Abstract
Background: This is an in vitro study to investigate the effects of ultrasonic scaling on the surface roughness and quantitative bacterial count on four different types of commonly used composite restorative materials for class V cavities.
Materials & Methods: Nanofilled, hybrid, silorane and flowable composites were tested. Forty extracted teeth served as specimen and were divided into 4 groups of 10 specimens, with each group receiving a different treatment and were examined by a Field emission scanning electron microscope. Bacterial suspension was then added to the pellicle-coated specimens, and then bacterial adhesion was analyzed by using image analyzing program.
Results: Flowable and silorane-based composites showed considerably smoother surfaces and lesser bacterial count in comparison to other types, proving that bacterial adhesion is directly proportional to surface roughness.
Conclusion: The use of ultrasonic scalers affects the surfaces of composite restorative materials. Routine periodontal scaling should be carried out very carefully, and polishing of the scaled surfaces may overcome the alterations in roughness, thus preventing secondary caries, surface staining, plaque accumulation and subsequent periodontal inflammation.
How to cite this article: Eid H A, Togoo R A, Saleh A A, Sumanth C R. Surface Topography of Composite Restorative Materials following Ultrasonic Scaling and its Impact on Bacterial Plaque Accumulation. An In-Vitro SEM Study. J Int Oral Health 2013; 5(3):13-19.
Key Words: : Surface roughness, Composites, Ultrasonic Scaling.
Introduction
Periodontal therapy and prophylaxis involve the removal of plaque, calculus and endotoxins from teeth or exposed root surfaces, either with hand- or machine-driven instruments.1 Ultrasonic scaling is one of the most widely used methods among the dental surgeons and oral hygienists due to the decreased time requirement and ease of application in comparison to hand instrumentation.2 The effects of ultrasonic scalers on oral hard and soft tissues are investigated and well documented. However, there is limited information regarding the effects of ultrasonic instrumentation on restorative materials.3 The cleaning procedures, however, may increase the surface roughness of restorations promoting plaque formation, thereby increasing the risk for both caries and periodontal inflammation.4
A large number of restorative materials have recently been marketed for esthetic restorations, and therefore practitioners have a variety of options when selecting materials and procedures for restoring teeth. In addition to conventional resin composites and glass ionomer cements, more-recently developed tooth-coloured filling materials, particularly resin-modified glass ionomer cements, polyacid-modified resin composites and flowable composites are introduced; with their own advantages and disadvantages. As calculus and plaque deposits are often heaviest in the cervical area of teeth, restorations of Class V cavities are frequently exposed to periodontal prophylaxis.5 Hence, the surface roughness of these materials as a result of these procedures is of considerable interest as it influences the esthetic appearance and longevity of restorations as well as plaque and stain retention.6
An additional issue is that the surface roughness of these restorative materials can promote biofilm formation.7 Increased surface roughness facilitates mechanical aggregation of the initial bacterial populations, which promotes the process of periodontal disease due to the retention of bacterial plaque. High substratum surface free energy and an increase in surface roughness facilitate plaque accumulation and increase the bacterial count.6 This in vitro study investigated the effects of ultrasonic scaling on the surface roughness and quantitative bacterial count on four different types of commonly used composite restorative materials for class V cavities.
Materials and Methods
The materials tested were nanofilled (Nano hybrid universal composite, Denmat Holdings Llc , Santa Maria, CA, USA), hybrid (Amelogen Hybrid, Ultradent, Istanbul, Turkey), silorane (Feltik P 90, 3M ESPE, Saint Paul, MN, USA) and flowable (Tetric flow, Ivoclar/Vivadent, Amherst, NY, USA) composites.
