Abstract
AIM: To evaluate if 3 mo oral supplementation with Eviendep® was able to reduce the number of duodenal polyps in familial adenomatous polyposis (FAP) patients with ileal pouch-anal anastomosis (IPAA).
METHODS: Eleven FAP patients with IPAA and duodenal polyps were enrolled. They underwent upper gastrointestinal (GI) endoscopy at the baseline and after 3 mo of treatment. Each patient received 5 mg Eviendep twice a day, at breakfast and dinner time, for 3 mo. Two endoscopists evaluated in a blinded manner the number and size of duodenal polyps. Upper GI endoscopies with biopsies were performed at the baseline (T0) with the assessment of the Spigelman score. Polyps > 10 mm were removed during endoscopy and at the end of the procedure a new Spigelman score was determined (T1). The procedure was repeated 3 mo after the baseline (T2). Four photograms were examined for each patient, at T1 and T2. The examined area was divided into 3 segments: duodenal bulb, second and third portion duodenum. Biopsy specimens were taken from all polyps > 10 mm and from all suspicious ones, defined by the presence of a central depression, irregular surface, or irregular vascular pattern. Histology was classified according to the updated Vienna criteria.
RESULTS: At baseline the mean number of duodenal detected polyps was 27.7 and mean sizes were 15.8 mm; the mean Spigelman score was 7.1. After polypectomy the mean number of duodenal detected polyps was 25.7 and mean sizes were 7.6 mm; the mean Spigelman score was 6.4. After 3 mo of Eviendep bid, all patients showed a reduction of number and size of duodenal polyps. The mean number of duodenal polyps was 8 (P = 0.021) and mean size was 4.4 mm; the mean Spigelman score was 6.6. Interrater agreement was measured. Lesions > 1 cm found a very good degree of concordance (kappa 0.851) and a good concordance was as well encountered for smaller lesions (kappa 0.641).
CONCLUSION: Our study demonstrated that short-term (90 d) supplementation with Eviendep® in FAP patients with IPAA and with recurrent adenomas in the duodenal mucosa, resulted effective in reducing polyps number of 32% and size of 51%.
Keywords: Familial adenomatous polyposis, Ileal pouch-anal anastomosis, Duodenal polyps, Eviendep
Core tip: Our open study demonstrated for the first time that short-term (90 d) supplementation with Eviendep® in familial adenomatous polyposis patients with ileal pouch-anal anastomosis and with recurrent adenomas in the duodenal mucosa, resulted effective in reducing polyps number of 32% and size of 51%. Eviendep® was easy to manage and its daily use was well tolerated by the patients. Its safety was guaranteed by its composition. Each ingredient is blended into the composition in a lower dose than the one otherwise needed for the single component to similarly exert the desired effect, thus leading to synergistic and/or potentiating effect, with the added advantage of higher safety, even over long-term exposure.
INTRODUCTION
Familial adenomatous polyposis (FAP) is a disease with autosomal dominant inheritance. It is caused by an alteration of the FAP gene that is located on chromosome 5q21, affecting roughly 1 in 15000 live births in the Northern European population. FAP shares its phenotype with biallelic Homolog Gene Mutation Carriers, characterized by the early onset of hundreds to thousands of adenomas throughout the colon, with a nearly 100% progression to colorectal cancer by the age of 35-45 years in untreated subjects[1-3]. Patients with FAP have a cumulative lifetime risk of over 80% of developing duodenal adenomas, the precancerous lesions of duodenal adenocarcinoma. Consequently, these patients have a 4% lifetime risk of peri-ampullary or duodenal adenocarcinoma[4,5].
Early prophylactic colorectal surgery changed the prognosis of patients with FAP, and nowadays desmoids and peri-ampullary duodenal cancers are the most common causes of death in these patients[6,7].
Nearly 100% of FAP patients will develop duodenal adenomatosis[5-11] with an estimated lifetime risk of progression to duodenal carcinoma of 5%-10%[5-12]. The severity of duodenal adenomatosis is graded according to the Spigelman classification[10], which ranges from grade 0 to IV and is based on the number, size and histopathological features of the duodenal adenomas. Patients with advanced Spigelman stages are most at risk of developing duodenal carcinoma[5,10]. Current guidelines recommend frequent endoscopic surveillance in these patients, which improved the prognosis through earlier detection of duodenal malignancy[13].
