Abstract
Shigella flexnerii and Escherichia coli were the most frequent Gram-negative bacteria found in the mouth cavity and cloacae of the turtles Podocnemis expansa and P. unifilis on beaches in the National Park of Araguaia, Brazil. Reptiles are known as Salmonella carriers, despite rarely isolated in these turtles.
Keywords: Salmonella, Shigella, Enterobacteriaceae, Turtles, Bananal Island
The association of reptiles with human pathogens, especially Salmonella, has been largely documented (3, 8). Mermin et al. (11) suggest that reptile and amphibian exposure is associated with ~74,000 Salmonella yearly infections in the United States. Turtles have been involved as vectors of salmonellae in captivity as well as in the wild (8, 16). Moreover, other human enteric pathogens have also been isolated in turtles (18, 19).
Studies regarding the microbiota of Brazilian reptiles in the wild are rare, but Serafini et al. (21) showed that the Pantanal alligator (Caiman crocodilus yacare) and the “jacarétinga” (Caiman crocodilus crocodilus) carry Aeromonas sp., Acinetobacter spp., Citrobacter freundii, Escherichia coli and Pseudomonas sp. Abalem de Sá and Solari (1) found Salmonella spp. in 39.1% of Brazilian and imported pet reptiles, including chelonians. Ferronato et al. (7) isolated E. coli, Klebsiella pneumoniae, Enterobacter aglomerans, C. freundii and Bacillus sp. in oral samples of Phrynops geoffroanus turtles.
Our work aims at detecting Enterobacteriaceae in the mouth cavity and the cloacae of nesting P. expansa and P. unifilis, which are extensively used as food as well as for manufacturing utilitarian handicrafts by local populations dwelling along the Amazon and Araguaia/Javaés river basins (17). This causes pressure toward extinction, and also raises the issue concerning health risk to those human populations in contact with these reptiles.
This study is located within the National Park region of the Araguaia Plains (Fig. 1). Field collections were carried out in August and November 2005 under research license 081/04 -IBAMA/RAN. Eighteen female specimens of P. expansa were caught on the beaches during oviposition, and 30 males and females of P. unifilis captured in the river water while feeding and submitted to cloacal and mouth cavity swabbing.
Figure 1.
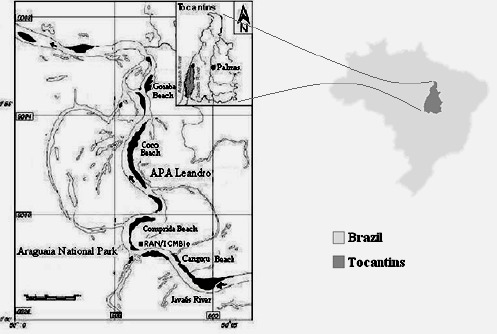
Location map of the study area at the National Park of Araguaia Plains with the nesting beaches of Canguçu, Comprida, Coco and Goiaba and the Javaés River. At the upper right side, the location of the National Park of Araguaia Plains in the Tocantins State, that is shown in the map of Brazil, at the right.
This research project has been approved by the Research Ethics Committee of the Federal University of Tocantins (Universidade Federal do Tocantins). The swabs were incubated in tubes containing Muller-Kauffmann tetrationate broth (Merck KGaA, Darmstadt, Germany) for one to four days at 35–37°C and taken to the laboratory where they were inoculated on Petri dishes containing EMB Agar (Merck KGaA, Darmstadt, Germany), McConckey Agar (Merck KGaA, Darmstadt, Germany) and SS Agar (Merck KGaA, Darmstadt, Germany) and then incubated for 24 h at 37°C. Typical colonies were purified and identified by Gram staining, TSI (Merck KGaA, Darmstadt, Germany) screening and API 20ETM (BioMerieux, Jacarepaguá, Rio de Janeiro, Brazil) kit tests. Only Gram-negative bacilli and cocobacilli were identified by API20E. APIWEBTM (BioMerieux, Jacarepaguá, Rio de Janeiro, Brazil) was used for identification. Results are expressed as the frequency of occurrence of each bacterial species found within each sampled individual, and two or more isolates from a single individual sample were considered as a single isolate. Salmonella serotyping was kindly carried out by LACEN-TO lab, according to standard procedures for human isolates.