Forty extracted teeth were sterilized according to OSHA and CDC regulations8 and they served as the specimen. Standard class V cavity preparations were done in each of them. The materials were overfilled into rectangular recesses (16 x 6 x 1.5 mm) of customized Teflon molds and covered with acetate strips (Polydentia SA, Mezzovico, Switzerland). A glass slide was placed over the acetate strips and pressure was applied to extrude excess material and to prevent oxygen inhibiting layer formation and then polymerized using a halogen light-curing unit (QHL75, Dentsply International, York, PA, USA) according to the manufacturer’s instructions, for 40 seconds. The specimens of each material were divided into 4 groups of 10 specimens, with each group receiving a different treatment and were examined by a Field emission scanning electron microscope (FESEM, S-4700, JEOL, Tokyo, Japan) at a voltage of 20 kV and a magnification of 600X.
Group A: Materials were filled in class V cavities without any further procedure acted as control group.
Group B: 24 hours after the restoration the specimen were subjected to ultrasonic scaling for three minutes.
Group C: Filled teeth immersed for 4 months in an acidic drink (Pepsi), then subjected to ultrasonic scaling for three minutes.
Group D: Filled teeth stored for 4 months in artificial saliva and mucin, before storage subjected to ultrasonic scaling for three minutes. The specimens were instrumented with a Piezo-electric ultrasonic scaling unit (EMS SA, Germany) under standard operating conditions (medium power setting, 0o angulation and standard lateral force along the long axis of the teeth). To avoid operator variation, the same operator performed the ultrasonication on all specimens, starting from the periphery to the centre of each specimen.
Test specimens of group D were covered with artificial saliva and mucin suspension (M2378, Type II; Sigma-Aldrich, St. Louis, Mo) (5 ml) in a petri dish and left for 1 hour to produce a pellicle. Type II mucin (140 mg) was added to 100 ml of artificial saliva. Artificial saliva was prepared as described in previous studies9: 8.4 mg NaF, 2560 mg NaCl, 332.97 mg CaCl2, 250.00 mg MgCl2 (6H2O), 189.48 mg KCl, 3015.00 mg CH3COOK, 772.00 mg K3PO4 (3 H2O), and 0.1 ml H3PO4 (85%) (Merck KGaA, Darmstadt,Germany). Bacterial suspension (109CFU/100 ml) was then added to the pellicle-coated specimens, and then bacterial adhesion was analyzed by using image analyzing program (Clemex Vision Lite 3.5; Clemex Technologies, Inc, Longueuil, Canada). Qualitative bacterial count is calculated as following:
Results
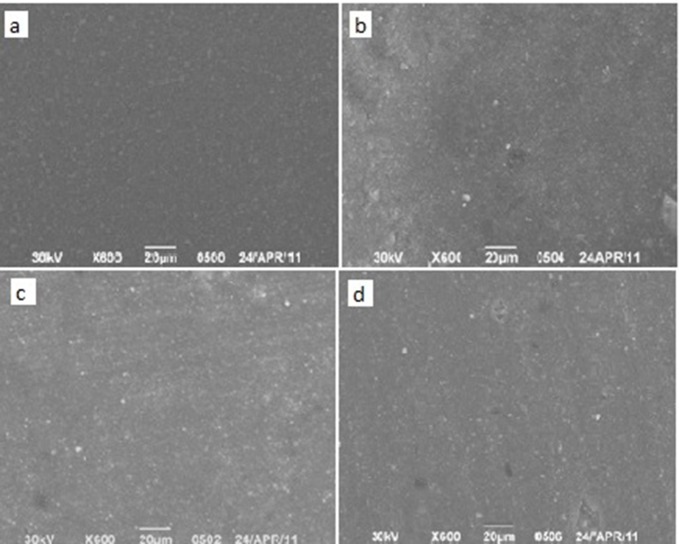
Group A: Scanning electron micrograph of the surface of Hybrid (a), silorane (b), nanofilled (c) and flowable composites (d) after their application (magnification 600 X) (Figure 1) revealed that nanofilled composite specimens had wider
Fig.1: SEM images of the surfaces of the un-treated composite specimen.

distribution in filler size and flowable composite specimens showed minimal amount of filler content, but both of them had smoother surfaces in comparison to silorane-based and regular hybrid composite surfaces.
