Abstract
AIM: To evaluate the ameliorative effect of naringenin (NG) during ulcerative colitis (UC) in rats.
METHODS: Rats were treated with three different doses (25, 50 and 100 mg/kg per day) of NG and a single dose of mesalazine (MES, 300 mg/kg per day) for seven days prior to ulcerative colitis induction by 4% acetic acid (AA). Twenty four hours after AA rectal administration, animals were scarified and the colonic tissues were dissected. Colonic mucus content was estimated using Alcian blue dye binding technique. In colon tissues, levels of total glutathione sulphadryls (T-GSH), non-protein sulphadryls (NP-SH) and thiobarbituric acid reactive substances (TBARS) were evaluated. The activities of the antioxidant enzymes, catalase (CAT) and superoxide dismutase (SOD) were measured. Concentrations of nucleic acids (DNA and RNA) and total protein were also estimated in colon tissues. Colonic levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), prostaglandin E2 (PGE2) and nitric oxide (NO) were estimated. In cross section of colitis tissue the histopathological changes were observed.
RESULTS: Colonic mucus content was decreased in AA compared to controls (587.09 ± 65.59 mg/kg vs 941.78 ± 68.41 mg/kg, P < 0.001). AA administration markedly reduced T-GSH (5.25 ± 0.37 nmol/L vs 3.04 ± 0.24 nmol/L, P < 0.01), NP-SH (3.16 ± 0.04 nmol/L vs 2.16 ± 0.30 nmol/L, P < 0.01), CAT (6.77 ± 0.40 U/mg vs 3.04 ± 0.2 U/mg, P < 0.01) and SOD (3.10 ± 0.11 U/mg vs 1.77 ± 0.18 U/mg, P < 0.01) while TBARS, TNF-α, IL-1β, IL-6, PGE2 and NO levels (15.09 ± 3.84 nmol/L vs 59.90 ± 16.34 nmol/L, P < 0.01; 113.56 ± 1.91 pg/mg vs 134.24 ± 4.77 pg/mg, P < 0.01; 209.20 ± 36.38 pg/mg vs 422.19 ± 31.47 pg/mg, P < 0.01; 250.83 ± 25.09 pg/mg vs 638.58 ± 115.9 pg/mg, P < 0.01; 248.19 ± 36.98 pg/mg vs 541.74 ± 58.34 pg/mg, P < 0.01 and 81.26 ± 2.98 mmol/g vs 101.90 ± 10.73 mmol/g, P < 0.001) were increased in colon of rats with UC compared controls respectively.Naringenin supplementation, significantly and dose dependently increased the colonic mucus content. The elevated TBARS levels were significantly decreased (39.35 ± 5.86 nmol/L, P < 0.05; 26.74 ± 3.17 nmol/L, P < 0.01 nmol/L and 17.74 ± 2.69 nmol/L, P < 0.01) compared to AA (59.90 ± 16.34 nmol/L) group while the decreased levels of T-GSH and NP-SH and activities of CAT and SOD found increased by NG treatments in dose dependent manner. The decreased values of nucleic acids and total protein in AA group were also significantly (P < 0.01) increased in all three NG supplemented groups respectively. NG pretreatment inhibited the TNF-α levels (123.76 ± 3.76 pg/mg, 122.62 ± 3.41 pg/mg and 121.51 ± 2.61 pg/mg vs 134.24 ± 4.78 pg/mg, P < 0.05) compared to AA group, respectively. Interleukins, IL-1β and IL-6 levels were also decreased in NG50 + AA (314.37 ± 16.31 pg/mg and 292.58 ± 23.68 pg/mg, P < 0.05) and NG100 + AA (416.72 ± 49.62 pg/mg and 407.96 ± 43.87 pg/mg, P < 0.05) when compared to AA (352.46 ± 8.58 pg/mg and 638.58 ± 115.98 pg/mg) group. Similar decrease (P < 0.05) was seen in PGE2 and NO values when compared to AA group. The group pretreated with MES, as a reference drug, showed significant (P < 0.01) protection against the changes induced in colon tissue by AA administration respectively.
CONCLUSION: In present study, NG produced antioxidant and anti-inflammatory effects demonstrating protective effect in inflammatory bowel disease.
Keywords: Naringenin, Ulcerative colitis, Inflammatory bowel disease, Oxidative stress
Core tip: Inflammatory bowel disease (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC), results in substantial morbidity and is difficult to treat. New strategies for adjunct therapies are needed. Systemic corticosteroids are highly effective at inducing clinical remission in cases of acute exacerbation of CD and UC; however, their use is limited by their frequent and sometimes severe side effects. Results of present study revealed that, naringenin has protective effects against acetic acid-induced UC by inhibiting inflammatory and oxidative bio-markers. Thus, it may pose promising outcomes for future clinical usage as a natural non-toxic effective supplement in IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a common chronic inflammatory disease of the gastrointestinal tract. Several etiological factors, such as genetic, immunological, and environmental have been linked with the pathophysiology of the disease[1]. There are two main subtypes of IBD; Crohn’s disease (CD) and ulcerative colitis (UC) having a combined prevalence of 150-250/100000 population[2,3]. Moreover, the prevalence of hospitalization due to CD and UC is estimated to be 50.1 and 50.6 per 100000 population, respectively[4]. UC involves only the colon and rectum. Although the etiology of UC is not completely understood, it has been commonly associated with reduced antioxidant capacity as well as increased free radical production such as reactive oxygen species (ROS)[5]. Over production of ROS leads to lipid peroxidation (LPO), which can inhibit cellular antioxidant capability finally resulting in prominent colonic inflammation[6]. Clinically, colitis patients were found to overproduce ROS and nitrogen species leading to LPO of membranes and attack on tissue proteins and DNA[7,8]. Endogenous antioxidant defenses against ROS production even in low concentrations influence on two main types: (1) enzymatic such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT); and (2) non-enzymatic such as glutathione (GSH) and ascorbic acid (vitamin C). It is suggested that inflammatory response amplification can induce inflammatory cells chemotaxis resulting in release of ROS and inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β, which triggers the pathological responses and symptoms during IBD[9]. Elevated levels of pro-inflammatory cytokines in both the IBD forms reported to have a vital role of such mediators, which also play in determining the severity of the disease[10]. Medications that have ability to inhibit the production of these inflammatory mediators are shown to be clinically effective, which indicate their contribution to IBD and other chronic inflammatory conditions aggravation[11].
Some natural products, such as flavonoids, are getting more attention as novel agents for therapeutically usage. Flavonoids are one of the most abundant natural antioxidants present in plants and the human diets. Naringenin (4,5,7-trihydroxy flavonone) a flavonoid, widely distributed in citrus fruits, tomatoes, cherries, and cocoa[12]. Several pharmacological studies revealed its effects including antidiabetic[13], antiatherogenic[14], antidepressant[15], immunomodulatory[14], antitumor[16], DNA protective[17], hypolipidemic[18] and peroxisome proliferator-activated receptors activator[19]. It has also been shown to have prominent antioxidant[20] and anti-inflammatory[21] potentials. InêsAmaro et al[22] reported that, naringenin (NG) has reducing effect on intestinal edema-induced by dextran sodium sulphate in mice.
