Abstract
A culture of Saccharomyces cerevisiae M30 entrapped in loofa-reinforced alginate was used for continuous ethanol fermentation in a packed-bed reactor with initial sugar concentrations of 200-248 g/L. Maximum ethanol productivity of 11.5 g/(L·h) was obtained at an ethanol concentration of 57.4 g/L, an initial sugar concentration of 220 g/L and a dilution rate (D) of 0.2 h-1. However, a maximum ethanol concentration of 82.1 g/L (productivity of 9.0 g/(L·h)) was obtained at a D of 0.11 h-1. Ethanol productivity in the continuous culture was 6-8-fold higher than that in the batch culture. Due to the developed carrier’s high biocompatibility, high porosity, and good mechanical strength, advantages such as cell regeneration, reusability, altered mechanical strength, and high capacity to trap active cells in the reactor were achieved in this study. The immobilized cell reactor was successfully operated for 30 days without any loss in ethanol productivity. The average conversion yield was 0.43-0.45 throughout the entire operation, with an immobilization yield of 47.5%. The final total cell concentration in the reactor was 37.3 g/L (17.7 g/L immobilized cells and 19.6 g/L suspended cells). The concentration of suspended cells in the effluent was 0.8 g/L.
Keywords: ethanol, loofa, alginate, immobilization, continuous
INTRODUCTION
Ethanol demand has continued to grow, both as an alternative fuel and as a petroleum fuel extender because of gasoline shortages. Ethanol production from renewable carbohydrate materials has attracted worldwide interest, and much research has focused on ethanol production using immobilized viable microbial cells in continuous systems. Continuous fermentation using immobilized cell (IC) carriers offers many advantages, such as higher productivity, relative ease of product separation, biocatalyst reuse, and high productivity. The immobilization of yeast cells inside the porous support material enhances cell stability (5).
Fermentative ethanol production by S. cerevisiae immobilized within alginate beads has been found to have a higher productivity than in a batch system (8). However, some limitations, such as gel degradation, low physical strength and severe mass transfer restrictions, were often observed when using alginate-based carriers. Dias et al. (3) reported a lower specific growth rate of immobilized yeast cells due to oxygen diffusion problems caused by entrapment in a Ba-alginate gel. In contrast, the loofa sponge was shown to be an excellent cell carrier for ethanol fermentation by flocculating the cells in a bubble column with an external loop for the recirculation of the fermentation broth (11). Its strength, abundance, low price, biodegradability, and natural origin are of great interest. However, a low-shear environment and a large aggregate of cells were required during the application of the loofa sponge to prevent excessive cell sloughing from the carrier (9, 10). In our previous study, immobilized yeast cells entrapped in loofa-reinforced alginate matrix (ALM) carriers were successfully developed for repeated batch ethanol fermentations in a 500-mL shake flask system (13). The carriers were simply fabricated by gelating a peripheral loofa sponge that had been previously dipped in an alginate/cell mixture. An ALM with dimensions of 9×9×3 mm3, which was comparable to a 2-mm-diameter alginate bead, was found to be effective for yeast immobilization. Moreover, after storage for 4 months, the ALM-immobilized cell culture was still active, and the stability of IC cultures in the ALM was higher than that of the suspended culture (13). The ALM structure proved to be more porous and less dense than a typical alginate bead, allowing for better internal mass-transfer diffusion. The aim of present investigation is to use an ALM carrier with dimensions of 20×20×3 mm3 for continuous ethanol fermentation in a packed-bed reactor (PBR) and evaluate its performance for long-term operation.
MATERIALS AND METHODS
Microorganisms and media
Saccharomyces cerevisiae M30 was selected for this study because of its high efficiency in ethanol production from molasses at high temperatures. Starter cultures were prepared by transferring cells from stock PDA slants to 150 mL of sterilized medium followed by incubation at 33°C at 150 rpm for 20 h. The medium for the starter culture contained 0.05% ammonium sulfate and 5% inverse sugar from palm sugar at pH 5.0. Subsequently, the resulting cell suspension was concentrated by decantation and then transferred to the main culture.