A new recent line of intervention focuses on the role of the estrogen receptors (ERs) in intestinal carcinogenesis[14-16]. A pivotal role of ER-β has been suggested in preventing malignant transformation of colon epithelial cells in humans[16]. Data confirm the involvement of ERs-β in colorectal carcinogenesis and suggest a possible explanation for the protective effect of estrogens in cancer development[17-19]. They also provided further support of the role of vegetable-rich diets in the prevention of bowel cancer, thanks to their high content of phytoestrogens. Phytoestrogens include a variety of vegetable derived compounds with estrogen-like chemical structure and differential selectivity to the two ERs, ERα and ERβ. Particularly the dietary flavonolignan silymarin and the lignans have been reported to exert selective agonism to the ERβ the former, and preferential selectivity the latter. Since the promotion and progression of carcinogenesis are susceptible to nutritional interventions[5], the aim of this study was to evaluate if 3 mo oral supplementation with a patented blend of phytoestrogens and indigestible and insoluble fibres (Eviendep®, CM&D Pharma Limited, United Kingdom) was able to reduce the number of duodenal polyps in FAP patients with ileal pouch-anal anastomosis (IPAA).
MATERIALS AND METHODS
Population
This study was conducted in FAP patients with IPAA. The patients were ongoing the surveillance program at our department by screening upper gastrointestinal (GI) endoscopies for the follow-up of duodenal adenoma (polyp) recurrence and progression to adenocarcinoma. Cardiovascular diseases and inadequate organ function were study exclusion criteria. All patients gave their informed consent.
Endoscopic and histological procedures
Endoscopies were performed after an overnight fast; patients were prepared by a light sedation (iv midazolam coupled with 20 mg of scopolamine N-butyl bromide) and were examined with an upper GI endoscopy (Olympus GIF 165) until the third portion of the duodenum.
The severity of duodenal polyposis was classically assessed using the Spigelman classification[10]. This classification system describes five stages in duodenal polyposis development. Points are accumulated for number, size and histology of adenomatous polyps. Spigelman stage I (1-4 points) indicates mild disease, whereas stage III-IV (> 6 points) implies severe duodenal polyposis. The traditional Spigelman classification classified adenomas into mild, moderate and severe dysplasia, whereas the updated classification distinguishes low- and high-grade dysplasia.
Upper GI endoscopies with biopsies were performed by the first operator (Calabrese C) at the baseline (T0) with the assessment of the Spigelman score. Polyps > 10 mm were removed during endoscopy and at the end of the procedure a new Spigelman score was determined (T1). The procedure was repeated 3 mo after the baseline (T2), which also coincided with the 3 mo oral supplementation of Eviendep.
The first operator together with another experienced endoscopist (Rizzello F) re-evaluated all the endoscopy videos and photos. They evaluated images in a blinded manner and scored the images separately. Each expert first evaluated them individually and then in case of disagreement, a consensus was reached afterward by discussion.
Four photograms were examined for each patient, at T1 and T2. The examined area (photogram) was divided into 3 segments: duodenal bulb, second and third portion duodenum. For each segment the two operators were asked to assess the total number of polyps observed and their sizes by using an open biopsy forceps (8 mm).
Lastly, biopsy specimens were taken from all polyps > 10 mm and from all suspicious ones, defined by the presence of a central depression, irregular surface, or irregular vascular pattern.
Histologic samples were processed by using standard procedures and evaluated by gastroenterology specialized pathologists. Histology was classified according to the epithelium type (tubular, tubulovillous, or villous adenoma) and the degree of dysplasia (none, low grade, high grade, or cancer according to the updated Vienna criteria).
Treatment procedures
Eviendep® was chosen for its specifically high content of phytoestrogens and fibres. It comprises the selective ERβ-targeted flavonolignan silymarin (qualified for a 30% content in silibinin) and lignans (qualified for at least 40% of secoisolariciresinol diglucoside), in combination with non-starch, insoluble and indigestible fibres (qualified for or less than 5% lignin content). Each patient received 5 mg Eviendep twice a day, at breakfast and dinner time, for 3 mo.