In several studies, pathogenesis of UC disease has demonstrated that excessive inflammation and oxidative stress play a significant role[23,24]. Amelioration of LPO as well as free radicals scavenging would provide a useful, protective and therapeutic treatment for UC. With respect to the high antioxidant capacity and anti-inflammatory activity, NG would be expected to reduce injury and/or improve tissue healing following injury from ulcerative colitis. In the present study we had evaluated the protective effect of NG during experimental ulcerative colitis and the possible mechanism of action.
MATERIALS AND METHODS
Animals and ethical approval
Eight weeks old male Wister albino rats weighting 250-280 g were received from Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Animals were housed under controlled environmental conditions (25 °C and a 12 h light/dark cycle). Animals had free access to Purina rat chow (Manufactured by Grain Silos and Flour Mills Organization, Riyadh, Saudi Arabia) and tap water. All experimental procedures and protocolsin this study including euthanasia were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (NIH Publications No. 80-23; 1996) as well as the Ethical Guidelines of the Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Induction of ulcerative colitis
Experimental ulceration in colon tissue was done according to the method described by Mousavizadeh et al[25] with slight modification. In brief, under light ether anesthesia rats were administered 2 mL of 4% acetic acid solution (v/v; Merck, Darmstadt, Germany) by transrectally using a (2.7 mm) soft pediatric catheter. After AA administration, rats were holed horizontally for 2 min to prevent AA leakage. Control animals underwent the same procedure using equal volume of normal saline instead of AA solution.
Experimental design
Forty two rats were divided into seven groups (six animals in each) as follows: (1) Control (Cont); (2) NG 100 mg/kg per day (NG100); (3) AA treated rats (AA); (4) NG 25 mg/kg per day + acetic acid (NG25 + AA); (5) NG 50 mg/kg per day + AA (NG50 + AA); (6) NG 100 mg/kg per day + AA (NG100 + AA); and (7) MES 300 mg/kg per day + AA (MES + AA). Naringenin (Sigma Aldrich, United States) and MES treatments were continued for 7 consecutive days by gavage[26]. At end of the treatment, ulcerative colitis was induced in all AA groups. Twenty four hours after the colitis induction, animals were sacrificed under deep anesthesia[27]. The colon (5-6 cm) specimens were dissected, washed with saline solution, imaged, weighted and small cross section was fixed in 10% formaldehyde solution for histopathological evaluation. The remaining tissues were stored at -75 °C (Ultra-low freezer, Environmental Equipment, Cincinnati, Ohio, United States) till analysis.
Evaluation of the adherent colonic mucus
The modified procedure of Popov et al[28] was used to determine adherent colonic mucus concentration. Briefly, a small portion of colon tissue was excised, weighted then transferred immediately to 1% Alcian blue solution (in 0.16 mol/L sucrose solution buffered with sodium acetate, pH 5) for 24 h. The excess dye was removed by rinsing with sucrose solution. The dye complexed with the gastric wall mucus was extracted using 10 mL of 0.5 mol/L MgCl2 solution. A 4 mL aliquot of blue extract was then shaken with an equal volume of diethyl ether. The resulting emulsion was centrifuged at 4000 rpm and the absorbance of the aqueous layer was recorded at 580 nm by using spectrophotometer (LKB-Pharmacia, Mark II, Ireland). The quantity of Alcian blue extracted (μg) per grams of wet colonic tissue was then calculated.
Histopathological investigations
Colon sections were fixed 10% neutral buffered formalin then put for 24 h in decal. Samples were then cut into several sections and embedded into paraffin wax blocks.Tissues were stained with haematoxylin and eosin and were mounted and observed microscopically for histopathological changes by a pathologist in blinded fashion.
Estimations of total glutathione sulphadryls and non-protein sulphadryls concentrations in colon
In colon tissues, total glutathione sulphadryls (T-GSH) and non-protein sulphadryls (NP-SH) levels were estimated according to the method described by Sedlak et al[29]. Tissues were homogenated in ice-cold 0.02 mol/L ethylenediamine tetra-acetic acid. An aliquots of 0.5 mL of tissue homogenate was mixed with 0.2 mol/L Tris buffer, pH 8.2 and 0.1 mL of 0.01 mol/L Ellman’s reagent, [5,5’-dithiobis-(2-nitr-benzoic acid)] (DTNB). Each sample tube was centrifuged at 3000 rpm at room temperature for 15 min the absorbance of the clear supernatant was measured using spectrophotometer (LKB-Pharmacia, Mark II, Ireland) at 412 nm. For NP-SH estimation, homogenate was diluted with distilled H2O and mixed with 1 mL of 50% trichloroacetic acid (TCA). The tubes were shaken intermittently for 10-15 min and centrifuged for 15 min at approximately 3000 g. Two milliliter of supernatant was then added to 4 mL of 0.4 mol/L Tris buffer (pH 8.9) then 0.1 mL DTNB added. The absorbance was read within 5 min of the addition of DTNB at 412 nm against a reagent blank.
Estimation of thiobarbituric acid reactive substances levels in colon
A thiobarbituric acid reactive substances (TBARS) assay kit (ZeptoMetrix, United States) was used to measure the LPO products, malondialdehyde (MDA) equivalents. Briefly, one hundred microliters of colon homogenate was added to 2.5 mL reaction buffer (provided by the kit) and heated at 95 °C for 60 min. After the mixture cooling, supernatant absorbance was measured at 532 nm using a spectrophotometer (LKB-Pharmacia, Mark II, Ireland). The LPO products are expressed in terms of nmoles MDA/mg protein.
Estimation of CAT and SOD activities in colon
Catalase activity in colon tissues was estimated by the method described by Aebi[30]. In brief, aliquot of 0.5 mL post-mitochondrial supernatant was mixed with 2.5 mL of 50 mmol/L phosphate buffer (pH 7.0) and 20 mmol/L H2O2. CAT activity was estimated spectrophotometrically following the decrease in absorbance at 240 nm and expressed in terms of units/mg protein as compared to a standard curve.
The SOD activity in colon tissue was measured by using the method described by Kono[31]. The principle of this method was that superoxide anions generated by the oxidation of hydroxylamine hydrochloride can mediate the reduction of nitrobluetetrazolium to blue formazon. The color was then measured at 560 nm under aerobic conditions. Addition of superoxide dismutase inhibited nitrobluetetrazolium reduction and the extent of this inhibition was taken as a measure of enzymatic activity. The SOD activity was expressed as units/mg protein.
