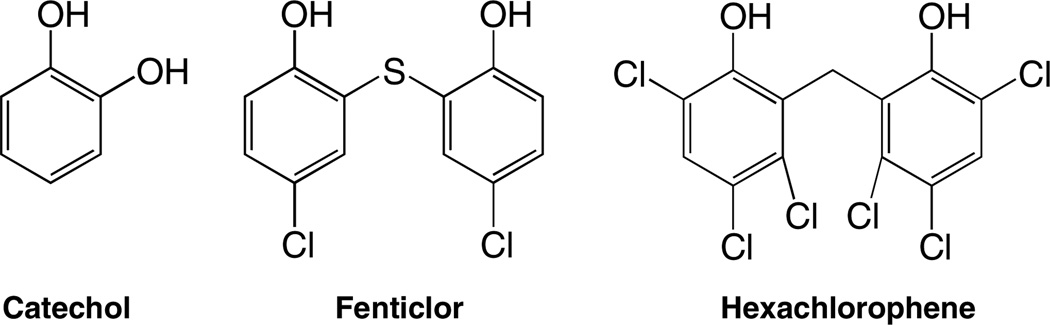
Based on recent successes with non-steroidal anti-inflammatory drugs (NSAIDs) such as salicylates, other bioactive compounds are being investigated for inclusion into polymeric backbones. The commercially utilized antiseptics shown in Scheme 2 below (catechol, fenticlor and hexachlorophene) were chosen because they are not salicylates; an alternate synthetic approach was necessary to accommodate the bisphenols.
Scheme 2.
Antiseptics selected for chemical incorporation into polymer backbones.
Antiseptic formulations are widely used in hospitals for topical and hard-surface applications to control infection and prevent nosocomial infections.[19] Multiple active chemical agents are found in these products, many which have been used for decades that include phenols for antisepsis, disinfection, and preservation. Most antiseptic agents demonstrate broad-spectrum antimicrobial activity, as compared to antibiotics that have specific intracellular targets.
Catechol is an astringent/antiseptic[20] and an important model for chemical incorporation into a polymeric backbone. Hexachlorophene and fenticlor are known antiseptics which are chemically classified as bis-phenols; bis-phenols are typically used as germicides, fungicides, algaecides, or wood preservatives.[21–23] Hexachlorophene has a strong bacteriostatic action against many gram-positive organisms (i.e., S. epidermis) and few gram-negative organisms.[24] It is widely used in medicated soaps and hand rinses such as pHisohex®. Fenticlor is known to affect metabolic activities of E. coli and S. aureus, and also used as a wood preservative.[23]
In this article, an alternate approach to incorporate bis-phenols into a polymer backbone is introduced. Previous approaches were limited to NSAIDs, specifically salicylates, that contain both a carboxylic acid and phenol, whereas the synthetic approach described herein may be applied to a broader range of di-functionalized molecules. Our longer term goal is to create polymeric forms of antiseptics that control infection via temporary coatings for topical and hard-surface applications. In this paper, the synthesis and characterization of antiseptic-derived poly(anhydrideesters) composed of catechol, fenticlor and hexachlorophene is described. The antiseptic molecules are both chemically and physically incorporated into a polymer matrix, and the in vitro drug release profiles compared.
Experimental
Materials
Hexachlorophene was purchased from ICN (Aurora, OH). Fenticlor was purchased from TCI (Portland, OR). Tetrahydrofuran (THF), pyridine, acetic anhydride, methylene chloride, and diethyl ether were purchased from Fisher (Fair Lawn, NJ). All other fine chemicals were obtained from Aldrich (Milwaukee, WI) and used as received.
Methods
Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Varian 200 MHz spectrometer. The deuterated solvent used to dissolve the samples (5–10 mg) was DMSO-d6, with the solvent as the internal reference. Infrared (IR) spectra were measured on a Thermo Nicolet/Avatar 360 FT-IR spectrometer, by depositing samples onto NaCl plates (if liquid) or solvent-casting samples from methylene chloride onto NaCl plates (if solid). Elemental analyses were provided by QTI (Whitehouse, NJ). Melting points were obtained with a Mel-Temp apparatus.