Thirteen species of Gram-negative bacteria were isolated from P. expansa and P. unifilis. Chromobacterium violaceum and P. aeruginosa along with two species of Citrobacter and one species of Salmonella were the most frequent bacteria from the mouth samples of 18 nesting P. expansa (Table 1). Five of the samples were negative for growth of enterobacteria and eight isolates could not be identified by the employed methods. Acinetobacter calcoaceticus and C. violaceum were the most frequent species in the mouth of P unifilis, and one Aeromonas and two Citrobacter species, E. coli, E. cloacae and Salmonella Choleraesuis subsp. arizonensis were isolated at low frequencies along with four unidentified ones. All samples were positive for the presence of enterobacteria. Shigella flexnerii and E. coli, which comprised 28 out of 32 isolates from cloacal samples of P. expansa. Cloacal samples of P. unifilis resulted in the isolation of S. flexnerii (nine isolates), E. coli (six isolates) and K. pneumoniae subsp. pneumoniae (six isolates) and one isolate each of C. youngae, H. alvei and S. ficaria. Seven isolates from cloacae of P. unifilis and four from P. expansa showed a similar and unknown profile (profile 1 in Table 1) in API20ETM.
Table 1.
Frequency of occurrence of bacterial species in mouth cavity and cloacae of P. expansa and P. unifilis adults in four beaches of the Javaés River border of National Park of Araguaia.
| Species | P. expansa (na = 18) | P. unifilis (n = 30) | ||
|---|---|---|---|---|
| Mouth | Cloaca | Mouth | Cloaca | |
| Aeromonas salmonicida subsp. salmonicida | 1 | |||
| Acinetobacter calcoaceticus | 4 | |||
| Chromobacterium violaceum | 4 | 4 | ||
| Citrobacter freundii | 1 | 1 | ||
| Citrobacter youngae | 1 | 1 | 1 | |
| Escherichia coli | 8 | 1 | 6 | |
| Enterobacter cloacae | 1 | |||
| Hafnia alvei | 1 | |||
| Klebsiella pneumoniae subsp. pneumoniae | 6 | |||
| Pseudomonas aeruginosa | 2 | 2 | 1 | |
| Salmonella Cholerasuis subsp. arizonae | 1 | 1 | ||
| Serratia ficaria | 1 | |||
| Shigella flexnerii | 20 | 9 | ||
| Non-identified profile 1 | 4 | 7 | ||
| Non-identified profile 2 to 3 | 8 | |||
| Non-identified profiles 4 to 8 | 12 | |||
| Total | 17 | 32 | 28 | 32 |
Number of samples.
The isolation of Shigella and Klebsiella associated with both turtle species may very well indicate health risk to humans consuming their meat. Shigella has rarely been reported as associated with turtles, however solely by Mahmoud et al. (10) who isolated Shigella spp. from the Chelonya midas oviductal fluid and by Dickinson et al. (6) from tortoises. David et al. (5) proposed that polluted water was the source of Shigella in the Nile tilapia in a Kenyan lake, but the Javaés river water presents as highly pristine, according to Morais et al. (13). Santoro et al. (18) verified that K. pneumoniae was the most common microbe identified and the Enterobacteriaceae family was the largest Gram-negative group of bacteria in 70 nesting green turtles (Chelonia mydas) from Tortuguero National Park, Costa Rica. Boede and Hernández (2) found Klebsiella spp. implicated in enteritis and dermatitis in the turtle Pseudemis scripta.
Similar to this study, E. coli was prevalent in the cloaca and feces of the estuarine diamondback terrapin (Malaclemys terrapin) (9) and Phrynops geoffroanus (7). Oros et al. (14) associated E. coli with lesions in Caretta caretta, and Raidal et al. (15) to juvenile mortality of Chelonia midas. Santoro et al. (19) found Aeromonas spp. and C. freundii but not S. flexnerii, E. coli or C. violaceum as frequent bacteria in Lepidochelys olivacea, a chelonian from Costa Rica.
Salmonella was not obtained from cloaca of the chelonians and only two isolates were obtained from mouth samples. Salmonella Choleraesuis subsp. arizonae was also isolated from P. unifilis eggs in the same area (13). It might be possible that the failure to detect Salmonella in cloacal samples was due to a methodological bias. Harwood et al. (9) argues that the method of cloacal swabs for sampling presents a great deal of variability due to individual recent activities. In this study, recent oviposition by an individual turtle was not unlikely since the majority of P. expansa individuals were females captured on nesting beaches. Also, wild turtles are believed to shed Salmonella at lower rates than captive turtles because they either lack exposure to stressors that increase shedding rates or because they are not natural carriers of the bacterium (16).
We conclude that Enterobacteriaceae are part of the normal microbiota of mouth and cloacae of Podocnemis expansa and P. unifilis in the pristine area of Araguaia National Park and surrounding Plains, since sand and water are not contaminated as shown by Morais et al. (13). In regard to the fact that the cold-blooded turtles shed coliform bacteria, including E. coli, in their cloacae may have public health significance.
ACKNOWLEDGEMENTS
This work was sponsored by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through grant #620009/2004–7. We would like to thank Dr. Adriana Malvasio and her team for help in the field work.