Statistical analysis
Statistical significance was determined using Student’s t-tests for paired and unpaired samples. Treatment results were compared by χ2 test for comparison of proportion with a 95% confidence interval (CI). All statistical analyses were 2-tailed, and significance was accepted at a P value < 0.05. To test the reproducibility of these findings, interrater agreement was calculated with kappa analysis. A score of < 0.20 was considered poor, 0.21 to 0.40 fair, 0.41 to 0.60 moderate, 0.61 to 0.80 good, and 0.81 to 1.00 very good. We performed all the statistical analyses using a statistical software package (SPSS Inc, Chicago, IL, United States).
RESULTS
Eleven patients (M/F 5/6; mean age 40.7 years, SD ± 13.6 years) met the inclusion criteria and were enrolled; they were followed prospectively between November 2012 and January 2013 at our department outpatients’ clinic. The mean age at colectomy was 23 ± 6.2 years and mean age of IPAA was 17.7 ± 8.5 years. Table 1 shows the demographic, clinical characteristics and polyps’ histological features.
Table 1.
Findings at baseline and patients characteristics in 11 jejunal polyposis patients
| Patient No. | Age, yr (gender) | Age at colectomy (yr) | No. of duodenal polyps | Max size of duodenal polyps (mm) | Spigelman score |
| 1 | 32 (F) | 18 | 53 | 12 | 8 |
| 2 | 57 (M) | 31 | 32 | 21 | 8 |
| 3 | 43 (F) | 21 | 21 | 22 | 8 |
| 4 | 31 (F) | 16 | 20 | 5 | 7 |
| 5 | 23 (M) | 22 | 30 | 12 | 7 |
| 6 | 29 (M) | 18 | 12 | 5 | 6 |
| 7 | 62 (M) | 32 | 19 | 23 | 7 |
| 8 | 31 (F) | 17 | 22 | 19 | 7 |
| 9 | 45 (M) | 28 | 19 | 12 | 6 |
| 10 | 35 (F) | 19 | 54 | 21 | 7 |
| 11 | 60 (F) | 31 | 23 | 22 | 7 |
F: Female; M: Male.
At baseline (T0) the mean number of duodenal detected polyps was 27.7 ± 13.8 years (range 12-54 years) and mean sizes were 15.8 ± 6.8 mm (range 5-23 mm); the mean Spigelman score was 7.1 ± 0.7 (range 6-8). Histology confirmed tubular adenomatous tissue with low-grade dysplasia.
After polypectomy (T1) the mean number of duodenal detected polyps was 25.7 ± 13.4 (range 10-51) and mean sizes were 7.6 ± 1.9 mm (range 5-10 mm); the mean Spigelman score was 6.4 ± 0.5 (range 6-7). After 3 mo of Eviendep bid (T2), all patients showed a reduction of number and size of duodenal polyps. The mean number of duodenal polyps was 8 ± 6.2 (range 2-19) (χ2 = 28.42, P = 0.021) and mean size was 4.4 ± 2.1 mm (range 2-9 mm); the mean Spigelman score was 6.6 ± 0.7 (range 4-6) (Table 2, Figure 1).
Table 2.
Findings at baseline after polypectomies and after 3 mo of treatment
| Patient No. | T1 | T2 | ||||
| Duodenal polyps (n) | Max size of duodenal polyps (mm) | Spigelman score | Duodenal polyps (n) | Max size of duodenal polyps (mm) | Spigelman score | |
| 1 | 50 | 10 | 7 | 19 | 4 | 5 |
| 2 | 30 | 8 | 7 | 10 | 8 | 6 |
| 3 | 20 | 9 | 6 | 4 | 9 | 5 |
| 4 | 18 | 5 | 6 | 2 | 3 | 4 |
| 5 | 29 | 8 | 7 | 19 | 4 | 5 |
| 6 | 10 | 5 | 6 | 4 | 2 | 4 |
| 7 | 18 | 8 | 6 | 10 | 4 | 5 |
| 8 | 19 | 10 | 6 | 4 | 4 | 4 |
| 9 | 17 | 8 | 6 | 2 | 3 | 4 |
| 10 | 51 | 8 | 7 | 10 | 4 | 5 |
| 11 | 21 | 5 | 7 | 4 | 4 | 4 |
Figure 1.