Determination of nucleic acids and total protein levels in colon
The method described by Bregman[32] was used to determine the levels of nucleic acids (DNA and RNA) in colon. In brief, colon tissues were homogenized in 4 mL ice-cold distilled water and 2 mL homogenate was suspended in 5 mL of 10% ice-cold trichloroacetic acid (TCA). After centrifugation, the pellet was extracted twice with 95% ethanol. Finally, the nucleic acids were extracted in 5% TCA. DNA was determined by treating the nucleic acid extract with diphenylamine reagent and measuring the intensity of blue color at 600 nm. For quantification of RNA, the nucleic acid extract was treated with orcinol reagent and the green color was read at 660 nm. Standard curves were used to determine the amounts of nucleic acids present. Total protein in colon was estimated by Lowry et al[33] method using Bovine plasma albumin as a standard.
Determination of inflammatory cytokines, PGE2 and NO levels in colon
In colon, TNF-α, IL-1β, IL-6 and PGE2 levels were assessed and quantified according to the method ofMousavizadeh et al[25] using enzyme-linked immunoabsorbent assay ELISA (R and D systems, United States). The results were expressed as pg/mg tissue. Levels of colonic nitric oxide were assayed by Griess reaction method using commercial kit (R and D systems, United States).
Statistical analysis
Data were expressed as mean ± SE. Statistical analysis was carried out using one-way ANOVA followed by Newman-Keuls post hoc test. P value of ≤ 0.05 was considered statistically significant. All statistics tests were conducted using Graph Pad Prism (version 5) software.
RESULTS
Acetic acid significantly (P < 0.01) increased the colonic weight as compared to control group. Pretreatment with NG following three doses and MES for 7 d, showed significant (P < 0.05) inhibition in weight increase while compared to AA group (Figure 1A). Mucus concentration was significantly (P < 0.01) reduced in AA administered group when compared to control animals. Pretreatment with higher doses (50 and 100 mg/kg) of NG and MES significantly elevated the reduced colonic mucus concentration (P < 0.05, P < 0.01 and P < 0.05, respectively) as compared to AA group (Figure 1B). The colon images were clearly showed the induction of ulceration and its protection by the treatments (Figure 1C).
Figure 1.
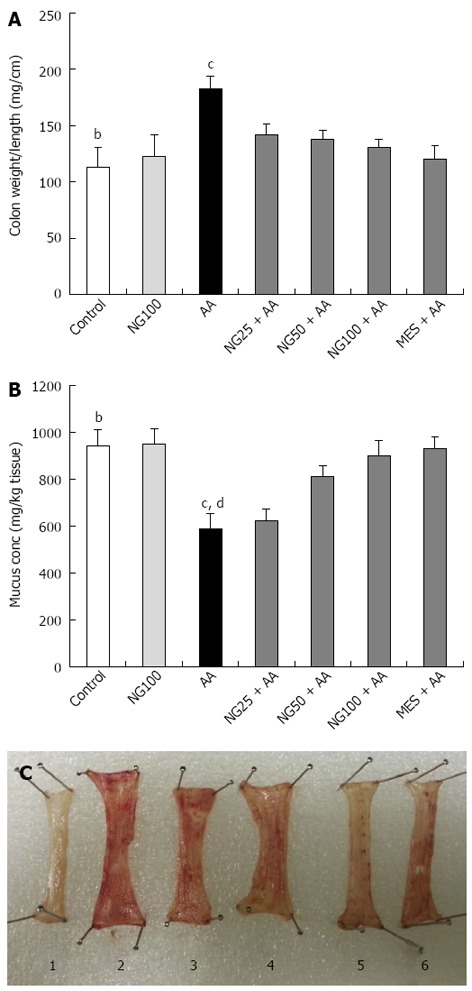
Effect of naringenin on colon weight/length (A), mucus concentration (B) and induction of ulceration and its protection by treatments in colonic tissue of rats with acetic acid-induced ulcerative colitis (C) (n = 6). Values in (A) and (B) are expressed as mean ± SE and analyzed using one way analysis of variance followed by Newman-Keuls post hoc test. bP < 0.01 control vs acetic acid (AA); cP < 0.05, dP < 0.01 AA vs naringenin (NG) 25 + AA, NG50 + AA, NG100 + AA or mesalazine (MES) + AA groups. Groups in (C) are arranged as follows: control (1), AA (2), NG25 + AA (3), NG50 + AA (4), NG100 + AA (5) and MES + AA (6).
Histopathological changes with their intensity are presented in Figure 2. Slide from control group, showing benign mucosal epithelium of tall columnar epithelial cells with goblet cells (Table 1 and Figure 2A). In the AA group, the slide revealed diffused active colitis with widely eroded mucosa with ulcerations and necrosis associated with edema, goblet cell hyperplasia, lymphoid follicular hyperplasia and transmural lymphoplasmacytic infiltrate with few intraepithelial neutrophilic cells within stromal (Table 1 and Figure 2B). In NG25 + AA group, slight healing epithelial cells with scattered superficial ulcers lined by colonic glands with reparative epithelial changes with hyperchromatic nuclei and infrequent mitosis and less goblet cells surrounded by transmucosal fewer lymphoplasmacytic infiltrate within stromal edemawas seen (Table 1 and Figure 2C). Slide from NG50 + AA group showed intestinal rat lined by healing epithelial cells with tall columnar epithelium, with superficial shredded epithelial cells, less eroded surface surrounded by few inflammatory edema and less necrosis with colonic gland showed reparative epithelial changes (Table 1 and Figure 2D). In higher dose of NG treatment (NG100 + AA) group, superficial tiny eroded mucosa with mucosal, hemorrhage, edema and scattered acute and chronic inflammatory cells infiltrate surrounding colonic glands with reparative epithelial changes and few goblet cells were seen (Table 1 and Figure 2E). Slide from MES + AA group revealed intestinal section with more better healed and improvement of intestinal mucosa compared to positive controlled sections with few mucosal lymphoplasmacytic infiltrate within stromal edema (Table 1 and Figure 2F).
Figure 2.