Cell immobilization
Sodium alginate (3% w/v) solution was formulated by dissolving Na-alginate powder in 0.9% (w/v) NaCl solution. It was autoclaved for 5 min at 121°C and stored overnight at 4°C to facilitate deaeration. To form an alginate-cell mixture, 5 mL of cell suspension was added to 50 mL of 3% (w/v) alginate solution. Loofa sponges were cut into small thin square pieces with dimensions of 19 × 19 × 2 mm3 using scissors. To form ALMs, 2 g of sterilized thin square loofa sponges were dipped into the alginate/cell mixture. The gel carriers were transferred to 1.5 % (w/v) CaCl2 solution and left to harden in this solution with mild stirring for 15 min. The carriers were then rinsed 3 times with 0.9% (w/v) NaCl solution. The carriers were prepared under aseptic conditions, and the average ALM dimensions were 20 × 20 × 3 mm3.
Fermentations
A 1-L (Ø= 5.7 cm; height= 43.4 cm) reactor column containing an immobilized cell bed of ALM carriers was used for the study. The experimental setup for the PBR with a packed volume of 32% (v/v) of the total bed volume is shown in Fig. 1. The temperature of the system was controlled at 32 ± 1°C by passing 28°C cooling water inside the reactor jacket. Initial sugar concentrations of 200, 220, and 240 g/L were continuously fed into the bottom of the reactor at dilution rates of 0.11, 0.16, 0.20, and 0.30 h-1. Samples (5 mL) were collected every 8 hours. The samples were frozen before determining the sugar, ethanol, and cell concentrations to enable simultaneous analysis of all samples.
Figure 1.
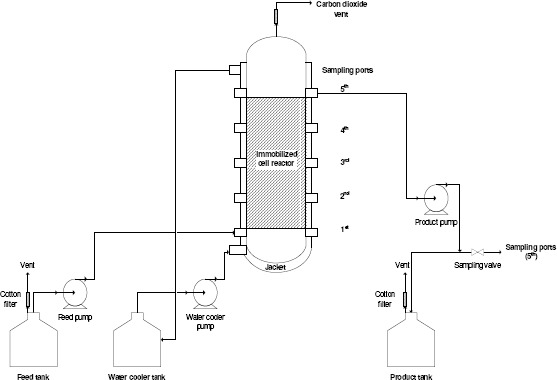
Schematic diagram of the immobilized-cell PBR.
Analytical methods
Free cell dry weight was determined from the absorbance measured at 660 nm by a UV-2450 UV-visible spectrophotometer and converted to dry cell concentration using a corresponding standard curve. For immobilized cells, a known mass of cell carriers was dissolved in 0.05 M sodium citrate. After the sponge was removed, immobilized cell concentrations were determined in a manner similar to that used for the free cells. Yeast cell viability was determined using methylene blue staining (2). The concentration of ethanol was determined using a gas chromatograph (model GC-7AG; Shimadzu, Kyoto, Japan) equipped with a flame ionization detector. To measure reducing sugar concentration, the sample solution was hydrolyzed in 33% HCl at 100ºC for 10 min and neutralized with NaOH solution. Reducing sugar content was then determined using the dinitrosalicylic acid method (6).
RESULTS
Batch fermentation
The purpose of this work was to expand upon our previous work (13) and develop an efficient, continuous process for producing ethanol from sugarcane molasses using an immobilized S. cerevisiae M30 culture. ALM was chosen based on its strong potential as a cell carrier. The ALM carrier has many advantages, including high regeneration ability, reusability, altered mechanical strength, and high ethanol productivity. Inspection of the cross section of the ALM carrier (Fig. 2) reveals the carrier’s structure, which is composed of loofa fiber, a layer of alginate gel, and a hollow space between the loofa fiber and alginate gel. The hollow space between the alginate and loofa fiber was found to be an ideal space for cell growth (13). Fig. 3 and 4 reveals that yeasts can also grow well in the alginate layer and in the hollow space of the core fiber. The cells had a normal oval shape, and filaments were observed connecting cells both to other cells and to cellulose fibers (Fig. 4). This filamentous structure likely promoted firm attachment of the cells both to the carrier and to cell aggregations.
Figure 2.