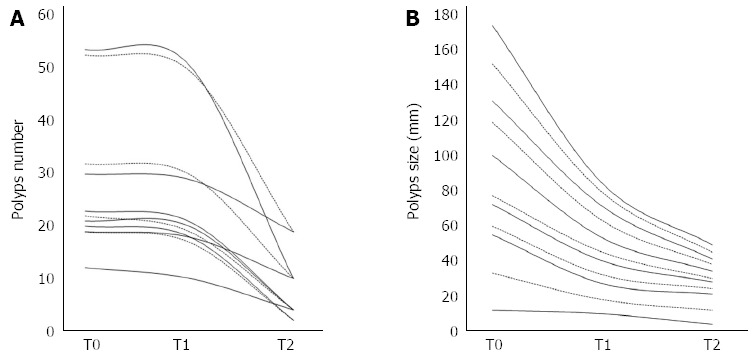
Changes in total polyps number and max size in all patients at baseline (T0), after polypectomy (T1) and after 3 mo of treatment (T2). A: Polyps number; B: Polyps size.
Interrater agreement was measured. Lesions > 1 cm found a very good degree of concordance (kappa value 0.851) and a good concordance was as well encountered for smaller lesions (kappa value 0.641).
All the eleven patients completed the study. Compliance was excellent; only one patient reported mild intestinal bloating, and the therapy was not discontinued.
DISCUSSION
Colonic surveillance programs and proctocolectomy with IPAA have improved the prognosis of patients with FAP. Current leading disease-related causes of death are desmoids tumours and duodenal adenocarcinomas. Duodenal cancer is nowadays the most important cause of death in FAP patients[13]. With respect to the duodenal manifestation of this disease, surveillance and prophylactic treatment strategies will hopefully further improve prognosis. The secondary chemoprevention of duodenal cancer identifies three different lines of intervention.
First chemopreventive strategy
Pharmacological intervention studies have been primarily focused on targeting the inflammatory pathway of cyclooxygenase-2 (COX-2), an enzyme with increased expression in experimental and human intestinal neoplasia. Most of these studies tested the efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) and acetylsalicylic acid on either adenoma regression or new polyps prevention[20]. Randomized placebo-controlled trials have demonstrated that the NSAIDs sulindac and the selective COX-2 inhibitors celecoxib and rofecoxib have chemopreventive efficacy in FAP, with a significative regression of polyps[21-23].
However, evaluations of the safety of the COX-2 inhibitors and NSAIDs showed an elevated risk of serious cardiac disorders, selected renal and hypertension events and a rebound effect on adenomas after the discontinuation of the treatments[24].
Second chemopreventive strategy
Dietary interventions are the second chemopreventive treatment line. The studies one these interventions are based on potential chemopreventive properties of several dietary components and on the evidence of epigenetic mutations of tumour suppressor genes in the intestinal mucosa after an unbalanced diet[25,26]. These studies forecasted an increase in servings/day of either fruit, vegetables, wholegrain and fibres in populations at risk of colorectal carcinoma, as well as the supplementation of specific nutrient blends.
Third chemopreventive strategy
The third new line of intervention focuses on the role of the ERs[27]. Since the discovery of ERs in the colonic tumour cells[28,29], several epidemiological and clinical studies have supported the idea that estrogens play a protective role in the pathogenesis of colorectal neoplastic lesions, suggesting their potential use in the prevention of colorectal cancer[16,30-33]. There is evidence of estrogens proliferative modulation not only on the usual estrogens responsive tissues[34] but also on other apparatuses[35].
Estrogens bind two types of receptors: estrogen receptor-alpha (ER-α), prevalent in the breast, bone, cardiovascular tissue, urogenital tract and central nervous system, and estrogen receptor-beta (ER-β), prevalent in the gut[36,37].
ER-β expression is significantly lower in colonic adenocarcinoma cells than in normal colonic epithelial cells and this reduction is directly correlated with the degree of tumour dedifferentiation[15]. Although ER expression has been widely investigated in colorectal cancer cells (CRCs)[15,38,39], few data are available about colorectal pre-cancerous lesions.