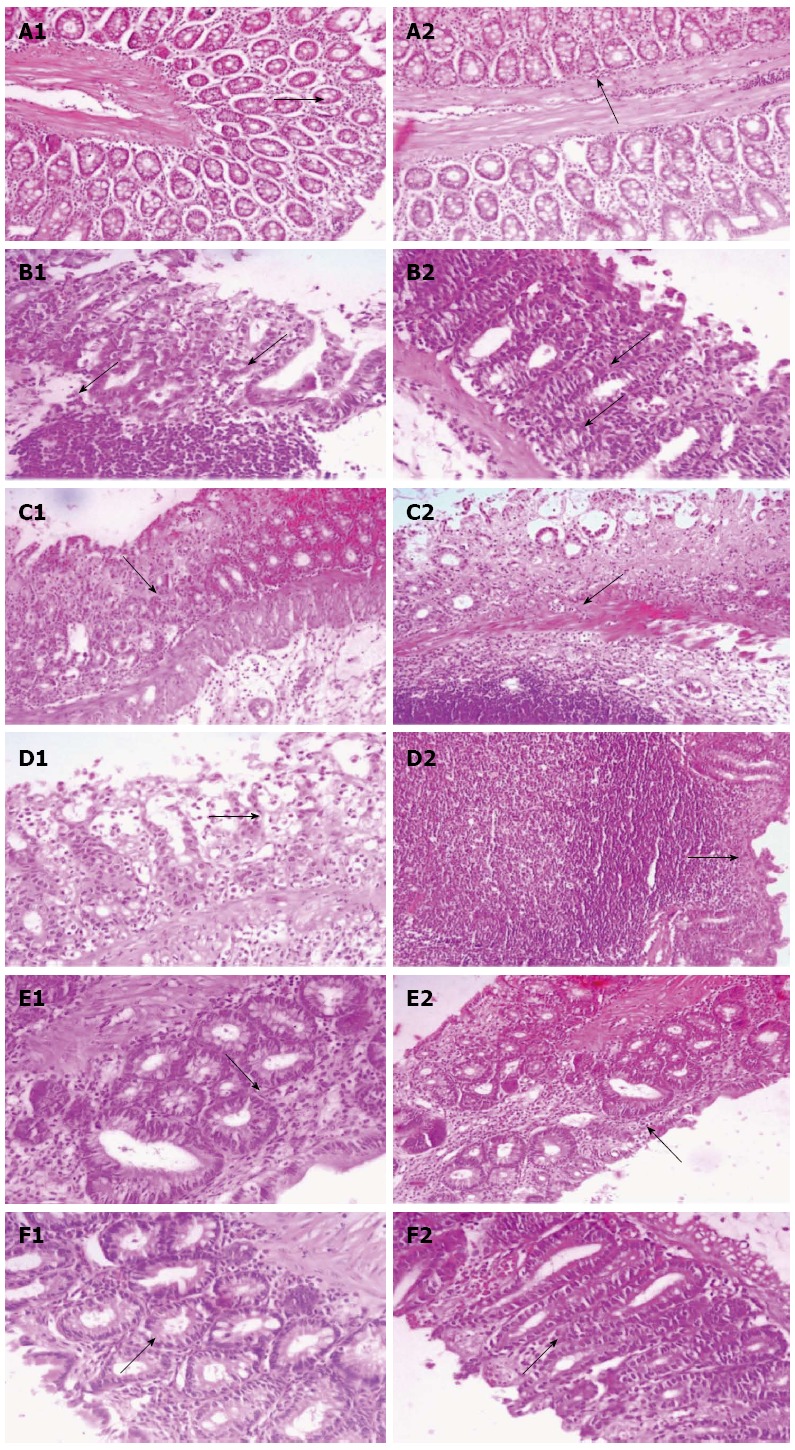
Histopathological changes with their intensity are presented. A-1 and 2: Histopathological colonic sections showing normal benign looking mucosa; B-1 and 2: Diffused active colitis with superficial erosions, stormal edema, dense acute and chronic inflammatory cells infiltrate with widely ulcerating mucosa; C-1 and 2: Reparative epithelial changes with little ulcer healing and inflammatory cells infiltrate; D-1 and 2: Reparative epithelial changes and healing ulcer with lymphoid follicle form; E-1 and 2: Healing ulcer and reparative epithelial changes; F-1 and 2: Attenuated cell damage with complete ulcer healing. A1-F1 (× 400), A2-F2 (× 200).
Table 1.
Effect of naringenin on microscopic scoring of histopathological sections of colonic tissue of rats with acetic acid-induced ulcerative colitis
| Groups | Ulceration | Hyperemia | Necrosis | Edema | Cellular infiltrate | Goblet cell hyperplasia |
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| AA | +++ | +++ | ++++ | +++ | ++++ | ++ |
| NG25 + AA | ++ | ++ | +++ | ++ | +++ | ++ |
| NG50 + AA | ++ | ++ | ++ | ++ | +++ | + |
| NG100 + AA | + | + | + | + | ++ | + |
| MES + AA | + | + | + | + | + | + |
MES: Mesalazine; AA: Acetic acid; NG: Naringenin; NG25: NG 25 mg/kg per day; NG50: 50 mg/kg per day; NG100: 100 mg/kg per day; 0: Normal; +: Mild; ++: Moderate; +++: Sever; ++++: Very sever.
Acetic acid administration resulted in a significant (P < 0.01) decrease in colon levels of both T-GSH and NP-SH when compared to control animals. Pretreatment with NG with higher doses (50 mg/kg and 100 mg/kg) significantly (P < 0.01) attenuated T-GSH and NP-SH (P < 0.05) the reduced levels as compared to AA group. Pretreatment with MES significantly inhibited the decreased levels of T-GSH and NP-SH (P < 0.001 and P < 0.05, respectively) (Figure 3A and B). Concentration of TBARS in the colons of AA treated rats were significantly (P < 0.01) increased compared to control animals. A significantly lower concentrations of TBARS values were found in NG25 + AA (P < 0.05), NG50 + AA (P < 0.01), NG100 + AA (P < 0.01) and MES + AA (P < 0.01) groups as compared to AA group (Figure 3C). CAT activity was significantly (P < 0.01) decreased in colon tissues of AA administered rats compared to control animals. Pretreatment with 50 mg/kg per day and 100 mg/kg per day of NG, significantly (P < 0.05) inhibited the decrease CAT activity in colon as compared to AA group (Figure 3D). SOD activity was significantly (P < 0.01) reduced in the colons of AA treated animals as compared to control rats. Group of rats pretreated with 100 mg/kg per day of NG for 7 d showed a significant (P < 0.05) increase in colon SOD activity while compared to AA treated animals (Figure 3E). Pretreatment with MES also markedly (P < 0.05 and P < 0.01, respectively) enhanced the CAT and SOD activities as compared to AA group (Figure 3D and E).
Figure 3.
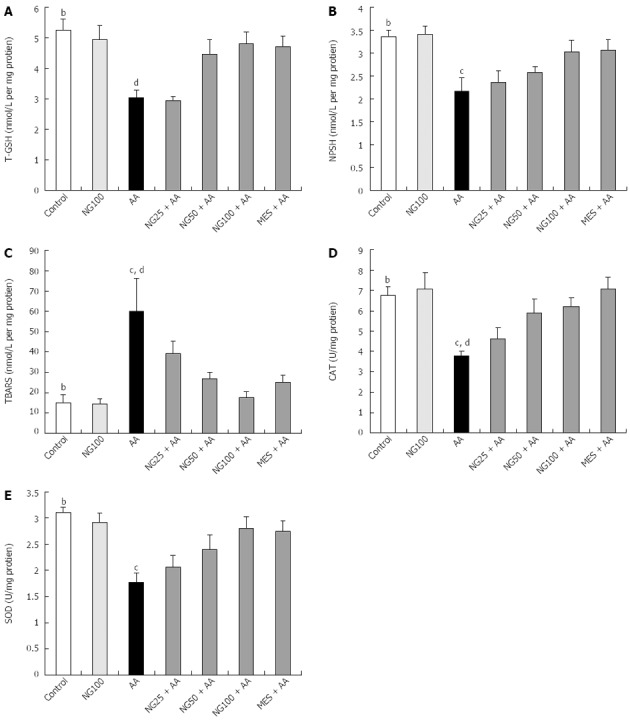
Effect of naringenin on total glutathione sulphadryls (A), non-protein sulphadryls (B) and thiobarbituric acid reactive substances levels (C) as well as catalase (D) and superoxide dismutase activities (E) in colonic tissue of rats with acetic acid-induced ulcerative colitis (n = 6). Values are expressed as mean ± SE and analyzed using one way analysis of variance followed by Newman-Keuls post hoc test. bP < 0.01 control vs AA; cP < 0.05, dP < 0.01 AA vs NG25 + AA, NG50 + AA, NG100 + AA or MES + AA groups. T-GSH: Total glutathione sulphadryls; NP-SH: Non-protein sulphadryls; TBARS: Thiobarbituric acid reactive substances; CAT: Catalase; SOD: Superoxide dismutase; AA: Acetic acid; UC: Ulcerative colitis; MES: Mesalazine; NG: Naringenin.