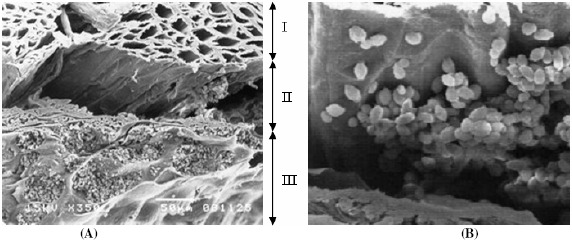
(A) Cross-section of the ALM after 72 h of ethanol fermentation. The ALM consisted of loofa fiber (I), a hollow space between the loofa fiber and alginate gel (II), and alginate gel (III). (B) A higher-magnification view of the hollow space.
Figure 3.
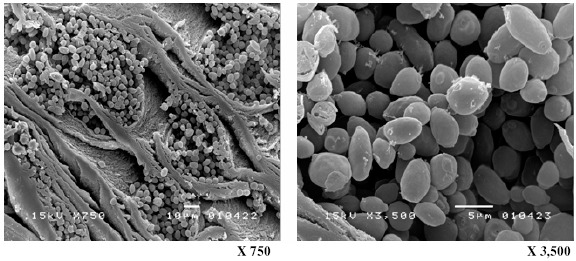
Yeast cells in the alginate gel layer of the ALM after 72 h of ethanol fermentation
Figure 4.
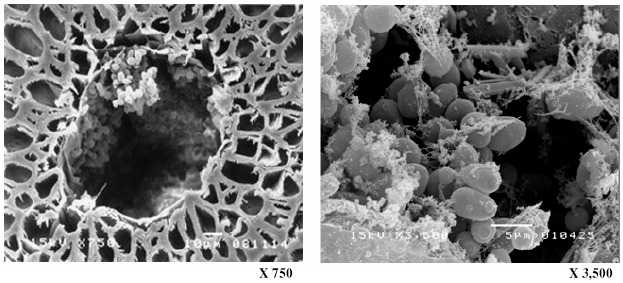
Yeast cells in the hollow core fibers of the ALM after 72 h of ethanol fermentation.
For more convenient preparation, the ALM carrier used in the PBR was modified into a square with dimensions of 20 × 20 × 3 mm3. Pre-examination was performed in 500-mL Erlenmeyer flasks containing 250 mL sterilized medium under controlled conditions of 220 g/L of initial sugar, initial pH of 5.0, 150 rpm, and 33°C. Ethanol production, growth rate, and immobilized yield (YI) using the squares with dimensions of 20 × 20 × 3 mm3 and 9 × 9 × 3 mm3 were comparable (Fig. 5). An ethanol concentration of 89-90 g/L was produced within 60 hours of batch fermentation, whereas the IC and free-cell concentrations were about 4.0 g/L and 1.0 g/L, respectively. No significant differences were observed in the data obtained from the ALM carriers of the two different sizes.
Figure 5.
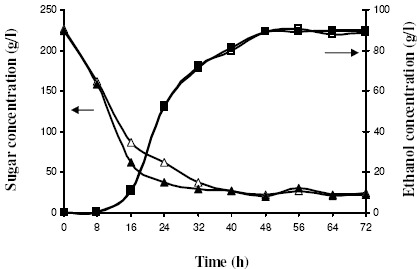
Batch fermentations using ALM carriers with dimensions of 9 × 9 × 3 mm3 (open symbols) and 20 × 20 × 3 mm3 (filled symbols); ∆, ▲= sugar: □,■= ethanol.
