Abstract
Autologous stem cell transplant (ASCT) is an effective treatment for multiple myeloma (MM). However the timing of ASCT in the era of novel agents (lenalidomide, thalidomide, bortezomib) is unknown. We retrospectively reviewed the outcome of MM patients who received novel agent based induction treatment and received first ASCT within 12 months of diagnosis (early ASCT, N = 102), or at a later date (late ASCT, N = 65). Median time to ASCT was 7.9 months vs. 17.7 months in the early vs. late ASCT. The 3 and 5 yr overall Survival (OS) from diagnosis was 90 and 63% versus 82 and 63% in early and late ASCT respectively (P=0.45). Forty-one and 36 patients in the early and late ASCT have relapsed or progressed with median time to relapse of 28 and 23 mos (p=0.055). On multivariable analysis, factors predictive of increased risk for progression were ISS stage III (p=0.007), and < VGPR post-ASCT (p<0.001). Factor predictive of worst outcomes for OS was being on hemodialysis (p=0.037). No superiority of one agent was seen. In summary, early or late ASCT is a viable option for MM patients receiving induction treatment with novel targeted therapies.
Keywords: Multiple Myeloma, Transplantation, Bortezomib, Lenalidomide
INTRODUCTION
Multiple myeloma (MM) represents approximately 10% of all hematologic malignancies with over 20,000 new diagnoses and greater than 10,000 deaths in the United States per year 1. The introduction of high dose therapy (HDT) and autologous stem cell transplant (ASCT) in the 1980s was a major advancement in MM treatment. Major randomized trials demonstrated improved progression free survival (PFS) and overall survival (OS) with ASCT compared to conventional therapy 2,3, while others showed only improved PFS with no significant survival benefit 4,5. A large scale meta-analysis of randomized trials demonstrated improved PFS but no overall survival between the two groups 6. Because of the improved PFS resulting in prolonged time off treatment without symptoms with improved quality of life, ASCT has become an integral part of treatment in patients with newly diagnosed MM. An early transplant was shown to significantly improve the period of time without symptoms, treatment and treatment related toxicity 4,7.
Over the last decade, there has been remarkable improvement in PFS and OS with the introduction of the novel agents – thalidomide, lenalidomide and bortezomib- in the treatment of MM, first for relapsed and refractory disease, and more recently as upfront therapy 8–15. The percentage of patients expected to achieve a very good partial response (VGPR) or higher before transplant has increased from 20% with vincristine, adriamycin, and dexamethasone (VAD) 16 to as high as 67% with novel agent based therapy 17. Even as novel agents have gained widespread use, early ASCT continues to be standard of care in transplant eligible patients based on current guidelines, and in clinical practice 18. However, there is an increasing trend towards delaying ASCT. Hence the timing of ASCT in the era of these novel agents is an important question. To date, there is no published randomized trial addressing this issue. We therefore reviewed the outcomes of MM patients seen at the Ohio State University between 2002 and 2009 who received induction treatment with novel agent-based therapy and compared those who had ASCT within 12 months (early ASCT) to those who had ASCT at a later date (late ASCT) from time of diagnosis.
PATIENTS AND METHODS
Patients
Between August 2002 and December 2009, 317 patients who met the diagnostic criteria for symptomatic multiple myeloma underwent HDT and ASCT at The Ohio State University Medical Center. Patients were eligible for ASCT if they had an ECOG performance status of 0–2, stable or better disease response, adequate cardiac (left ventricular ejection fraction ≥45%), pulmonary (diffusing capacity of the lung for carbon monoxide ≥50%), hepatic (bilirubin, transaminases < 2 times upper limit of normal) function and no uncontrolled infection. Only patients (n=167) who received thalidomide, bortezomib or lenalidomide based therapy (named as the novel agents) and who did not receive consolidation or maintenance therapy after ASCT were included in this analysis. The main reason for late ASCT was late referral by treating physician.
Hematopoietic Stem Cell Collection
G-CSF-mobilized peripheral blood progenitor cells were collected using standard mobilization protocol and apheresis techniques. All patients signed informed consent according to our institutional and the National Marrow Donor Program guidelines. The study was approved by the Institutional Review Board at The Ohio State University.