There was a significant (P < 0.001) decrease in colon levels of DNA, RNA and total protein in AA administered group as compared to control animals. Pretreatment with NG (100 mg/kg per day) or MES (300 mg/kg per day) significantly (P < 0.01) increased the DNA content in colon tissue compared to AA group. The RNA levels in NG higher doses and MES groups found significant (P < 0.01) elevation compared to AA group. Total protein levels were also significantly (P < 0.05) increased in NG50 + AA, NG100 + AA and MES + AA groups compared to AA group (Table 2).
Table 2.
Effect of naringenin on DNA, RNA and total protein levels in colonic tissue of rats with acetic acid-induced ulcerative colitis
| Groups | DNA (μg/100 mg wet tissue) | RNA (μg/100 mg wet tissue) | Total protein (mg/100 mg wet tissue) |
| Control | 652.05 ± 17.12b | 378.51 ± 38.44b | 3.0 ± 0.19b |
| NG100 | 688.84 ± 47.69 | 380.24 ± 48.96 | 2.43 ± 0.31 |
| AA | 222.69 ± 18.78d | 167.35 ± 15.16d | 0.95 ± 0.08c |
| NG25 + AA | 293.93 ± 33.49 | 208.68 ± 25.05 | 1.32 ± 0.13 |
| NG50 + AA | 338.54 ± 21.44 | 250.06 ± 10.11 | 2.02 ± 0.46 |
| NG100 + AA | 415.50 ± 41.51 | 298.34 ± 12.92 | 2.15 ± 0.27 |
| MES + AA | 425.04 ± 38.23 | 307.44 ± 18.31 | 2.14 ± 0.26 |
Values are expressed as mean ± SE (n = 6) and analyzed using one way ANOVA followed by Newman-Keuls post hoc test.
P < 0.01 control vs acetic acid (AA);
P < 0.05,
P < 0.01 AA vs Naringenin (NG) 25 + AA, NG50 + AA, NG100 + AA or mesalazine (MES) + AA groups.
Pro-inflammatory cytokines including TNF-α, IL-1β and IL-6 levels produced significant (P < 0.01) increase in AA-induced ulcerative colitis and levels found significantly (P < 0.05 and P < 0.01) diminished in NG higher doses and MES pretreated groups as compared to AA group, respectively (Figure 4A-C). Similar changes in PGE2 levels were seen in colon tissue of rats (Figure 4D). In colon tissue, NO levels were significantly (P < 0.01) increased AA group compared to controls. The elevated NO levels were markedly (P < 0.05) reduced in NG and MES treated group compared AA group respectively (Figure 4E).
Figure 4.
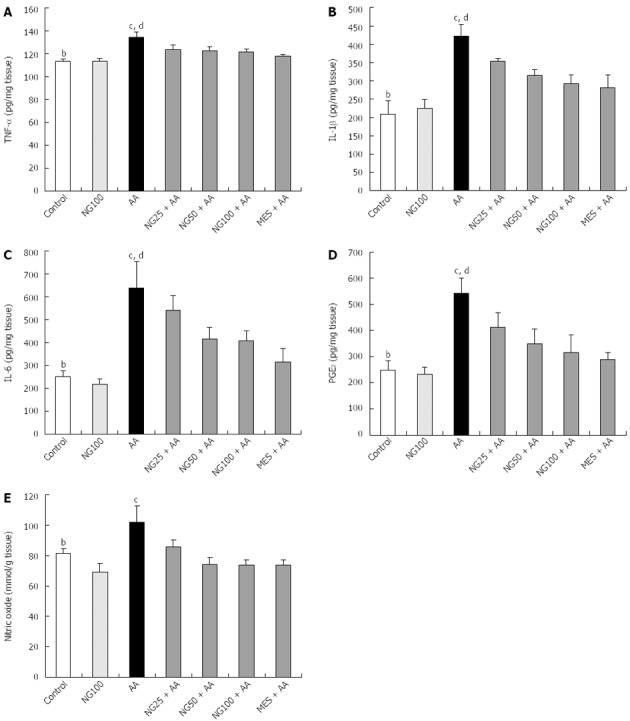
Effect of naringenin on tumor necrosis factor-α (A), interleukin-1β (B), interleukin-6 (C), prostaglandin E2 (D) and nitric oxide (E) levels in colonic tissue of rats with acetic acid-induced ulcerative colitis (n = 6). Values are expressed as mean ± SE and analyzed using one way analysis of variance followed by Newman-Keuls post hoc test. bP < 0.01 control vs AA; cP < 0.05, dP < 0.01 AA vs NG25 + AA, NG50 + AA, NG100 + AA or MES + AA groups. TNF-α: Tumor necrosis factor-α; IL-1β: Interleukin-1β; IL-6: Interleukin-6; PGE2: Prostaglandin E2; AA: Acetic acid; NG: Naringenin; MES: Mesalazine.
DISCUSSION
Present investigation outlines the anti-ulcerogenic effect of NG against experimentally induced UC in rats as a model for IBD. The preventative effect of NG was confirmed by histological evaluation and also using MES as a standard drug. Seven days pretreatment with NG significantly reduced the AA-induced colonic mucus content and prevented oxidative and inflammatory response in dose dependent manner.
Ulcerative colitis is characterized by mucosal inflammation and ulcerations with a variable extent and severity[34]. Rectal administration of 4% AA to experimental rodents to induce UC is a well-established animal model, which phenotypically resembles human colon inflammation[35]. It also causes colonic epithelial lesions and necrosis associated with neutrophils and macrophages infiltration to the damaged colon indicating inflammatory conditions[28,35]. In present study, the 4% AA administration resulted a significant increase in colonic weight and induced sever ulceration and tissue necrosis associated with inflammatory infiltrate and goblet cell hyperplasia as indicated in the results of the histopathologicalestimations. Similar pathological impairments were reported in earlier studies using the same animal model[26,36]. Application of AA in the present study disturbed the colonic mucus, which is in agreement with Popov et al[28]. Colonic mucus plays an important protective role against chemically induced ulceration which may also facilitate the repair of the damaged epithelium[37]. Although, numerous pharmacotherapies have been suggested for UC treatment, the side effects or toxicity of these medications are a major clinical problem[38]. That is why naturally occurring products such as flavonoids are now suggested as an alternative option beside the conventional therapies[39]. Indeed, earlier experimental studies demonstrated flavonoids such as quercitrin, kushenin, kaempferol and baicalin to promote UC healing[40-43].