Weight-average molecular weights (Mw) were determined by gel permeation chromatography (GPC) on a Perkin-Elmer liquid chromatography system consisting of a Series 200 refractive index detector, a Series 200 LC pump, and an ISS 200 advanced sample processor. A Dell OptiPlex GX110 computer running Perkin-Elmer TurboChrom 4 software was used for data collection and processing, and to automate the analysis via Perkin-Elmer Nelson 900 Series Interface and 600 Series Link. Polymers were dissolved in methylene chloride (5 mg/mL) and filtered through 0.45 µm poly(tetrafluoroethylene) (PTFE) syringe filters (Whatman, Clifton, NJ) before elution. Samples were resolved on a Jordi divinylbenzene mixed-bed GPC column (7.8×300 mm) (Alltech Associates, Deerfield, IL) at 25 °C, with methylene chloride as eluent at a flow rate of 0.5 mL/min. Molecular weights were calibrated relative to narrow molecular weight polystyrene standards (Polysciences, Dorval, Canada) and reported as weight-averaged molecular weights (Mw) and polydispersity index (PDI).
Thermal analyses were performed on a Perkin-Elmer system consisting of Pyris 1 DSC and TGA 7 analyzers with TAC 7/DX instrument controllers. Perkin-Elmer Pyris software was used for data collection on a Dell OptiPlex GX110 computer. For differential scanning calorimetry (DSC), samples (5 mg) were heated under dry nitrogen gas. Data were collected at heating and cooling rates of 10 °C/min with a two-cycle minimum. For thermogravimetric analysis (TGA), samples (10 mg) were heated under dry nitrogen gas. Data were collected at a heating rate of 10 °C/min. Decomposition temperatures were defined as the onset of decomposition.
Solubility of the antiseptics was determined by UV measurements in PBS (pH = 7.4). UV analysis was performed on a DU Series 520 ultraviolet-visible spectrometer (Beckman Coulter, Fullerton, CA) with quartz cuvettes. Saturated solutions were calibrated against solutions of known concentrations. Sessile contact angles were measured in triplicate on prepared-polymer discs using a model 100 goniometer (Rame-Hart, Mountain Lakes, NJ) using phosphate buffer solution (pH = 7.4).
Diacid synthesis (6)
Antiseptic (5 mmol) was dissolved in a solution of THF (10 mL) and triethylamine (10 mL). Glutaric anhydride (10 mmol) was added to the reaction mixture at room temperature. The reaction was stirred for 1–3 d, and then poured over an ice-water slush (200 mL). After acidifying to pH~2 with concentrated hydrochloric acid, diacid was isolated by vacuum filtration, washed with water (3×50 mL) and dried under vacuum. Any changes to the reaction are noted below.
Catechol-glutaric diacid (6a)
Yield: 86 % (white powder).1H-NMR (DMSO-d6): δ 7.30 (s,4H,ArH), 2.63 (t,4H,CH2), 2.35 (t,4H,CH2), 1.82 (m,4H,CH2). IR (NaCl, cm−1): 3600-3000 (COOH), 1763 (C=O, acid), 1697 (C=O, ester). Elem. Anal. Calcd.: C, 56.83%; H, 5.32%. Found: C, 56.71%; H, 5.18%. Tm = 93–95 °C.
Fenticlor-glutaric diacid (6b)
Yield: 85 % (white powder).1H-NMR (DMSO-d6): δ 7.53 (d,2H,ArH), 7.45 (s,2H,ArH), 7.29 (d,2H,ArH), 2.57 (t,4H,CH2), 2.30 (t,4H,CH2), 1.82 (m,4H,CH2). IR (NaCl, cm−1): 3600-3200 (COOH), 1756 (C=O, acid), 1699 (C=O, ester), 638 (C-S, aryl sulfide), 1099 (C-Cl, aryl chloride). Elem. Anal. Calcd.: C, 51.28%; H, 3.88%; Cl, 13.76%. Found: C, 51.02%; H, 3.72%; Cl, 13.61%. Tm = 122–124 °C.