Continuous fermentation
Effect of dilution rate and initial sugar concentration: Continuous ethanol fermentations using cane molasses in a 1-L packed-bed reactor were performed at a temperature of 32 ± 1°C and an initial pH of 5.0. The initial reducing sugar concentrations were 202, 222, and 248 g/L at dilution rates ranging from 0.11-0.30 h-1. The effects of dilution rate on ethanol concentration and ethanol productivity are presented in Fig. 6 and Fig. 7, respectively. The ethanol concentration decreased as the dilution rate increased, as is commonly observed. When the initial sugar concentration (S0) was at 200–220 g/L, the ethanol productivity increased linearly with the dilution rate, from 0.1 to 0.2 h-1, and then remained nearly constant. However, at the very high initial sugar concentration of 248 g/L, the ethanol productivity was limited to 8.0 g/(L·h). The optimal S0 for ethanol productivity was 220 g/L. Under steady-state conditions and at varying dilution rates of 0.11, 0.16, 0.20 and 0.30 h-1, the average ethanol concentrations in the effluent were 82.1, 66.1, 57.4 and 37.2 g/L, respectively, corresponding to ethanol productivities (PE) of 9.0, 10.6, 11.5 and 11.2 g/(L·h), respectively. The ethanol conversion yield (YE/S) was almost constant at 0.45±0.02. An optimal ethanol productivity of 11.5 g/(L·h) was obtained at an ethanol concentration of 57.4 g/L, an initial sugar concentration of 220 g/L, and D of 0.2 h-1, whereas the maximum ethanol concentration of 82.1 g/L (PE = 9.0 g/(L·h)) was obtained at a D of 0.11 h-1. At the end of the procedure (360 h), the concentrations of immobilized cells, suspended cells in the reactor, and suspended cells in the effluent were 16.0±0.4, 12.3±0.5 and 0.6±0.1 g/L, respectively, with an average immobilized yield of 56.5%.
Figure 6.
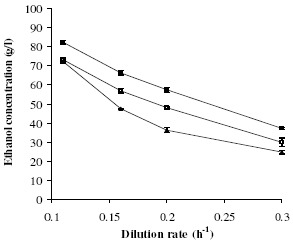
Ethanol concentration at dilution rates of 0.1–0.3 h- and initial sugar concentrations of 200 g/L (□), 220 g/L (■), and 248 g/L (▲).
Figure 7.
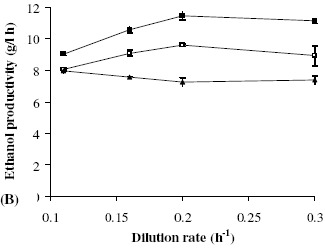
Ethanol productivity at dilution rates of 0.1–0.3 h-1 and initial sugar concentrations of 200 g/L (□), 220 g/L (■), and 248 g/L (▲).
Effect of superficial velocity: The design of packing materials that achieve ideal conditions, which correspond to plug flow and a uniform distribution of fluid across the cross-section of the column, is important for a PBR. The ethanol and reducing sugar concentrations measured from the five sampling ports on both sides of the PBR after the system reached steady state revealed a satisfactorily uniform distribution of the fluid. The ethanol concentration profiles in a plug-flow reactor packed with immobilized cells entrapped in ALM are shown in Fig. 6. Usually, for anaerobic ethanol fermentation with high substrate concentrations, it is likely that external mass transfer is not limiting, at least for a large portion of the packed bed (15). The external mass transfer coefficient can be enhanced by increasing the liquid superficial velocity. In this study, the effect of liquid superficial velocity was examined. Liquid superficial velocity (VS, cm/h)) was calculated from:
where Q is the volumetric flow rate of the fluid (cm3/h), A is the cross-sectional area of the bed (cm2 ), and φ is the packed-bed porosity (the volume of voids per volume of reactor).
Based on the results, the effect of superficial velocity on ethanol concentration profile can be divided into two sub-effects, one with S0 of 200-220 g/L and the other with S0 of 248 g/L. Fig. 8(A) and Fig. 8(B) depict the results with S0 at 200 g/L and 220 g/L, respectively. The results revealed an increase in ethanol concentration as the liquid superficial velocity was increased from 4.8 cm/h to 6.9 cm/h. However, no significant enhancement in ethanol production was observed when the liquid superficial velocity was further increased to 8.7-13.0 cm/h. Based on the result with S0 at 200-220 g/L, the external mass transfer resistance exhibited some negative effects on the overall fermentation rate of the packed bed reactor when the liquid creeping over the static solid particles had a velocity of less than about 7 cm/h. However, at a high initial sugar concentration at S0 of 248 g/L, decreases in sugar consumption and ethanol production were observed as the liquid superficial velocity increased (Fig. 8(C)). The inhibitory effects of high initial sugar and high ethanol concentrations have been previously reported (12). Therefore, mass transfer resistance had a positive effect on ethanol fermentation at very high sugar concentrations due to a reduction of sugar-induced inhibition.
Figure 8.