Previous studies demonstrated that NG administration effectively protected the experimentally induced gastric lesions and ulcers[44,45]. Protection against experimental UC induced by NG was accompanied by restoration of the increased colon thickening in AA group, which is an indirect assessment of colon inflammation. Microscopic scoring of the histopathological sections confirmed the protective action of NG as it decreased colonic tissue ulceration, necrosis and inflammation in dose dependent manner. Motilva et al[45] reported that NG treatment increased the gastric mucus levels in rats induced gastric lesions by absolute ethanol. In the present study, NG was also found to inhibit the depletion of colonic mucus caused by AA treatment. This protective activity could be attributed to its antioxidant and anti-inflammatory properties.
Oxidative stress is known to play an important role in IBD initiation and progression[46]. Experimentally induced colitis in animals is characterized by oxidative damage and an imbalance between oxidant and antioxidant substances[47]. The AA-induced colitis model is known to cause vascular dilatation and white blood cells accumulation, as well as an increase in blood flow, leading to increased production of oxygen and hence the excessive generation of free radical and ROS[35,48]. Several studies have indicated the vital role that free radicals play in the pathogenesis of mucosal injuries[49,50]. Moreover, free radicals and ROS were reported in colorectal specimens of ulcerative colitis[51,52]. The first line of oxidative defense system against free radicals is the sulphadryls groups in peptide namely GSH or NP-SH. It is widely distributed in all biological tissues and work as a non-enzymatic antioxidant. GSH inhibits ROS oxidative injuries directly via its sulfhydryl group and indirectly as a cofactor or a coenzyme in ROS enzymatic detoxification process[53,54]. Another line in oxidative defense system is the enzymatic antioxidants. Examples for important antioxidant enzymes are SOD, CAT, and GPx[55]. In present study, levels and activities of non-enzymatic and enzymatic defense systems were severely decreased in the colon of AA administered animals indicating oxidative cellular injury. Furthermore, free radicals are known to attack lipid contents of cellular membranes leading to activation of LPO process and cellular damage. Therefore, the concentrations of LPO specific products such as TBARS were elevated, while the critical cellular macro- and micro-molecules such as nucleic acids and total proteins levels were decreased in the present work indicating cellular oxidative injury and cytotoxicity. These results, which are in agreement with previous findings, suggest the harmful effects of AA on cellular macromolecules and its ability to impair the epithelial cell integrity and hinder mucosal recovery[23,36].
In present study, NG was able to attenuate AA induced oxidative damage and injury of colon tissues confirming its strong antioxidant and anti-inflammatory properties. It has seen in earlier studies that NG markedly increased the antioxidant markers such as GSH, NP-SH levels and SOD, and CAT activities[12,56,57]. Han et al[56] found that NG pretreatment can increase the activity of antioxidant enzyme GPx, which suggest the ability of NG to attenuate oxidative stress by decreasing the lipid peroxide level and to inhibit accumulation of free radicals generation during LPO process[57]. In the current study, NG treatment significantly corrected the impaired levels of nucleic acids and total protein in colon tissue suggesting the cytoprotective properties of the naturally occurring flavonoids. The antioxidant activity of NG depends mainly on the presence of B-ring catechol group, which can stabilize a radical species by donating hydrogen (H + )[12].
Inflammatory cytokines are known to play a crucial role in modulating mucosal immune system where the neutrophils and macrophages are responsible for disrupting epithelial integrity and causing colon injury[58]. The pathogenesis of UC is characterized by migration of granulocytes and other leukocytes to the inflamed mucosa and superficial ulcers leading to increased levels of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β[59,60]. In present study, the elevated colon level of TNF-α, IL-6 and IL-1β in AA administered group is an evidence for epithelial cell necrosis, edema, and neutrophil infiltration, which is also supported by the histopathological results. These results are in accordance with earlier experimental and clinical studies[6,28,36,61]. The reported increased levels of colonic PGE2 in AA group of animals is in agreement with Otani et al[62], where the enhanced level of PGE2 was attributed to its overproduction rather than decreased metabolism, both of which are mediated by pro-inflammatory cytokines. Naringenin was found in the current and earlier studies to inhibit the level of inflammatory cytokines including TNF-α, IL-6 and IL-1β[63]. The anti-inflammatory properties of naringenin were suggested to be through several mechanisms including increased phosphorylation of ERK 5 and P38 MAPK and inhibition of NF-κB and activator protein-1 signaling[64,65]. Additionally, naringenin, which present in high concentrations in citrus fruits, was found to block NF-κB activation resulting in down regulation of the downstream target genes of NF-κB such as iNOS and COX-2 expression[66]. These enzymes catalyze oxidative stress-induced production of NO and prostaglandins respectively, which are known as an important inflammatory mediators in the pathogenesis of colitis[63,67]. These findings are in agreement with our results where pretreatment with naringenin significantly ameliorated AA induced elevation of the level of PGE2 and NO in rats’ colon.
In conclusion, the present study revealed that NGprotects the AA-induced ulcerative colitis by inhibiting inflammatory and oxidative bio-markers. Finally, our results may pose promising outcomes for future clinical usage of NG as a natural non-toxic effective supplement in IBD.
COMMENTS
Background
The pathogenesis of inflammatory bowel disease (IBD) such as ulcerative colitis (UC) is usually associated with reduced antioxidant capacity. Generation of free radicals like reactive oxygen species (ROS) leads to lipid peroxidation, which inhibits cellular antioxidant capability, resulting in prominent colonic inflammation. There is a great need to search for safe and tolerable compounds for the management of IBD to reduce patient compliance as well as the adverse effects of conventional treatments. Naringenin (NG) is a naturally occurring flavonoid that can be extracted from citrus fruits, tomatoes, cherries, grapefruit, and cocoa. Like most of the flavonoids, NG was experimentally found to have several pharmacological potentials, including antioxidant, antitumor and anti-inflammatory because of NG has properties to produce sufficient hydroxyl (-OH) substitutions, which give it the capability to scavenge ROS. Thus, it has considered that NG may diminish and/or improve pathological conditions where oxidation or inflammation is deemed to play a vital role, like in case of IBD.
Research frontiers
In the present study, NG was orally (gavage) treated with three doses (25, 50 and 100 mg/kg per day body weight) for 7 consecutive days, 24 h later UC was induced by 4% acetic acid. In colitis tissue, Alcian blue, pro-oxidative and inflammatory biomarkers were estimated. The biochemical alterations were further justified with histopathological changes.
Innovations and breakthroughs
NG pretreatment clearly revealed the protection against acetic acid-induced UC in animal model. Antioxidant and anti-inflammatory properties of NG are suggested to be the key for these effects as NG significantly reduced oxidative stress and inflammatory biomarkers in a dose dependent manner.