Preparative Regimen and Supportive Care
All patients received conditioning regimen with melphalan 200mg/m2 except for patients with CrCl < 50ml/min including patients on hemodialysis, in which case melphalan140 mg/m2 was given. Patients received infection prophylaxis with antiviral (valacyclovir) and antifungal (fluconazole). Filgrastim 5ug/kg was administered subcutaneously daily from day 1 after ASCT until recovery of absolute neutrophil count (ANC) to >1.5 × 109/L for 3 days. Blood products were irradiated and leukopore filtered. Antiviral was continued for 3–6 months after neutrophil recovery.
Engraftment, Response and Outcome
Response, relapse and disease progression were defined based on the international myeloma working group response criteria (IMWG)19. Patients were classified into high risk (FISH detection of t(4;14), t(14;16), t(14;20), deletion 17p, conventional karyotyping of hypodiploidy or deletion 13), or standard risk (all others)20. Neutrophil engraftment was defined as the first of 3 consecutive days with an ANC≥0.5 × 109/L. Failure to engraft by day 30 was considered primary graft failure. Platelet engraftment was defined as the first of 7 consecutive days with a platelet of ≥ 20 × 109/L without platelet transfusion.
Statistical Methods
Primary endpoints were OS and PFS. Secondary endpoints were relapse and treatment related mortality (TRM). OS was measured from the day of MM diagnosis to death from any cause, with censoring performed at date of last contact. PFS was determined from the day of stem cell infusion to the day of documented relapse or progression. Death from any cause other than relapse was classified as TRM. Actuarial survival curves were estimated using the method of Kaplan-Meier. The univariate associations between survival curves and other categorical variables were determined with the log-rank test. Covariates identified as having an influence on survival by univariate analysis (p-value <0.05) were analyzed using Cox proportional hazards model. Step-down regression method was used to build parsimonious statistical models. Patient characteristics were also summarized (median and range for continuous variables, frequency for categorical variables). Categorical variables were analyzed by the Fisher’s exact or chi-square test, whichever was appropriate. Bonferroni method was used to adjust for multiplicity21. Statistical data was analyzed using the commercial statistical package SAS for Windows® Version 9.2 (SAS Institute Inc., Cary, NC, USA). Statistical significance was determined at the 0.05 level.
RESULTS
Patient Characteristics
Out of 317 MM patients who underwent first ASCT, 150 were excluded (94 patients did not receive novel agent-based induction therapy, 4 patients were on study incorporating Allogeneic SCT after ASCT, and 52 patients participated on CALGB 100104 study incorporating lenalidomide versus placebo as maintenance post ASCT). Of the167 remaining patients, we compared the outcomes of those who received ASCT within 12 months of diagnosis (early ASCT, N=102) to those who received ASCT at a later date (late ASCT, N=65). No patient received maintenance treatment post ASCT. Patient baseline characteristics at diagnosis are summarized in Table I. There was no statistically significant difference between the groups in age, race, gender, performance status, comorbidity index score, stage of disease at diagnosis, cytogenetic and fluorescent in-situ-hybridization (FISH), or dose of melphalan conditioning. In the early group 46% of patients were in VGPR or greater at the time of ASCT compared to 30% in the late gp (p=0.036). The median time from diagnosis to transplant was 7.9 months (range 3.5–12) in the early ASCT and 17.7 months (range 12.3–89.4) in the late ASCT. As first line therapy, 69% of early ASCT vs 55% of late ASCT received 2-drug therapy and 31% vs 45% received 3-drug therapy. Forty three patients in the late ASCT received a second line therapy. Nine (8.9%) patients in early ASCT and 7 (10.8%) in late ASCT were on dialysis. More patients in the late ASCT had thalidomide based induction therapy (66%), whereas more patients in the early ASCT had bortezomib based induction (63%), with both groups equally exposed to lenalidomide based induction therapy.
Table I.