REFERENCES
- 1.Acevedo F., Gentina J.C., Valencia P. Optimization of pulp density and particle size in the biooxidation of a pyritic gold concentrate by Sulfolobus metallicus. World J. Microbiol. Biotechnol. 2004;20(8):865–869. [Google Scholar]
- 2.Brierley C.L., Brierley J.A. A chemoautotrophic and thermophilic microorganism isolated from an acid hot spring. Can. J. Microbiol. 1973;19:183–188. doi: 10.1139/m73-028. [DOI] [PubMed] [Google Scholar]
- 3.Claus H., Akca E., Debaerdemaeker T., Evrard C., Declercq J.P., Konig H. Primary structure of selected archaeal mesophilic and extremely thermophilic outer surface layer proteins. Syst. appl. microbiol. 2002;25(1):3–12. doi: 10.1078/0723-2020-00100. [DOI] [PubMed] [Google Scholar]
- 4.Cytryn E., Minz D, Oremland R.S., Cohen Y. Distribution and diversity of archaea corresponding to the limnological cycle of a Hypersaline Stratified Lake (Solar Lake, Sinai, Egypt) Appl. Environ. Microbiol. 2000;66(8):3269–3276. doi: 10.1128/aem.66.8.3269-3276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dempers C.J.N., Breed A.W., Hansford G.S. The kinetics of ferrous-iron oxidation by Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans: effect of cell maintenance. Biochem. Eng. J. 2003;16(3):337–346. [Google Scholar]
- 6.Ding J., He H., Zhang C., Yu Y., Qiu G. Isolation and characterization of YNTC-1, a novel Alicyclobacillus sendaiensis strain. J. Cent. South Univ. T. 2008;15(4):508–514. [Google Scholar]
- 7.Dopson M., Lindstrom E.B. Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl. Environ. Microbiol. 1999;65(1):36–40. doi: 10.1128/aem.65.1.36-40.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dopson M., Baker-Austin C., Hind A., Bowman J.P, Bond P.L. Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl. Environ. Microbiol. 2004;70(4):2088. doi: 10.1128/AEM.70.4.2079-2088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falco L., Pogliani C., Curutchet G., Donati E. A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 2079. 2003;71(1–2):31–36. [Google Scholar]
- 10.Fowler T.A., Crundwell F.K. Leaching of zinc sulfide by Thiobacillus ferrooxidans: bacterial oxidation of the sulfur product layer increases the rate of zinc sulfide dissolution at high concentrations of ferrous ions. Appl. Environ. Microbiol. 1999;65(12):5285–5292. doi: 10.1128/aem.65.12.5285-5292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J., Zhang C.G., Wu X.L., Wang H.H., QIU G.Z. Isolation and identification of a strain of Leptospirillum ferriphilum from an extreme acid mine drainage site. Ann. Microbiol. 2007;57(2):171–176. [Google Scholar]
- 12.Geng A., Soh A.E.W., Lim C.J., Loke L.C.T. Isolation and characterization of a phenol-degrading bacterium from an industrial activated sludge. Appl. Microbiolm. Biotechnol. 2006;71(5):728–735. doi: 10.1007/s00253-005-0199-z. [DOI] [PubMed] [Google Scholar]
- 13.Gomez E., Ballester A., Blazquez M.L., Gonzalez F. Silver-catalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms. Hydrometallurgy. 1999;51(1):37–46. [Google Scholar]
- 14.Goto K., Matsubara H., Mochida K., Matsumura T., Hara Y., Niwa M., Yamasato K. Alicyclobacillus herbarius sp. nov., a novel bacterium containing omega-cycloheptane fatty acids, isolated from herbal tea. Int. J. Syst. Evol. Microbiol. 2002;52(1):109–113. doi: 10.1099/00207713-52-1-109. [DOI] [PubMed] [Google Scholar]
- 15.Hallberg K.B., Johnson D.B. Biodiversity of acidophilic prokaryotes. Adv. appl. Microbiol. 2001;49:37–84. doi: 10.1016/s0065-2164(01)49009-5. [DOI] [PubMed] [Google Scholar]
- 16.He Z.G., Zhong H., Li Y. Acidianus tengchongensis sp. nov., a new species of acidothermophilic archaeon isolated from an acidothermal spring. Curr. Microbiol. 2004;48(2):159–163. doi: 10.1007/s00284-003-4155-9. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov I.T. Derivative conductometry profile of thermal alterations in cellular membranes—a possible relationship between membrane alterations, cellular proliferation capacity and maximum temperature of growth. J. Therm. Biol. 2002;27(2):137–149. [Google Scholar]