Hexachlorophene-glutaric diacid (6c)
Pyridine was used as base. Yield: 87 % (white powder).1H-NMR (DMSO-d6): δ 8.05 (s,2H,ArH), 4.65 (s,2H,CH2), 2.52 (t,4H,CH2), 2.27 (t,4H,CH2), 1.76 (m,4H,CH2). IR (NaCl, cm−1): 3650-3200 (COOH), 1773 (C=O, acid), 1707 (C=O, ester), 1105 (C-Cl, aryl chloride). Elem. Anal. Calcd.: C, 43.51.%; H, 2.84%; Cl, 33.49%. Found: C, 43.39%; H, 2.70%; Cl, 33.31%. Tm = 95–98 °C.
Monomer synthesis (7)
The diacid (6a–c) (2 g) was added to an excess of acetic anhydride (50 mL), then stirred at room temperature until an homogeneous solution was observed. The monomer (7a–c) was isolated by removing excess acetic anhydride under vacuum.
Catechol-glutaric monomer (7a)
Yield: Quantitative (pale yellow oil).1H-NMR (DMSO-d6): δ 7.32 (s,4H,ArH), 2.67 (m,8H,CH2), 2.23 (s,6H,CH3), 1.90 (m,4H,CH2). IR (NaCl, cm−1): 1829 (C=O, anhydride), 1771 (C=O, ester), 1753 (C=O, anhydride). Td = 199 °C.
Fenticlor-glutaric monomer (7b)
Yield: Quantitative (pale yellow oil).1H-NMR (DMSO-d6): δ 7.53 (d,2H,ArH), 7.45 (s,2H,ArH), 7.29 (d,2H,ArH), 2.57 (t,4H,CH2), 2.30 (t,4H,CH2), 2.23 (s,6H,CH3), 1.82 (m,4H,CH2). IR (NaCl, cm−1): 1834 (C=O, anhydride), 1779 (C=O, ester), 1763 (C=O, anhydride), 1098 (C-Cl, aryl chloride), 641 (C-S, aryl sulfide). Td = 371 °C.
Hexachlorophene-glutaric monomer (7c)
Yield: Quantitative (pale yellow oil).1H-NMR (DMSO-d6): δ 8.05 (s,2H,ArH), 4.65 (s,2H,CH2), 2.52 (t,4H,CH2), 2.27 (t,4H,CH2), 2.23 (s,6H,CH3), 1.76 (m,4H,CH2). IR (NaCl, cm−1): 1838 (C=O, anhydride), 1790 (C=O, ester), 1771 (C=O, anhydride), 1108 (C-Cl, aryl chloride). Td = 268 °C.
Polymer synthesis (8)
Monomer (7a–c) (1 g) was placed in a 2-necked round bottom flask, which was heated to 160 °C using a temperature controller (Cole Parmer) in a silicone bath under high vacuum (< 2 mmHg) for 3 h. During this time, the melt was actively stirred at ~100 rpm by an overhead stirrer (T-line Laboratory Stirrer, Talboys Engineering). The polymer (8a–c) was cooled to room temperature, dissolved in a minimal volume of methylene chloride (5 ml), and precipitated into a 20-fold excess of diethyl ether (100 ml).
Catechol-glutaric polymer (8a)
Yield: Quantitative (beige solid).1H-NMR (DMSO-d6): δ 7.30 (s,4H,ArH), 2.63 (t,4H,CH2), 2.35 (t,4H,CH2), 1.82 (m,4H,CH2). IR (NaCl, cm−1): 1829 (C=O, anhydride), 1771 (C=O, ester), 1753 (C=O, anhydride). Tg = 23 °C. Td = 361 °C. Mw = 11400, PDI = 1.5.
Fenticlor-glutaric polymer (8b)
Yield: Quantitative (beige solid). 1H-NMR (DMSO-d6): δ 7.53 (d,2H,ArH), 7.45 (s,2H,ArH), 7.29 (d,2H,ArH), 2.57 (t,4H,CH2), 2.30 (t,4H,CH2), 1.82 (m,4H,CH2). IR (NaCl, cm−1): 1834 (C=O, anhydride), 1779 (C=O, ester), 1763 (C=O, anhydride), 1098 (C-Cl, aryl chloride), 641 (C-S, aryl sulfide). Tg = 84 °C. Td = 378 °C. Mw = 10200, PDI = 1.4.