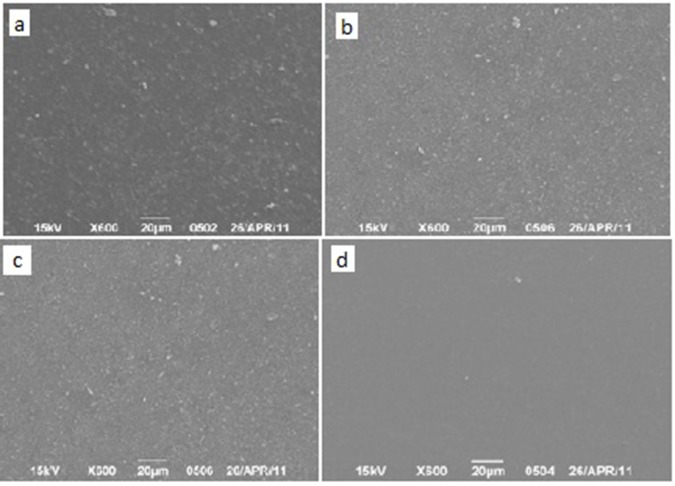
Group B: All samples showed a kind of surface roughness and exposure of their fillers with the exception of Flowable composite specimens. They showed the smoothest surface and looked un-affected by scaling procedure. Nanofilled composite surfaces showed irregularities and a kind of fibre-like fillers. Silorane-based showed more homogenous distribution in comparison to the regular hybrid composite surfaces (Figure 2).
Fig.2: SEM images of the surfaces of the specimen after 24 hours of ultrasonic scaling.
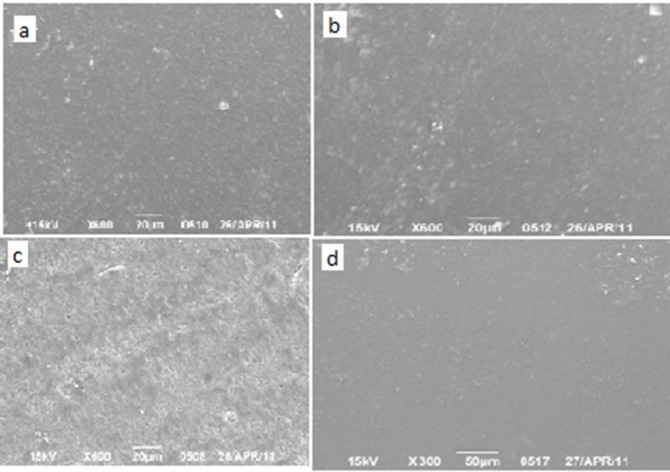
Group C: Scaling after a storage period of 4 months caused a kind of surface roughness and exposure of composite fillers. Flowable composite again showed the smoothest surface among the examined specimens. Nanofilled composite surfaces showed surface crazing and surface irregularities. Hybrid showed higher filler exposure rate in comparison to silorane-based hybrid composite (Figure 3).
Fig.3: SEM images of surfaces of the specimen after storage in an acidic drink for 4 months and scaling.
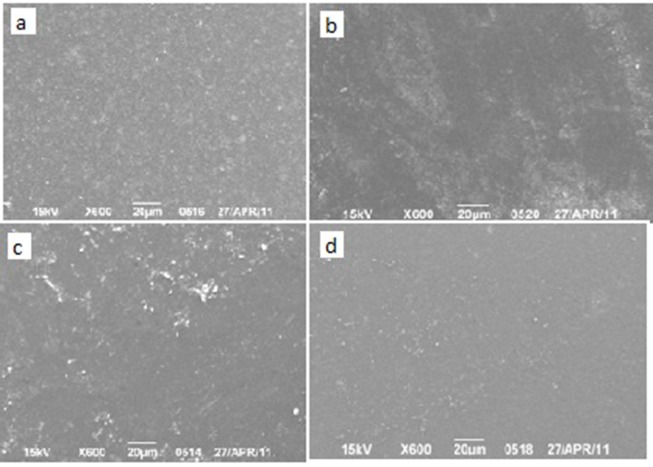
Group D: Scaling after a storage period of 4 months and pH cycling caused higher degree of filler exposure. All of them showed a degree of surface roughness. Both flowable and silorane-based composites owned smoother surfaces in comparison to other types. Nanofilled composite surfaces showed a kind of surface irregularities and exposure of filler clusters. Regular hybrid showed higher filler exposure rate in comparison to silorane-based hybrid composite (Figure 4).