Recently data confirm the involvement of ERs-β in colorectal carcinogenesis and suggest a possible explanation for the protective effect of oestrogens on cancer development[17-19]. They also further support the role of vegetable-rich diets in the prevention of bowel cancer, thanks to their high content of phytoestrogens[5].
In particular milk thistle, traditionally used as an antioxidant and antifibrotic agent in chronic liver disease[14], is the source of silymarin, an ER-β selective-agonist[15]. Silymarin has been documented to be an effective chemopreventive in the intestinal tumour progression[15,16,39]. The lignans, non-soluble dietary fibres has been reported to be similarly effective in the chemoprevention of CRC[17,40] most likely for their ability to absorb potential carcinogens in the intestinal lumen[17-18]. Barone et al[41] demonstrated with animal studies that ER-β expression is amenable to dietary modulation to regress and/or oppose the progression of the adenoma-adenocarcinoma sequence. In a randomized, double-blind and placebo controlled study in patients undergoing surveillance colonoscopy because of recurrent sporadic adenopolyposis, a two months supplementation of Eviendep® (5 g bid) was able to specifically induce the expression of the ERβ in the colon mucosa, with optimal tolerability and safety[42]. The same group recently demonstrated that the oral supplementation of Eviendep to patients undergoing surveillance colonoscopy because of recurrent sporadic adenopolyposis was able to significantly increase the expression of ER-β in the colon mucosa, with optimal tolerability and safety[42].
At the same time, Yamada et al[43] performed a study with capsule endoscopy and found that patients with duodenal polyps had a larger number of polyps in the small intestine than those without duodenal polyps. In our experiences 8 of the 11 patients enrolled were previously investigated by capsule endoscopy. In our subset of patients there was no evidence of polyps in the small intestine.
In conclusion, our study demonstrated for the first time that short-term (90 d) supplementation with Eviendep® in FAP patients with IPAA and with recurrent adenomas in the duodenal mucosa, resulted effective in reducing polyps number by 32% and size by 51%. Eviendep® was easy to manage and its daily use was well tolerated by the patients. Its safety was guaranteed by its composition. Each ingredient is blended into the composition in a lower dose than the one otherwise needed for the single component to similarly exert the desired effect, thus leading to synergistic and/or potentiating effect, with the added advantage of higher safety, even over long-term exposure. Further molecular studies are needed to better confirm the role of estrogens in the duodenal mucosa, but we do believe that they also play a central role in duodenal carcinogenesis in the colon.
COMMENTS
Background
Familial adenomatous polyposis (FAP) is a disease with autosomal dominant inheritance. Early prophylactic colorectal surgery changed the prognosis of patients with FAP, and duodenal cancer is nowadays the most important cause of death in FAP patients.
Research frontiers
The recent discover of the involvement of estrogen receptors (ERs)-β in colorectal carcinogenesis suggests a possible explanation for the protective effect of estrogens in cancer development. This also provided further support of the role of vegetable-rich diets in the prevention of bowel cancer, thanks to their high content of phytoestrogens (ER-β selective agonists).
Innovations and breakthroughs
Several epidemiological and clinical studies have supported the idea that estrogens play a protective role in the pathogenesis of colorectal neoplastic lesions, suggesting their potential use in the prevention of colorectal cancer. The aim of this study was to evaluate if dietary supplementation with phytoestrogens, selective agonists of the estrogen receptor, was able to prevent as well the progression of carcinogenesis in duodenal polyps.
Applications
Their study demonstrated that short-term supplementation with phytoestrogens in FAP patients with ileal pouch-anal anastomosis (IPAA) is effective in reducing duodenal polyps number and size.
Peer review
The authors investigated the effects of short-term (90 d) supplementation with Eviendep on the reduction of the number and size of duodenal polyps in FAP patients who had undergone IPAA.