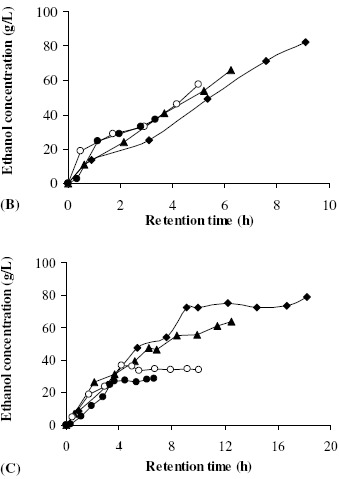
The steady-state ethanol concentration profiles at initial sugar concentrations of 200 g/L (A), 220 g/L (B), and 248 g/L (C) and at superficial velocities of 4.8 cm/h (♦), 6.9 cm/h (▲), 8.7 cm/h (○), and 13.0 cm/h(●).
Stability test: The long-term stability of the immobilized yeast cells entrapped in ALM carriers during continuous ethanol fermentation was examined using an initial sugar concentration of 220 g/L at a constant D of 0.11 h-1. After 30 days of operation, the degradation of alginate films due to cell growth and CO2 production was observed and resulted in cell leakage. Such leaks could also be observed from the cell suspensions in the reactor and in the effluent. Fig. 9(A) and Fig. 9(B) depict the suspended yeasts in the reactor and in the effluent, respectively. The suspended cells in the reactor appeared healthy and retained their normal oval shape. In addition, aggregations of yeast cells with filamentous connections were observed. These cell filaments were barely observed in the alginate gel layer. Therefore, it is possible that the formation of these filaments may be activated by the cellulose fibers of the loofa sponge. It was found that almost all of the aggregated cells were trapped in the packed bed. However, the suspended cells in the effluent were thinner, much smaller, and non-aggregated. The use of a hemacytometer and methylene blue staining to discriminate between dead and live cells revealed that almost all of the cells (>95%) in the reactor were alive, but more than 70% of cells in the effluent were dead.
Figure 9.
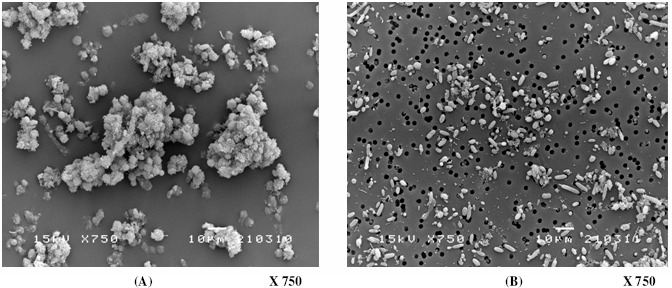
Suspended cells in the PBR (A) and in the effluent (B) after 30 days of continuous fermentation.
The robust performance of the immobilized cells in the alginate-loofa cube during continuous ethanol fermentation in the PBR was confirmed by satisfactory operational stability during 30-day fermentation at a D of 0.11 h-1. There was no significant decline in productivity during continuous operation, and an average ethanol productivity of 8.7 g/ (L·h) was achieved at an average ethanol concentration of 79.3 g/L. The average conversion yield was 0.43-0.45 throughout the entire operation, with an immobilization yield of 47.5%. The final total cell concentration in the reactor was 37.3 g/L (17.7 g/L immobilized cells and 19.6 g/L suspended cells). The concentration of suspended cells in the effluent was 0.8 g/L.
DISCUSSION
The data presented in this work demonstrate that continuous ethanol production from molasses using immobilized S. cerevisiae M30 cells entrapped in ALM (20 × 20 × 3 mm3) is promising. Compared to the batch fermentation, higher ethanol productivity (6-8-fold) was obtained by continuous fermentation in the PBR. A maximum productivity of 11.5 g/L h at an ethanol concentration of 57.4 g/L was obtained using an initial sugar concentration of 220 g/L at a D of 0.20 h-1, whereas a maximum ethanol concentration of 82.1 g/L was obtained at a D of 0.11 h-1. The steady-state ethanol concentration in the effluent of the packed-bed column obtained in this work was reasonably high compared to those described in previous reports (1, 4, 7, 8, 14, 16). The experimental results revealed that the ALM was suitable for yeast immobilization in a continuous PBR. With its favorable mechanical properties, high biocompatibility, and superior porous structure, the ALM is a system with high cell density, high ethanol production, and high stability. Furthermore, the ALM should be applied as a cell carrier for efficient production in other fermentation systems.