Hexachlorophene-glutaric polymer (8c)
Yield: Quantitative (beige solid). 1H-NMR (DMSO-d6): δ 8.05 (s,2H,ArH), 4.65 (s,2H,CH2), 2.52 (t,4H,CH2), 2.27 (t,4H,CH2), 1.76 (m,4H,CH2). IR (NaCl, cm−1): 1838 (C=O, anhydride), 1790 (C=O, ester), 1771 (C=O, anhydride), 1108 (C-Cl, aryl chloride). Tg = 66 °C. Td = 376 °C. Mw = 15900, PDI = 1.6.
Substrate preparation for degradation studies
For the in vitro degradation of the poly(anhydride-esters), the polymers (approximately 300 mg) were pressed into 13 mm diameter×1 mm thick discs by using an IR pellet die (International Crystal Laboratories, Garfield, NJ) with a hydraulic press (Carver, Wabash, IN). A force of 12,000 psi was applied for 10 minutes at room temperature. The same processing parameters for all the polymers were used, despite the concern that room temperature compression may yield non-homogenous films. All three polymers were compressed at room temperature (~22 °C), which was generally near or below the Tg values of the polymers (23, 66 and 84 °C). Generally, the films appeared cohesive and did not mechanically fail during the degradation process.
For substrates containing physically admixed fenticlor and hexachlorophene, the appropriate weight percent of antiseptic (56 wt.% and 64 wt.%, respectively) was added into the catechol-derived poly(anhydride-ester) (8a). The appropriate weight percentages were determined by calculating the antiseptic (4) composition within the polymer (8).
In vitro degradation studies
Hydrolytic degradation was conducted in triplicate, the polymer discs were placed in 10 mL of phosphate buffer solution (pH=7.4) to ensure sink conditions. Degradation media consisted of phosphate buffer solution (PBS) containing 0.1 M potassium hydrogen phosphate and 0.1 M potassium dihydrogen phosphate. If necessary, the pH was adjusted to 7.4 with 1.0 N sodium hydroxide. Samples were incubated at 37 °C in an orbital incubator-shaker (New Brunswick Scientific, Edison, NJ) at 60 rpm for 12 weeks (84 days). At designated time points, the buffer solution was exchanged, and the degradation media analyzed. Antiseptic release was monitored by UV [λ = 235 nm (catechol), 300 nm (fenticlor) and 305 nm (hexachlorophene)] with a DU Series 520 ultraviolet-visible spectrometer (Beckman Coulter, Fullerton, CA) with quartz cuvettes.
Results and discussion
Polymer synthesis
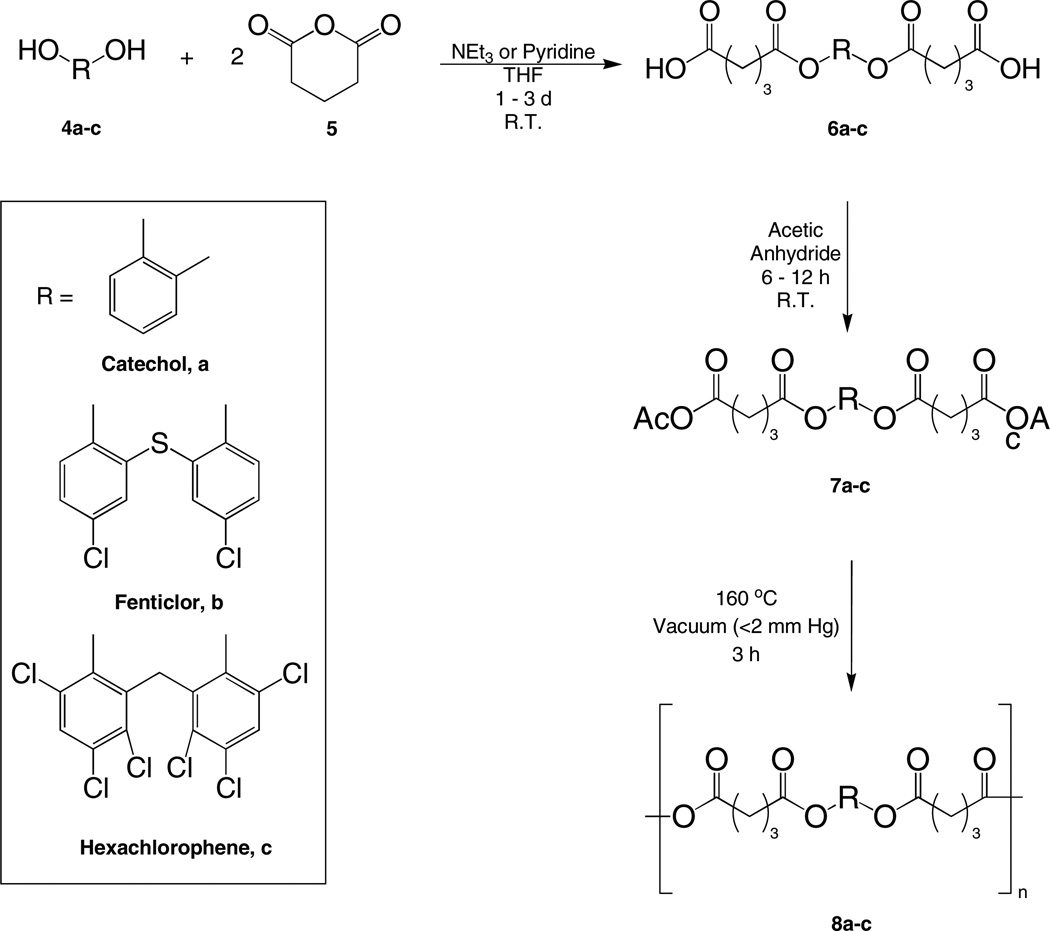
The polymers described herein were prepared by melt condensation because consistently higher molecular weight polymers were obtained using melt condensation polymerization methods relative to solution polymerization.[25] An outline of the antiseptic poly(anhydride-ester) (8) synthesis, starting from the relevant antiseptic (4a–c) is shown in Scheme 3.