Footnotes
P- Reviewers Bassorgun CI, Di Leo A, Nagayama S S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Bodmer WF, Bailey CJ, Bodmer J, Bussey HJ, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- 2.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 3.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 4.Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358–364. doi: 10.1016/s0016-5107(99)70013-1. [DOI] [PubMed] [Google Scholar]
- 5.Björk J, Akerbrant H, Iselius L, Bergman A, Engwall Y, Wahlström J, Martinsson T, Nordling M, Hultcrantz R. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127–1135. doi: 10.1053/gast.2001.28707. [DOI] [PubMed] [Google Scholar]
- 6.Iaquinto G, Fornasarig M, Quaia M, Giardullo N, D’Onofrio V, Iaquinto S, Di Bella S, Cannizzaro R. Capsule endoscopy is useful and safe for small-bowel surveillance in familial adenomatous polyposis. Gastrointest Endosc. 2008;67:61–67. doi: 10.1016/j.gie.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Ruo L, Coit DG, Brennan MF, Guillem JG. Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg. 2002;6:671–675. doi: 10.1016/s1091-255x(02)00045-8. [DOI] [PubMed] [Google Scholar]
- 8.Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–641. doi: 10.1136/gut.50.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latchford AR, Neale KF, Spigelman AD, Phillips RK, Clark SK. Features of duodenal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2009;7:659–663. doi: 10.1016/j.cgh.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 11.Langers AM, De Vos tot Nederveen Cappel WH, Veenendaal RA, Bonsing BA, Hardwick JC, Vasen HF. Double balloon endoscopy for detection of small-bowel adenomas in familial adenomatous polyposis after pancreaticoduodenectomy according to Whipple. Endoscopy. 2008;40:773–774. doi: 10.1055/s-2008-1077487. [DOI] [PubMed] [Google Scholar]
- 12.Mönkemüller K, Fry LC, Ebert M, Bellutti M, Venerito M, Knippig C, Rickes S, Muschke P, Röcken C, Malfertheiner P. Feasibility of double-balloon enteroscopy-assisted chromoendoscopy of the small bowel in patients with familial adenomatous polyposis. Endoscopy. 2007;39:52–57. doi: 10.1055/s-2006-945116. [DOI] [PubMed] [Google Scholar]
- 13.Bülow S, Christensen IJ, Højen H, Björk J, Elmberg M, Järvinen H, Lepistö A, Nieuwenhuis M, Vasen H. Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis. 2012;14:947–952. doi: 10.1111/j.1463-1318.2011.02844.x. [DOI] [PubMed] [Google Scholar]
- 14.Linsalata M, Russo F, Cavallini A, Berloco P, Di Leo A. Polyamines, diamine oxidase, and ornithine decarboxylase activity in colorectal cancer and in normal surrounding mucosa. Dis Colon Rectum. 1993;36:662–667. doi: 10.1007/BF02238593. [DOI] [PubMed] [Google Scholar]
- 15.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000;60:245–248. [PubMed] [Google Scholar]
- 16.Weyant MJ, Carothers AM, Mahmoud NN, Bradlow HL, Remotti H, Bilinski RT, Bertagnolli MM. Reciprocal expression of ERalpha and ERbeta is associated with estrogen-mediated modulation of intestinal tumorigenesis. Cancer Res. 2001;61:2547–2551. [PubMed] [Google Scholar]
- 17.Javid SH, Moran AE, Carothers AM, Redston M, Bertagnolli MM. Modulation of tumor formation and intestinal cell migration by estrogens in the Apc(Min/+) mouse model of colorectal cancer. Carcinogenesis. 2005;26:587–595. doi: 10.1093/carcin/bgh346. [DOI] [PubMed] [Google Scholar]
- 18.Seidlová-Wuttke D, Becker T, Christoffel V, Jarry H, Wuttke W. Silymarin is a selective estrogen receptor beta (ERbeta) agonist and has estrogenic effects in the metaphysis of the femur but no or antiestrogenic effects in the uterus of ovariectomized (ovx) rats. J Steroid Biochem Mol Biol. 2003;86:179–188. doi: 10.1016/s0960-0760(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 19.Di Leo A, Barone M, Margiotta M. Effect of selective agonist for the estrogen receptor β (Silymarin) upon colonic cell migration in normal mice. Dig Liv Dis. 2006;38:S105. [Google Scholar]