Fig.4: SEM images of surfaces of the specimen after scaling and storage in artificial saliva and mucin for 4 months.
The bacterial adhesion analyzed with image analyzing program revealed that Flowable and silorane-based composites comparatively had lesser bacterial count (Table 1).
| Table 1: Showing the Bacterial Count of the Samples | |||||
| Sample | No of G -ve colonies | No of G +ve colonies | Total count of G +ve | Total count of G -ve | Dilution |
| Flowable | 44 | 10 | 10-7 | 4.4*109 | 1*109 |
| Silorane | 27 | 16 | 10-7 | 2.7*109 | 1.6*109 |
| Nanofilled | 211 | 125 | 10-7 | 2.1*1010 | 1.2*1010 |
| Hybrid | 299 | 112 | 10-7 | 2.9*1010 | 1.1*108 |
Discussion
One of the main etiological factors of periodontal disease is the formation and maturation of biofilm. The principal objective of treatment in periodontitis is the periodic removal of plaque and calcified deposits from teeth and restorations. This procedure is usually accomplished by sonic and ultrasonic scaling systems that may inadvertently affect not only dental tissues but also the restorative materials. The vibration of ultrasonic scaler inserts operate between 18,000 and 45,000 cycles per second and attains results similar to hand instruments in removing plaque, calculus and endotoxins.10 The cleaning procedures, however, may increase surface roughness of dental restorations, which will influence bacterial colonization and increase the rate of plaque formation.11, 12 As calculus and plaque deposits are often heaviest in the cervical area of teeth, restorations of Class V cavities are frequently exposed to periodontal prophylaxis. In this study, we tested the effect of ultrasonic instruments on composite restorative materials used for cervical lesions in vitro.
In the current study, all the test materials presented acceptable surface roughness before instrumentation. Nanofilled composite specimens showed wider distribution in filler size, and flowable composite specimens showed minimal amount of filler content. Both showed smoother surfaces in comparison to both silorane-based and regular hybrid composite surfaces (Figure 1). 24 hours after instrumentation, all samples showed exposure of their fillers. However, flowable composite specimens were an exception. They showed the smoothest surface and looked un-affected by scaling procedure. Nanofilled composite surfaces showed some irregularities and a kind of fiber-like fillers. Silorane-based showed more homogenous distribution in comparison to the regular hybrid composite surfaces (Figure 2). The dissimilarity in surface roughness for the different resins is mainly attributed to the differences in the size and content of filler particles. Flowable composites are characterized by lower filler loading and by greater proportion of diluent monomers in the formulation. They were traditionally created by retaining the same small particle size of the conventional hybrid composites, but reducing the filler content, and allowing the increased resin content to reduce the viscosity of the mixture.13 However their various mechanical properties such as flexural strength and wear resistance have been reported to be generally inferior if compared to those of the conventional composites. For these reasons, flowable composites have been suggested to be filling materials for low-stress applications and in situations with difficult access or those requiring good penetration. Ikeda et al.14 outlined some of the clinical indications for flowable resin composites: Composite or crown margin repairs; pit and fissure sealing; preventive resin restorations; air abrasion cavity preparations; cavity lining; porcelain repairs; enamel defects; incisal edge repairs in anterior sites; small Class III and Class V restorations.