Scheme 3.
Synthesis of antiseptic-based poly(anhydride-esters).
The antiseptic molecule (4a–c) was first dissolved in a mixture of THF and base (triethylamine or pyridine) to give a clear solution. The choice of base was dependent upon the antiseptic solubility in the base/THF solution. Next, glutaric anhydride (5) was added to the reaction mixture and stirred for several days at room temperature. The hydroxyl groups of the antiseptic molecule 4 reacted with the anhydride group of 5 via ring-opening reaction in the presence of base to yield the antiseptic diacid (6a–c). This method eliminates further purification, except for washing with an appropriate solvent, because of the large solubility differential between the product (6a–c) and the reaction’s potential by-products (i.e., monosubstituted antiseptic-glutaric acid). Conversions are quantitative and isolated yields are greater than 85 %. Next, 6a–c was activated with acetic anhydride at room temperature to give the mixed anhydride monomer (7a–c). Then, melt condensation of the antiseptic-glutaric monomer (7a–c) at 160 °C for 3 hours in vacuo (<2 mmHg) yielded the corresponding antiseptic poly(anhydride-ester) (8a–c). By chemically incorporating antiseptic molecules (4a–c) into a polyanhydride backbone, a high amount of drug can be integrated into the polymer. For example, 64 % by weight of polymer 8c is composed of hexachlorophene (4c).
The molecular weights obtained for these polymers were typically above 10,000 corresponding to > 20 repeat units. Higher molecular weights may be achieved by optimizing the polymerization (e.g., longer polymerization time) as noted with most melt condensation polymers. In this study, however, each monomer was polymerized for only 3 hours to maintain constant polymerization conditions. Molecular weight values for these poly(anhydride-esters) are typical for aromatic polyanhydrides synthesized by melt condensation.[26–28] The glass transition temperature (Tg) for these polymers ranged from 23 °C to 84 °C, with no indication of a melting temperature (Tm). Molecular weight and thermal properties are summarized in Table 1. Catechol-derived poly(anhydride-ester) (8a) gave the lowest Tg (23 °C) and the fenticlor-derived polymer (8b) yielded the highest Tg (84 °C). The onset of thermal decomposition temperatures (Td) varied by approximately 20 °C. Polymer 8a began to thermally decompose around 361 °C, 8b around 378 °C, and the onset for 8c around 376 °C.