ACKNOWLEDGEMENTS
This work was supported by the Thailand Research Fund (TRF) and Chulalongkorn University (contract grant number RSA5080011).
REFERENCES
- 1.Amutha R., Gunasekaran P. Production of ethanol from liquefied cassava starch using co-immobilized cells of Zymomonas mobilis and Saccharomyces diastatcus. J. Biosci. Bioeng. 2001;92:560–564. doi: 10.1263/jbb.92.560. [DOI] [PubMed] [Google Scholar]
- 2.Bai F. W., Chena L. J., Zhangc Z., Andersona W. A., Moo-Young M. Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J. Biotechnol. 2004;110:287–293. doi: 10.1016/j.jbiotec.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Dias J.C.T., Rezende R.P., Linardi V.R. Effects of immobilization in Ba-alginate on nitrile-dependent oxygen uptake rates of Candida guilliermondii. Braz. J. Microbiol. 2001;32:221–224. [Google Scholar]
- 4.Goksungur Y., Zorlu N. Production of ethanol from beet molasses by Ca-alginate immobilized yeast cell in a packed-bed reactor. Turk. J. Biol. 2001;25:265–275. [Google Scholar]
- 5.Kiyohara P.K., Lima U.A., Santos H.S., Santos P.S. Comparative study between yeasts immobilized on alumina beads and on membranes prepared by two routes. Braz. J. Microbiol. 2003;34:129–137. [Google Scholar]
- 6.Miller G.L. Use of dinitrosalicylic acid reagent for determination reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- 7.Monte Alegre R., Rigo M., Joekes I. Ethanol fermentation of a diluted molasses medium by Saccharomyces cerevisiae immobilized on chrysotile. Braz. Arch. Biol. Technol. 2003;46:751–757. [Google Scholar]
- 8.Najafpour G., Younesi H., Syahidah K., Ismail K. Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresour. Technol. 2004;92:251–260. doi: 10.1016/j.biortech.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Ogbonna J.C., Tomiyama S., Tanaka H. Development of a method for immobilization of non-flocculating cells in loofa (luffa cylindrical) sponge. Process. Biochem. 1996;31:737–744. [Google Scholar]
- 10.Ogbonna J.C., Tomiyama S., Liu Y.C., Tanaka H. Efficient Production of ethanol by cells immobilized in loofa (Luffa cylindrical) sponge. J. Ferment. Bioeng. 1997;84:271–274. [Google Scholar]
- 11.Ogbonna J.C., Mashima H., Tanaka H. Scale up of fuel ethanol production from sugar beet juice using loofa sponge immobilized bioreactor. Bioresour. Technol. 2001;76:1–8. doi: 10.1016/s0960-8524(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 12.Phisalaphong M., Srirattana N., Tanthapanichakoon W. Mathematical modeling to investigate temperature effect on kinetic parameters of ethanol fermentation. Biochem. Eng. J. 2006;28:36–43. [Google Scholar]
- 13.Phisalaphong M., Bundiraharijo R., Bangrak P., Mongkolkajit J., Limtong S. Alginate-Loofa as carrier matrix for ethanol production. J. Biosci. Bioeng. 2007;104:214–217. doi: 10.1263/jbb.104.214. [DOI] [PubMed] [Google Scholar]
- 14.Valach M., Navratil M., Horvathova V., Zigova J., Sturdik E., Hrabarova E., Gemeiner P. Efficiency of a fixed-bed and gas-lift three-column reactor for continuous production of ethanol by pectate and alginate immobilized Saccharomyces cerevisiae cells. Chem. Pap. 2006;60:154–159. [Google Scholar]
- 15.Vega J.L., Clausen E.C., Gaddy J.L. Biofilm reactors for ethanol production. Enzyme Microb. Technol. 1988;10:390–402. [Google Scholar]
- 16.Wang B., Ge X.M., Li N., Bai F.W. Continuous ethanol fermentation coupled with recycling of yeast flocs. Chin. J. Biotechnol. 2006;22:816–821. doi: 10.1016/s1872-2075(06)60059-9. [DOI] [PubMed] [Google Scholar]