As class V restorative materials, resin composites are subjected to action of acidic substances, which may affect their behaviour. The composite resins are frequently subjected to certain deleterious actions in the oral cavity through the processes of abrasion (brushing), attrition and erosion (citrus drinks, fruit, soft drinks). Furthermore, the materials are exposed to exogenous substances containing a variety of chemicals, including acids, bases, salts, alcohol, oxygen, etc. entering the environment during oral food and fluid intake and oral hygiene. The chemical and duration of exposure are important determinants that may have some influence on the polymer chain molecules of materials.15 Accordingly, all the four materials showed more roughness on surface and exposure of fillers after acidic drink immersion for 4 months. Flowable composite showed the smoothest surface among the examined specimens. Nanofilled composite surfaces showed surface crazing and surface irregularities. Hybrid showed higher filler exposure rate in comparison to silorane-based hybrid composite (Figure 3). Neamat et al.16 have shown that fillers tend to fall out from resin materials and the matrix component decomposes when exposed to low pH environments. Many soft drinks are acidic and the pH is 3.0 or lower. This means that drinking acidic drinks over a long period and with continuous sipping can erode the tooth enamel and the resin material as well.16
The present study investigated the bacterial growth on the four types of composite resins in short term. Group D specimens showed higher degree of filler exposure and a higher degree of surface roughness in comparison to other groups. Both flowable and silorane-based composites showed considerably smoother surfaces and lesser bacterial count in comparison to other types. Nanofilled composite surfaces showed a kind of surface irregularities and exposure of filler clusters. Regular hybrid showed higher filler exposure rate in comparison to silorane-based hybrid composite (Figure 4). Surface roughness of restorative materials has been reported as a factor contributing to bacterial adherence in several studies.17 In a study carried out by Ikeda et al, it was reported that smooth resin composite surfaces exhibited lower bacterial adherence and ac-cumulation in comparison to rougher resin composite surfaces.14 Moreover, Ono et al demonstrated that smooth resin composite surfaces have an important role in retarding the biofilm adherence and growth.18
Conclusion
Flowable and silorane-based composites showed considerably smoother surfaces and lesser bacterial count in comparison to other types, proving that bacterial adhesion is directly proportional to surface roughness. The present data, although, may not be directly applicable to clinical conditions, but still suggests that the use of ultrasonic scalers affects the surfaces of composite restorative materials. In terms of surface roughness, it is recommended that routine periodontal scaling should be carried out very carefully, and that polishing of the scaled surfaces may overcome the alterations in roughness, thus preventing secondary caries, surface staining, plaque accumulation and subsequent periodontal inflammation.
Acknowledgments
The authors would like to acknowledge the contribution of Dr. Refaat El-Najar, SEM Unit, College of Medicine, King Khalid University, for his valuable help in preparation of samples and interpreting results of SEM.
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
Contributor Information
A. Eid Hossam, Department of Oral Medicine & Periodontology, Faculty of Dentistry, Suez Canal University, Ismailia, Egypt; Assistant Professor, Department of Preventive Dental Sciences, King Khalid University College of Dentistry, Abha, Saudi Arabia.
A. Togoo Rafi, Department of Preventive Dental Sciences, King Khalid University College of Dentistry Abha, Saudi Arabia.
A Saleh Ahmed, Department of Restorative Dental Sciences, King Khalid University College of Dentistry, Abha, Saudi Arabia; Lecturer, Department of Operative Dentistry, Faculty of Dentistry, Mansura University, Egypt.
Phani CR Sumanth, Department of Restorative Dental Sciences, King Khalid University College of Dentistry, Abha, Saudi Arabia.