- 20.Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–622. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 22.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 23.Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, Wakabayashi N, Shen Y, Zimmerman S, Godio L, et al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857–860. doi: 10.1136/gut.50.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 25.van Engeland M, Derks S, Smits KM, Meijer GA, Herman JG. Colorectal cancer epigenetics: complex simplicity. J Clin Oncol. 2011;29:1382–1391. doi: 10.1200/JCO.2010.28.2319. [DOI] [PubMed] [Google Scholar]
- 26.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9:385–391. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 28.Sica V, Nola E, Contieri E, Bova R, Masucci MT, Medici N, Petrillo A, Weisz A, Molinari AM, Puca GA. Estradiol and progesterone receptors in malignant gastrointestinal tumors. Cancer Res. 1984;44:4670–4674. [PubMed] [Google Scholar]
- 29.Francavilla A, Di Leo A, Polimeno L, Conte D, Barone M, Fanizza G, Chiumarulo C, Rizzo G, Rubino M. Nuclear and cytosolic estrogen receptors in human colon carcinoma and in surrounding noncancerous colonic tissue. Gastroenterology. 1987;93:1301–1306. doi: 10.1016/0016-5085(87)90259-9. [DOI] [PubMed] [Google Scholar]
- 30.Di Leo A, Messa C, Russo F, Misciagna G, Guerra V, Taveri R, Leo S. Prognostic value of cytosolic estrogen receptors in human colorectal carcinoma and surrounding mucosa. Preliminary results. Dig Dis Sci. 1994;39:2038–2042. doi: 10.1007/BF02088144. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez E, La Vecchia C, Braga C, Talamini R, Negri E, Parazzini F, Franceschi S. Hormone replacement therapy and risk of colon and rectal cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:329–333. [PubMed] [Google Scholar]
- 32.Notarnicola M, Gristina R, Messa C, Cariola F, Fiorente P, Caruso ML, Gentile M, Di Leo A. Oestrogen receptors and microsatellite instability in colorectal carcinoma patients. Cancer Lett. 2001;168:65–70. doi: 10.1016/s0304-3835(01)00494-3. [DOI] [PubMed] [Google Scholar]
- 33.Woodson K, Lanza E, Tangrea JA, Albert PS, Slattery M, Pinsky J, Caan B, Paskett E, Iber F, Kikendall JW, et al. Hormone replacement therapy and colorectal adenoma recurrence among women in the Polyp Prevention Trial. J Natl Cancer Inst. 2001;93:1799–1805. doi: 10.1093/jnci/93.23.1799. [DOI] [PubMed] [Google Scholar]
- 34.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B, Gunduz N, Saffer EA, Zheng S. Relation of estrogen and its receptor to rat liver growth and regeneration. Cancer Res. 1984;44:2410–2415. [PubMed] [Google Scholar]
- 36.Gustafsson JA. Estrogen receptor beta--a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- 37.Dechering K, Boersma C, Mosselman S. Estrogen receptors alpha and beta: two receptors of a kind? Curr Med Chem. 2000;7:561–576. doi: 10.2174/0929867003375010. [DOI] [PubMed] [Google Scholar]
- 38.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 39.Di Leo A, Messa C, Cavallini A, Linsalata M. Estrogens and colorectal cancer. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:1–12. doi: 10.2174/1568008013341749. [DOI] [PubMed] [Google Scholar]
- 40.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 41.Barone M, Tanzi S, Lofano K, Scavo MP, Pricci M, Demarinis L, Papagni S, Guido R, Maiorano E, Ingravallo G, et al. Dietary-induced ERbeta upregulation counteracts intestinal neoplasia development in intact male ApcMin/+ mice. Carcinogenesis. 2010;31:269–274. doi: 10.1093/carcin/bgp275. [DOI] [PubMed] [Google Scholar]
- 42.Principi M, Di Leo A, Pricci M, Scavo MP, Guido R, Tanzi S, Piscitelli D, Pisani A, Ierardi E, Comelli MC, et al. Phytoestrogens/insoluble fibers and colonic estrogen receptor β: Randomized, double-blind, placebo-controlled study. World J Gastroenterol. 2013;19:4325–4333. doi: 10.3748/wjg.v19.i27.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada A, Watabe H, Iwama T, Obi S, Omata M, Koike K. The prevalence of small intestinal polyps in patients with familial adenomatous polyposis: a prospective capsule endoscopy study. Fam Cancer. 2013:Epub ahead of print. doi: 10.1007/s10689-013-9668-1. [DOI] [PubMed] [Google Scholar]