Patient Baseline Characteristics
| SCT≤ 12 mos | SCT>12 mos | ||||
|---|---|---|---|---|---|
| N=102 or 61% | % | N=65 or 39% | % | P value | |
| Age | |||||
| Median | 56.6 | 55.3 | |||
| Range | 29 – 76 | 41–80 | |||
| Age>60/≤60 | 30/72 | 29/71 | 20/45 | 31/69 | 0.85 |
| Sex Male/Female | 59/43 | 58/42 | 39/26 | 60/40 | 0.78 |
| Race AA/C/O | 23 / 78 / 1 | 23 /76 /1 | 12 /53/ 0 | 18/82/0 | 0.73 |
| KPS Median (range) | 80 (60 –100) | 90 (70–100) | |||
| NCI-CI Median (range) | 3 (0–7) | 3 (0–6) | |||
| β2M > 3.5 | 31/71 | 43 | 18/47 | 38 | 0.71 |
| DS Stage 3 | 80 | 78 | 50 | 77 | 0.73 |
| DS 1and 2 | 22 | 15 | |||
| ISS stage 1/2/3 | 23/30/22 | 23/29/22 | 14/15/12 | 22/23/18 | 0.91 |
| ISS stage unknown | 27 | 26 | 24 | 37 | |
| Melphalan 140/200 | 19/83 | 19/81 | 11/54 | 17/83 | 0.78 |
| Prior treatment 1/2/3/4/5 | 74/27/1 | 73/26/1 | 22/22/15/4/2 | 34/34/23/6/2 | |
| Prior treatment 1/>1 | 74/28 | 73/27 | 22/43 | 34/66 | <0.001 |
| Thalidomide | 39 | 38 | 43 | 66 | |
| Bortezomib | 64 | 63 | 32 | 49 | |
| lenalidomide | 34 | 33 | 18 | 28 | |
| Standard/High Risk Cytogenetic | 67/31 | 68/32 | 40/20 | 67/33 | 0.82 |
| p53 deletion | 13 | 13 | 5 | 8 | 0.44 |
| deletion 13 karyotype | 11 | 11 | 7 | 11 | 0.91 |
| deletion 13 FISH | 32 | 31 | 24 | 37 | 0.33 |
| hyperdiploid | 24 | 23 | 15 | 23 | 0.91 |
| Complex Karyotype | 21 | 21 | 14 | 21 | |
| Disease Subtype | |||||
| IgG | 53 | 52 | 41 | 63 | 0.024 |
| IgA | 20 | 20 | 11 | 17 | |
| Light chain only | 25 | 24 | 7 | 11 | |
| Nonsecretory | 4 | 4 | 2 | 3 | |
| Others/unknown | 4 | 6 | |||
| Disease Status at SCT | |||||
| CR | 17 | 17 | 4 | 6 | 0.12 |
| VGPR | 30 | 29 | 15 | 23 | |
| CR+VGPR | 47 | 46 | 19 | 30 | 0.036 |
| PR | 43 | 42 | 34 | 52 | |
| SD | 12 | 12 | 11 | 17 | |
| Unknown/not evaluated | 1 |
Mos stands for months; SCT stem cell transplant; AA African American; C Caucasian; O other; KPS karnofsky performance score; BMI body mass index; β2M beta-2 microglobulin; DS durie salmon; ISS international scoring system; FISH florescent in-situ hybridization; CR complete response; VGPR very good partial response; PR partial response; SD stable disease.
Engraftment
All patients achieved engraftment except one patient who died 7 days post transplant. Median time to neutrophil and platelet engraftments was 11 and 13 days respectively.
Response
After a median follow up of 30.5 months (range 7–94) from diagnosis and 23.2 months (range 0.2–83) from ASCT in the early ASCT and 52 months (range 20–183) from diagnosis and 29 months (3.0–93) from ASCT in the late ASCT, the overall response rate post ASCT (ORR = CR +VGPR + PR) was similar (99% vs. 97%, p=0.56), but with a statistically significant greater proportion of patients in the early ASCT obtaining CR (50% vs. 28%, p=0.007) (Table II). In the early ASCT, 77% of patients obtained a VGPR or better response post ASCT compared to 55% in the late ASCT (p=0.003). In both groups, the number of patients obtaining a CR post ASCT increased by at least 3 fold from pre-ASCT status. The 100 day and 1 year NRM were 3%(3 patients) and 4% (4 patients) in the early ASCT vs.0 and 1.5% (1 patient) in the late ASCT. All patients died of Infection.
Table II.