Applications
The present data shows that NG has a promising protective effect against experimentally-induced UC in animal model. Thus, NG could be recommended for its use as potential alternative and complementary therapy for IBD after confirmation of the obtained findings by clinical trials.
Peer review
The preclinical preventative properties of NG against UC are outlined in present study. Also the possible pharmacological mechanisms of action responsible for these effects are evaluated. Overall, this study proofed that NG is an effective and safe compound that worth to be investigated in future clinical trials for its colonic anti-ulcerogenic properties.
Footnotes
Supported by The Deanship of Scientific Research at King Saud University for its funding this research through the research group project, No. RGP-VPP-266
P- Reviewers Auci DL, de Medina FS, Tebo AE S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Selling JH, Pasricha PJ. Pharmacotherapy of inflammatory bowel disease. In: Gilman’s G, editor. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill Publications; 2006. p. 1009. [Google Scholar]
- 2.Parkes M, Jewell D. Ulcerative colitis and Crohns disease: molecular genetics and clinical implications. Expert Rev Mol Med. 2001;2001:1–18. doi: 10.1017/S146239940100391X. [DOI] [PubMed] [Google Scholar]
- 3.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 4.Button LA, Roberts SE, Goldacre MJ, Akbari A, Rodgers SE, Williams JG. Hospitalized prevalence and 5-year mortality for IBD: record linkage study. World J Gastroenterol. 2010;16:431–438. doi: 10.3748/wjg.v16.i4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 6.Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, Tahan V, Uzun H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011;54:333–338. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371–2384. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tüzün A, Erdil A, Inal V, Aydin A, Bağci S, Yeşilova Z, Sayal A, Karaeren N, Dağalp K. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–572. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 9.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- 10.Bertevello PL, Logullo AF, Nonogaki S, Campos FM, Chiferi V, Alves CC, Torrinhas RS, Gama-Rodrigues JJ, Waitzberg DL. Immunohistochemical assessment of mucosal cytokine profile in acetic acid experimental colitis. Clinics (Sao Paulo) 2005;60:277–286. doi: 10.1590/s1807-59322005000400004. [DOI] [PubMed] [Google Scholar]
- 11.Andoh A, Yagi Y, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008;14:5154–5161. doi: 10.3748/wjg.14.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain A, Yadav A, Bozhkov AI, Padalko VI, Flora SJ. Therapeutic efficacy of silymarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol Environ Saf. 2011;74:607–614. doi: 10.1016/j.ecoenv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Andrade RR, Sánchez-Salgado JC, Navarrete-Vázquez G, Webster SP, Binnie M, García-Jiménez S, León-Rivera I, Cigarroa-Vázquez P, Villalobos-Molina R, Estrada-Soto S. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obes Metab. 2008;10:1097–1104. doi: 10.1111/j.1463-1326.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Lisa J, Wilcox M, Borradaile W. Antiatherogenic Properties of Naringenin, a Citrus Flavonoid. Cardiovasc Drug Rev. 1999;17:160–178. [Google Scholar]
- 15.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 16.Jong-Hwa P, Jin-Woo L, Hyun-Dong P, Ssang-Goo C, Seung-Yeol N, Yong-Sun P, Ye-Sun H. Cytotoxic Effects of 7-O-Butyl Naringenin on Human Breast Cancer MCF-7 Cells. Food Sci Biotechnol. 2010;19:717–724. [Google Scholar]
- 17.Oršolić N, Gajski G, Garaj-Vrhovac V, Dikić D, Prskalo ZŠ, Sirovina D. DNA-protective effects of quercetin or naringenin in alloxan-induced diabetic mice. Eur J Pharmacol. 2011;656:110–118. doi: 10.1016/j.ejphar.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA, Huff MW. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009;58:2198–2210. doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PLoS One. 2010;5:e12399. doi: 10.1371/journal.pone.0012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahigude A, Bhutada P, Kaulaskar S, Aswar M, Otari K. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience. 2012;226:62–72. doi: 10.1016/j.neuroscience.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Hirai S, Kim YI, Goto T, Kang MS, Yoshimura M, Obata A, Yu R, Kawada T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007;81:1272–1279. doi: 10.1016/j.lfs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 22.InêsAmaro M, Rocha J, Vila-Real H, Eduardo-Figueira M, Mota-Filipe H, Sepodes B, Ribeiro MH. Anti-inflammatory activity of naringin and the biosynthesisednaringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res Int. 2009;42:1010–1017. [Google Scholar]
- 23.Cetinkaya A, Bulbuloglu E, Kantarceken B, Ciralik H, Kurutas EB, Buyukbese MA, Gumusalan Y. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci. 2006;51:488–494. doi: 10.1007/s10620-006-3160-9. [DOI] [PubMed] [Google Scholar]
- 24.Koch TR, Yuan LX, Stryker SJ, Ratliff P, Telford GL, Opara EC. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig Dis Sci. 2000;45:1814–1819. doi: 10.1023/a:1005517824877. [DOI] [PubMed] [Google Scholar]
- 25.Mousavizadeh K, Rahimian R, Fakhfouri G, Aslani FS, Ghafourifar P. Anti-inflammatory effects of 5-HT receptor antagonist, tropisetron on experimental colitis in rats. Eur J Clin Invest. 2009;39:375–383. doi: 10.1111/j.1365-2362.2009.02102.x. [DOI] [PubMed] [Google Scholar]
- 26.Harputluoglu MM, Demirel U, Yücel N, Karadağ N, Temel I, Firat S, Ara C, Aladağ M, Karincaoğlu M, Hilmioğlu F. The effects of Gingko biloba extract on acetic acid-induced colitis in rats. Turk J Gastroenterol. 2006;17:177–182. [PubMed] [Google Scholar]
- 27.Kannan N, Guruvayoorappan C. Protective effect of Bauhinia tomentosa on acetic acid induced ulcerative colitis by regulating antioxidant and inflammatory mediators. Int Immunopharmacol. 2013;16:57–66. doi: 10.1016/j.intimp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Popov SV, Markov PA, Nikitina IR, Petrishev S, Smirnov V, Ovodov YS. Preventive effect of a pectic polysaccharide of the common cranberry Vaccinium oxycoccos L. on acetic acid-induced colitis in mice. World J Gastroenterol. 2006;12:6646–6651. doi: 10.3748/wjg.v12.i41.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 30.Aebi H. Catalase. In: Bergmeyer HV, editor. Methods in enzymatic analysis. New York: Verlag Chemie-Academic Press; 1974. pp. 674–684. [Google Scholar]