Table 1.
Molecular weight and thermal properties of polymer 8.
| Antiseptic-based Poly(anhydride-ester) (PAE) |
Mw | Tg (°C) | Td (°C) |
|---|---|---|---|
| Catechol-PAE (8a) | 11,400 | 23 | 361 |
| Fenticlor-PAE (8b) | 10,200 | 84 | 378 |
| Hexachlorophene-PAE (8c) | 15,900 | 66 | 376 |
Polymer degradation
The chemically incorporated antiseptics (4) were anticipated to have a slower release from compressed matrices than physically admixed antiseptics because both the anhydride and ester bonds must be hydrolyzed to release the antiseptic. In contrast, the physically admixed antiseptics can diffuse from the polymer matrix without necessarily hydrolyzing chemical bonds. The catechol-derived poly(anhydride-ester) (8a) was used as a control polymer to physically incorporate fenticlor (4b) and hexachlorophene (4c) at 56 wt% and 64 wt%, respectively.
Poly(anhydride-esters) derived from fenticlor (8b) and hexachlorophene (8c) were anticipated to be relatively slow degrading polymers in aqueous media, with respect to the poly(anhydride-ester) derived from catechol (8a), due to solubility differences between the antiseptics in phosphate buffered saline (PBS). Catechol (4a) is the most soluble (461 mg/mL), followed by hexachlorophene (4c) and fenticlor (4b) at 0.14 and < 0.1 mg/mL, respectively. Sessile-drop contact angle measurements also indicated that catechol-derived polymers (8a) were the most hydrophilic and fenticlor-derived polymers were most hydrophobic (Table 2).
Table 2.
Sessile-drop contact angle (CA) measurements of antiseptic-based poly(anhydrideesters) in PBS (pH 7.4).
| Antiseptic-based Poly(anhydride-ester) (PAE) | PBS CA (°) |
|---|---|
| Catechol-PAE (8a) | 81 |
| Fenticlor-PAE (8b) | 117 |
| Hexachlorophene-PAE (8c) | 115 |
| Fenticlor (4b) physically admixed with catechol- PAE (8a) | 113 |
| Hexachlorophene (4c) physically admixed with catechol-PAE (8a) | 108 |
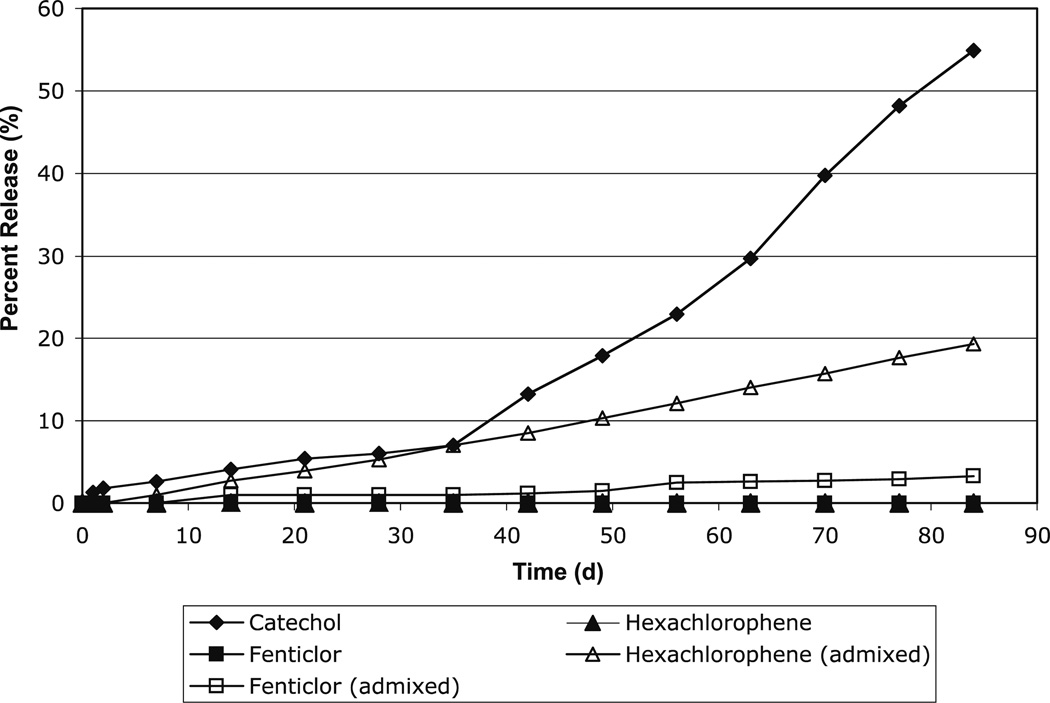
Release of the antiseptics (4) was monitored by analyzing the polymer degradation media by UV, based on the λmax for each antiseptic: 235 nm for catechol, 300 nm for fenticlor, and 305 nm for hexachlorophene. Over the 12-week period, cumulative release of the antiseptics ranged from 0 – 55 % as shown in Figure 1.