References:
- 1.P Axelsson, J Lindhe. Effect of controlled oral hygiene procedures on caries and periodontal diseases in adults results after 6 years . J Clin Periodontol . 1981;8(3):239–248. doi: 10.1111/j.1600-051x.1981.tb02035.x. [DOI] [PubMed] [Google Scholar]
- 2.P Mourouzis, EA Koulaouzidou, L Vassiliadias, LH Antoniades. Effect of sonic scaling on the surface roughness of restorative materials. J Oral Sci. 2009;51(4):607–614. doi: 10.2334/josnusd.51.607. [DOI] [PubMed] [Google Scholar]
- 3.YL Lai, YC Lin, CS Chang, SY Lee. Effects of sonic and ultrasonic scaling on the surface roughness of tooth-colored restorative materials for cervical lesions . Oper Dent. 2007;32(3):273–278. doi: 10.2341/06-77. [DOI] [PubMed] [Google Scholar]
- 4.W Teughels, N Van Assche, I Sliepen, M Quirynen. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 5.AU Yap, CW Sau, KW Lye. Effects of finishing/polishing time on surface characteristics of tooth-coloured restoratives. J Oral Rehabil. 1998;25(6):456–461. doi: 10.1046/j.1365-2842.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 6.M Quirynen, CM Bollen. The influence of surface roughness and surface-free energy on supra and sub-gingival plaque formation in man . A review of the literature. J Clin Periodontol. 1995;22(1):1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 7.KN Leknes, T Lie, UM Wikesjo, GC Bogle, KA Selvig. Influence of tooth instrumentation roughness on sub-gingival microbial colonizationJ Periodontol . 1994;65(4):303–308. doi: 10.1902/jop.1994.65.4.303. [DOI] [PubMed] [Google Scholar]
- 8.Association Document on Handling extracted teeth by American Dental . Available at: http: //www.ada.org /sections/professional Resources/pdfs/cdc_handling_extracte d.pdf [Google Scholar]
- 9.F Aykent, I Yondem, AG Ozyesil, SK Gunal, MC Avunduk, S Ozkan. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion . J Prosthet Dent. 2010;103(4):221–227. doi: 10.1016/S0022-3913(10)60034-0. [DOI] [PubMed] [Google Scholar]
- 10.M Folwaczny, U Merkel, A Mehl, R Hickel. Influence of parameters on root surface roughness following treatment with a magnetostrictive ultrasonic scaler: An in vitro study J Periodontol. 2004;75(9):1221–1226. doi: 10.1902/jop.2004.75.9.1221. [DOI] [PubMed] [Google Scholar]
- 11.EJ Bjornson, DE Collins, WO Engler. Surface alteration of composite resins after curette, ultrasonic, and sonic instrumentation: An in vitro study . Quintessence Int. 1990;21(5):381–389. [PubMed] [Google Scholar]
- 12.NE Jotikasthira, T Lie, KN Leknes. Comparative in vitro studies of sonic, ultrasonic and reciprocating scaling instruments. J Clin Periodontol. 1992;19(8):560–569. doi: 10.1111/j.1600-051x.1992.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 13.C Poggio, A Dagna, M Chiesa, M Colombo, A Scribante. Surface roughness of flowable resin composites eroded by acidic and alcoholic drinks. J Conserv Dent. 2012;15(2):137–140. doi: 10.4103/0972-0707.94581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.I Ikeda, M Otsuki, A Sadr, T Nomura, R Kishikawa, J Tagami. Effect of filler content of flowable composites on resin-cavity interface. Dent Mater J. 2009;28(6):679–685. doi: 10.4012/dmj.28.679. [DOI] [PubMed] [Google Scholar]
- 15.AC Rocha, CS De Lima, MC Santos, MA Montes. Evaluation of surface roughness of a nanofill resin composite after simulated bushing and immersion in mouth rinses, alcohol and water. Materials Research. 2010;13(1):77–80. [Google Scholar]
- 16.AB Neamat, L Han, A Okamoto, M Iwaku. Effect of alcoholic and low pH soft drinks on fluoride release from compomer. J Esthet Dent. 2000;12(2):97–104. doi: 10.1111/j.1708-8240.2000.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 17.S Kimyai, F Lotfipour, R Pourabbas, A Sadr, S Nikazar, M Milani. Effect of two prophylaxis methods on adherence of Streptococcus mutans to microfilled composite resin and giomer surfaces. Med Oral Patol Oral Cir Bucal. 2011;16(4):561–567. doi: 10.4317/medoral.16.e561. [DOI] [PubMed] [Google Scholar]
- 18.M Ono, T Nikaido, M Ikeda, S Imai, N Hanada, J Tagami, K Matin. Surface properties of resin composite materials relative to biofilm formation. Dent Mater J. 2007;26:613–622. doi: 10.4012/dmj.26.613. [DOI] [PubMed] [Google Scholar]