- 18.Jantzen E., Sonesson A., Tangen T., Eng J. Hydroxy-fatty acid profiles of Legionella species: diagnostic usefulness assessed by principal component analysis. J. Clin. Microbiol. 1993;31(6):1413–1419. doi: 10.1128/jcm.31.6.1413-1419.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson D.B., Hallberg K.B. The microbiology of acidic mine waters. Research in Microbiology. 2003;154(7):466–473. doi: 10.1016/S0923-2508(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 20.Plumb J.J., Haddad C.M., Gibson J.A.E., Franzmann P.D. Acidianus sulfidivorans sp. nov., an extremely acidophilic, thermophilic archaeon isolated from a solfatara on Lihir Island, Papua New Guinea, and emendation of the genus description. Int. J. Syst. Evol. Microbiol. 2007;57(7):1418–1423. doi: 10.1099/ijs.0.64846-0. [DOI] [PubMed] [Google Scholar]
- 21.Poulin R., Lawrence R.W. Economic and environmental niches of biohydrometallurgy. Miner. Eng. 1996;9(8):799–810. [Google Scholar]
- 22.Rawlings D.E. Heavy metal mining using microbes. Annu. Rev. Microbiol. 2002;56:65–91. doi: 10.1146/annurev.micro.56.012302.161052. [DOI] [PubMed] [Google Scholar]
- 23.Rawlings D.E., Johnson D.B. The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology. 2007;153(2):315–324. doi: 10.1099/mic.0.2006/001206-0. [DOI] [PubMed] [Google Scholar]
- 24.Rohwerder T., Gehrke T., Kinzler K., Sand W. Bioleaching review part A. Appl. Microbiol. Biotechnol. 2003;63(3):239–248. doi: 10.1007/s00253-003-1448-7. [DOI] [PubMed] [Google Scholar]
- 25.Schippers A., Sand W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999;65(1):319–321. doi: 10.1128/aem.65.1.319-321.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segerer A., Neuner A., Kristjansson J.K., Stetter K.O. Acidianus infernusgen. nov., sp. nov., and Acidianus brierleyi comb. nov.: facultatively aerobic, extremely acidophilic thermophilic sulfur-metabolizing archaebacteria. Int. J. Syst. Evol. Microbiol. 1986;36(4):559–564. [Google Scholar]
- 27.Shi S., Fang Z. Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans. Hydrometallurgy. 2004;75(1–4):1–10. [Google Scholar]
- 28.Shi S., Fang Z., Ni J. Bioleaching of marmatite flotation concentrate with a moderately thermoacidophilic iron-oxidizing bacterial strain. Miner. Eng. 2005;18(11):1127–1129. [Google Scholar]
- 29.Tsuruoka N., Isono Y., Shida O., Hemmi H., Nakayama T., Nishino T. Alicyclobacillus sendaiensis sp. nov., a novel acidophilic, slightly thermophilic species isolated from soil in Sendai, Japan. Int. J. Syst. Evol. Microbiol. 2003;53(4):1081–1084. doi: 10.1099/ijs.0.02409-0. [DOI] [PubMed] [Google Scholar]
- 30.Watling H.R., Perrot F.A., Shiers D.W. Comparison of selected characteristics of Sulfobacillus species and review of their occurrence in acidic and bioleaching environments. Hydrometallurgy. 2008;93(1–2):57–65. [Google Scholar]
- 31.Xia J.L., Peng A.A., He H., Yang Y., Liu X.D., Qiu G.Z. A new strain Acidithiobacillus albertensis BY-05 for bioleaching of metal sulfides ores. T. Nonferr. Metal. Soc. 2007;17(1):168–175. [Google Scholar]
- 32.Yoshida N., Nakasato M., Ohmura N., Ando A., Saiki H., Ishii M., Igarashi Y. Acidianus manzaensis sp. nov., a novel thermoacidophilic Archaeon growing autotrophically by the oxidation of HB2B with the reduction of FeP3+P. Curr. Microbiol. 2006;53(5):406–411. doi: 10.1007/s00284-006-0151-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R., Xia J., Peng J., Zhang Q., Zhang C., Nie Z., Qiu G. A new strain Leptospirillum ferriphilum YTW315 for bioleaching of metal sulfides ores. Trans. Nonferrous Met. Soc. 2010;20(1):135–141. China. [Google Scholar]
- 34.Zhou H., Zhang R., Hu P., Zeng W., Xie Y., Wu C., Qiu G. Isolation and characterization of Ferroplasma thermophilum sp. nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite. J. Appl. Microbiol. 2008;105(2):591–601. doi: 10.1111/j.1365-2672.2008.03807.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Q.G., Bo F., Hong Bo Z., Xi L., Jian G., Fei Fei L., Xin Hua C. Isolation of a strain of Acidithiobacillus caldus and its role in bioleaching of chalcopyrite. World J. Microbiol. Biotechnol. 2007;23(9):1217–1225. [Google Scholar]