Figure 1.
Antiseptic release from polymer disks over 12 weeks at 37 °C for catechol-PAE (8a), fenticlor-PAE (8b), fenticlor admixed into 8a, hexachlorophene-PAE (8c), and hexachlorophene admixed into 8a.
As is common among polyanhydrides,[2,3,12,29] the polymers exhibited a lag time, where polymer degradation does not significantly occur during the first 1 to 3 days of incubation. Polymer 8a exhibited drug release of 55 % over the 12 weeks, while the remaining polymers (8b and 8c) exhibited no release of the chemically incorporated antiseptics. The physically admixed antiseptics exhibited moderate drug release (from 3 to 19 %).
Three effects were observed: (i) the most water-soluble drug, catechol (4), was released most quickly into the degradation media; (ii) the more hydrophobic polymers, 8b and 8c, displayed minimal drug release after the 12-week incubation; and (iii) physically admixed antiseptic molecules are released into the degradation media at a faster rate than the same molecules that are chemically incorporated into a polymer backbone. Due to the low solubility of the antiseptics (4a–c) in PBS (pH = 7.4) and hydrophobicity of the polymers (8a–c), a 12-week period was not sufficient to complete the degradation of these polymer matrices. We estimate that complete polymer degradation would occur on the month-to-year time scale.
Conclusions
A series of biodegradable polymers derived from antiseptic compounds 4 (Scheme 2) were synthesized under similar polymerization conditions. The molecular weight of the polymers ranged from 10200 to 15900 with glass transition temperatures ranging from 23 to 84 °C. In vitro degradation of the antiseptic-derived poly(anhydride-esters) was conducted over a 12-week period in PBS (pH = 7.4). For comparison, the antiseptics were chemically incorporated into a polymer backbone (as outlined in Scheme 3) and also physically admixed into a polyanhydride matrix. The least hydrophobic catecholderived poly(anhydride-ester) (8a) released 55 % of the total catechol incorporated into the polymeric backbone over the 12-week period. Conversely, the more hydrophobic fenticlor- and hexachlorophene-derived poly(anhydride-esters) (8b and c, respectively) did not release fenticlor and hexachlorophene into the degradation media at detectable levels. When physically admixed into polymer 8a, fenticlor and hexachlorophene were released at low levels (3 and 19 %, respectively) after 12 weeks of incubation.
Based upon these results, the antiseptic-derived polymers may be useful as slow-release coatings to control infection in the surgical suite and prevent nosocomial infections. Currently, these antiseptic-derived poly(anhydride-esters) are being tested against gram-positive and gram–negative organisms to assess their ability to prevent biofilm formation and subsequent infections.
Acknowledgements
The authors thank the National Institutes of Health (DE 13205) for financial support.