- 31.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 32.Bregman A. Laboratory Investigation and Cell Biology. New York: John Wiley and Sons; 1983. [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.D’Argenio G, Mazzone G, Tuccillo C, Ribecco MT, Graziani G, Gravina AG, Caserta S, Guido S, Fogliano V, Caporaso N, et al. Apple polyphenols extract (APE) improves colon damage in a rat model of colitis. Dig Liver Dis. 2012;44:555–562. doi: 10.1016/j.dld.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann RM, Morgan Martins MI, Tieppo J, Fillmann HS, Marroni NP. Effect of Boswellia serrata on antioxidant status in an experimental model of colitis rats induced by acetic acid. Dig Dis Sci. 2012;57:2038–2044. doi: 10.1007/s10620-012-2134-3. [DOI] [PubMed] [Google Scholar]
- 36.El-Abhar HS, Hammad LN, Gawad HS. Modulating effect of ginger extract on rats with ulcerative colitis. J Ethnopharmacol. 2008;118:367–372. doi: 10.1016/j.jep.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Al-Rejaie SS, Abuohashish HM, Ahmed MM, Aleisa AM, Alkhamees O. Possible biochemical effects following inhibition of ethanol-induced gastric mucosa damage by Gymnema sylvestre in male Wistar albino rats. Pharm Biol. 2012;50:1542–1550. doi: 10.3109/13880209.2012.694894. [DOI] [PubMed] [Google Scholar]
- 38.Sands BE. Therapy of inflammatory bowel disease. Gastroenterology. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- 39.Langmead L, Dawson C, Hawkins C, Banna N, Loo S, Rampton DS. Antioxidant effects of herbal therapies used by patients with inflammatory bowel disease: an in vitro study. Aliment Pharmacol Ther. 2002;16:197–205. doi: 10.1046/j.1365-2036.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez de Medina F, Vera B, Gálvez J, Zarzuelo A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002;70:3097–3108. doi: 10.1016/s0024-3205(02)01568-0. [DOI] [PubMed] [Google Scholar]
- 41.Tang Q, Fan H, Shou Z, Liu X. [Study on protective mechanism of kushenin injection on colonic mucosa of experimental colitis rats] Zhongguo Zhong Yao Zazhi. 2012;37:1814–1817. [PubMed] [Google Scholar]
- 42.Park MY, Ji GE, Sung MK. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig Dis Sci. 2012;57:355–363. doi: 10.1007/s10620-011-1883-8. [DOI] [PubMed] [Google Scholar]
- 43.Dai SX, Zou Y, Feng YL, Liu HB, Zheng XB. Baicalin down-regulates the expression of macrophage migration inhibitory factor (MIF) effectively for rats with ulcerative colitis. Phytother Res. 2012;26:498–504. doi: 10.1002/ptr.3581. [DOI] [PubMed] [Google Scholar]
- 44.Parmar NS. The gastric anti-ulcer activity of naringenin, a specific histidine decarboxylase inhibitor. Int J Tissue React. 1983;5:415–420. [PubMed] [Google Scholar]
- 45.Motilva V, Alarcón de la Lastra C, Martín MJ. Ulcer-protecting effects of naringenin on gastric lesions induced by ethanol in rat: role of endogenous prostaglandins. J Pharm Pharmacol. 1994;46:91–94. doi: 10.1111/j.2042-7158.1994.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 46.Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease--radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 47.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 48.Closa D, Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56:185–191. doi: 10.1080/15216540410001701642. [DOI] [PubMed] [Google Scholar]
- 49.Isozaki Y, Yoshida N, Kuroda M, Takagi T, Handa O, Kokura S, Ichikawa H, Naito Y, Okanoue T, Yoshikawa T. Effect of a novel water-soluble vitamin E derivative as a cure for TNBS-induced colitis in rats. Int J Mol Med. 2006;17:497–502. [PubMed] [Google Scholar]
- 50.Yoshikawa T, Ueda S, Naito Y, Takahashi S, Oyamada H, Morita Y, Yoneta T, Kondo M. Role of oxygen-derived free radicals in gastric mucosal injury induced by ischemia or ischemia-reperfusion in rats. Free Radic Res Commun. 1989;7:285–291. doi: 10.3109/10715768909087953. [DOI] [PubMed] [Google Scholar]
- 51.Bitiren M, Karakilcik AZ, Zerin M, Ozardali I, Selek S, Nazligül Y, Ozgonul A, Musa D, Uzunkoy A. Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol Trace Elem Res. 2010;136:87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- 52.Ademoglu E, Erbil Y, Tam B, Barbaros U, Ilhan E, Olgac V, Mutlu-Turkoglu U. Do vitamin E and selenium have beneficial effects on trinitrobenzenesulfonic acid-induced experimental colitis. Dig Dis Sci. 2004;49:102–108. doi: 10.1023/b:ddas.0000011610.47179.0b. [DOI] [PubMed] [Google Scholar]
- 53.Sivaprasad R, Nagaraj M, Varalakshmi P. Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem. 2004;15:18–23. doi: 10.1016/j.jnutbio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Sivaprasad R, Nagaraj M, Varalakshmi P. Lipoic acid in combination with a chelator ameliorates lead-induced peroxidative damages in rat kidney. Arch Toxicol. 2002;76:437–441. doi: 10.1007/s00204-002-0350-x. [DOI] [PubMed] [Google Scholar]
- 55.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Han XZ, Gao S, Cheng YN, Sun YZ, Liu W, Tang LL, Ren DM. Protective effect of naringenin-7-O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. Biosci Trends. 2012;6:19–25. doi: 10.5582/bst.2012.v6.1.19. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res. 2012;146:354–359. doi: 10.1007/s12011-011-9268-6. [DOI] [PubMed] [Google Scholar]
- 58.Grisham MB, Yamada T. Neutrophils, nitrogen oxides, and inflammatory bowel disease. Ann N Y Acad Sci. 1992;664:103–115. doi: 10.1111/j.1749-6632.1992.tb39753.x. [DOI] [PubMed] [Google Scholar]
- 59.Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–322. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 60.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 61.Stucchi A, Reed K, O’Brien M, Cerda S, Andrews C, Gower A, Bushell K, Amar S, Leeman S, Becker J. A new transcription factor that regulates TNF-alpha gene expression, LITAF, is increased in intestinal tissues from patients with CD and UC. Inflamm Bowel Dis. 2006;12:581–587. doi: 10.1097/01.MIB.0000225338.14356.d5. [DOI] [PubMed] [Google Scholar]
- 62.Otani T, Yamaguchi K, Scherl E, Du B, Tai HH, Greifer M, Petrovic L, Daikoku T, Dey SK, Subbaramaiah K, et al. Levels of NAD( + )-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G361–G368. doi: 10.1152/ajpgi.00348.2005. [DOI] [PubMed] [Google Scholar]
- 63.Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–931. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 64.Yang J, Li Q, Zhou XD, Kolosov VP, Perelman JM. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol Cell Biochem. 2011;351:29–40. doi: 10.1007/s11010-010-0708-y. [DOI] [PubMed] [Google Scholar]
- 65.Bodet C, La VD, Epifano F, Grenier D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J Periodontal Res. 2008;43:400–407. doi: 10.1111/j.1600-0765.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 66.Park HY, Kim GY, Choi YH. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-κB and inhibiting mitogen-activated protein kinases. Int J Mol Med. 2012;30:204–210. doi: 10.3892/ijmm.2012.979. [DOI] [PubMed] [Google Scholar]
- 67.Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]