References
- 1.Langer R. Piskin E, Hoffmann A, Nijhoft M, editors. Biopolymers in controlled release systems. Polymer biomaterials. 1986 [Google Scholar]
- 2.Rosen H, Chang J, Wnek G, Linhardt R, Langer R. Biomaterials. 1983;4:131. doi: 10.1016/0142-9612(83)90054-6. [DOI] [PubMed] [Google Scholar]
- 3.Leong K, D'Amore P, Maletta M, Langer R. J Biomed Mater Res. 1986;20:51. doi: 10.1002/jbm.820200106. [DOI] [PubMed] [Google Scholar]
- 4.Park Y, Nam K, Ha S, Pai C, Chung C, Lee S. J Control Rel. 1997;43:151. [Google Scholar]
- 5.Guo W, Huang K, Tang R, Chi Q. Polymer. 2004;45:5743. [Google Scholar]
- 6.Quick D, Macdonald K, Anseth K. J Control Rel. 2004;97:333. doi: 10.1016/j.jconrel.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Kim G, Richards A, Cima M, Langer R. Polymer. 2004;45:3157. [Google Scholar]
- 8.Shikanov A, Vaisman B, Krasko M, Nyska A, Domb A. J Biomed Mater Res: Part A. 2004;69A:47. doi: 10.1002/jbm.a.20101. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G, Aponte M, Ramirez C. Polym Mater Sci Eng. 2003;89:618. [Google Scholar]
- 10.Thanos C, Liu Z, Goddard M, Reineke J, Bailey N, Cross M, Burrill R, Mathiowitz E. J Pharm Sci. 2003;92:1677. doi: 10.1002/jps.10446. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Fiegel J, Krauland E, Hanes J. Biomaterials. 2002;23:4425. doi: 10.1016/s0142-9612(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 12.Domb A, Gallardo C, Langer R. Macromolecules. 1989;22:3200. [Google Scholar]
- 13.Yeagy B, Prudencio A, Schmeltzer R, Uhrich K, Cook T. J Microencapsulation. doi: 10.1080/02652040600776481. (in press) [DOI] [PubMed] [Google Scholar]
- 14.Anastasiou T, Uhrich K. J Polym Sci Part A: Polym Chem. 2003;41:3667. [Google Scholar]
- 15.Erdmann L, Uhrich K. Biomaterials. 2000;20:1941. doi: 10.1016/s0142-9612(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 16.Prudencio A, Schmeltzer R, Uhrich K. Macromolecules. 2005;38:6895. doi: 10.1021/ma048051u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner E, Twardowski T, Whitaker K, Uhrich K. Proc ASME Intern Mech Eng Congress. 2003;44130:1. [Google Scholar]
- 18.Harten R, Svach D, Schmeltzer R, Uhrich K. J Biomed Mater Res: Part A. 2005;72A:354. doi: 10.1002/jbm.a.30184. [DOI] [PubMed] [Google Scholar]
- 19.Larson E. Apic infection control & applied epidemiology: Principles & practices. St. Louis: Mosby-Year Book; 1996. [Google Scholar]
- 20.Hugo W. Disinfection mechanisms. Oxford, England: Blackwell Science; 2000. [Google Scholar]
- 21.Diarra M, Lavoie M, Jaques M, Darwish I, Dolence J, Ghosh A, Ghosh M, Miller M, Malouin F. Antimicro Agents Chemother. 1996;40:2610. doi: 10.1128/aac.40.11.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y, Lim J, Yeo J, Bang C, Kim S, Lee T, Oh S, Moon Y, Lee C. J Antibiotics. 1996;49:499. doi: 10.7164/antibiotics.49.499. [DOI] [PubMed] [Google Scholar]
- 23.Goswami J, Liu J, Doyle A. US PN 550416. 2000 [Google Scholar]
- 24.Darouiche R, Raad I. US PN 84595. 1997 [Google Scholar]
- 25.Schmeltzer R, Anastasiou T, Uhrich K. Polym Bull. 2003;49:441. doi: 10.1007/s00289-003-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campo C, Anastasiou T, Uhrich K. Polym Bull. 1999;42:61. [Google Scholar]
- 27.Anastasiou T, Uhrich K. Macromolecules. 2000;33:6217. [Google Scholar]
- 28.Domb A, Langer R. J Polym Sci, Part A: Polym Chem. 1987;25:3373. [Google Scholar]
- 29.Whitaker-Brothers K, Uhrich K. J Biomed Mater Res. 2004;70A:309. doi: 10.1002/jbm.a.30083. [DOI] [PubMed] [Google Scholar]