Response, Mortality, Relapse
| SCT ≤12 mos | SCT >12 mos | ||||
|---|---|---|---|---|---|
| % | % | P value | |||
| N= 102 | N=65 | ||||
| Median Time from Dx to SCT | 7.9 mos | 17.7 mos | |||
| Range | 3.5–12.0 mos | 12.3–89.4 mos | |||
| Median follow up from Dx | 30.5 mos | 52 mos | |||
| Range | 7–94mos | 20–183 mos | |||
| Median Follow up from SCT | 23.2 mos | 29 mos | |||
| Range | 0.2–83 mos | 3.0–93 mos | |||
| Response | |||||
| ORR | 100 | 99 | 63 | 97 | 0.56 |
| CR | 51 | 50 | 18 | 28 | 0.007 |
| VGPR | 27 | 27 | 18 | 28 | |
| CR+VGPR | 78 | 77 | 36 | 55 | 0.003 |
| PR | 22 | 22 | 27 | 41 | |
| SD | 1 | 1 | 2 | 3 | |
| 100 day NRM | 3 | 3 | 0 | 0 | |
| 1 yr NRM | 4 | 4 | 1 | 1.5 | |
| Total Death | 15 | 15 | 25 | 38 | 0.001 |
| Death due to disease progression | 11 (73%) | 23 (92%) | |||
| Other | 4 (27%) | 2 (8%) | |||
| # Relapse/ Progression of Disease | 41 | 40 | 36 | 55 | 0.055 |
| Median time to relapse/progression | 28 mos | 23 mos | |||
| Median OS from Dx (Range) | NR (6–92) | 83 mos (19–179) | 0.45 | ||
| 1 yr OS from Dx | 96% | 100% | |||
| 3 yr OS from Dx | 90% | 82% | |||
| 5 yr OS from Dx | 63% | 63% | |||
| Median PFS (Range) | 28 mos (0–73) | 18 mos (2–91) | 0.11 | ||
| 1 yr PFS | 80% | 66% | |||
| 3 yr PFS | 32% | 28% | |||
| 5 yr PFS | 25% | 23% |
Mos stands for months; SCT stem cell transplant; Dx diagnosis; ORR overall response rate; CR complete response; VGPR very good partial response; PR partial response; SD stable disease; NRM non relapse mortality; OS overall survival; PFS progression free survival.
Relapse, Progression-Free and Overall Survival
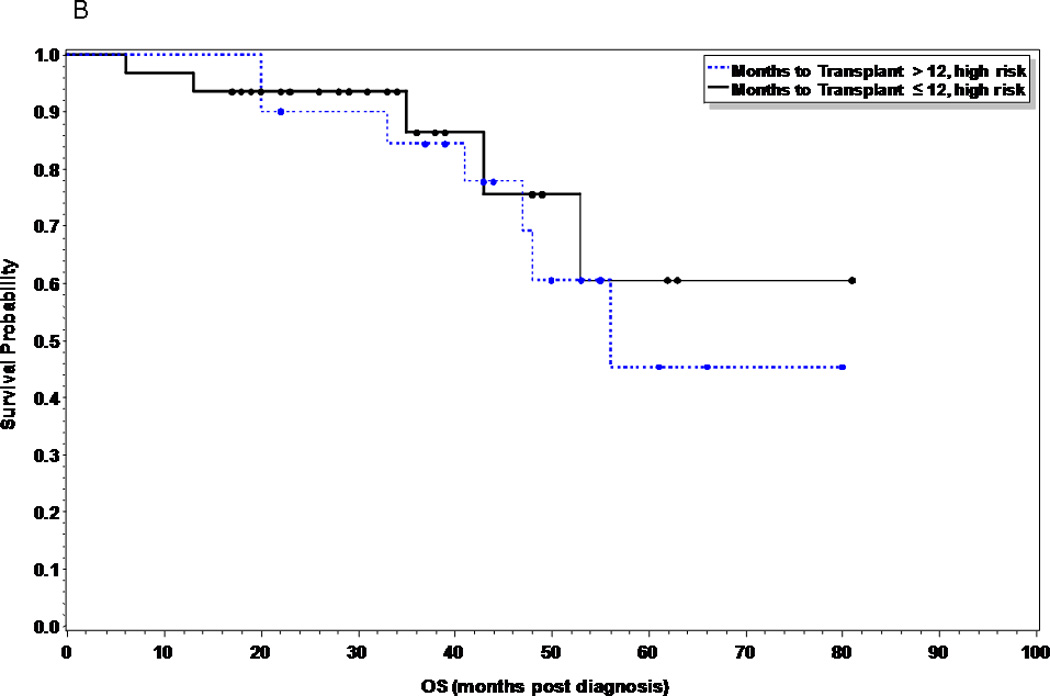
At the time of this analysis, 41 patients (40%) and 36 patients (55%) in the early and late ASCT respectively had relapsed (p=0.055), with a median time to relapse of 28 months and 23 months respectively. Of the 15 patients (15%) in the early ASCT and 25 (38%) patients in late ASCT who died, 11 (73%) and 23 (92%) were due to disease progression (Table II). There was no statistical significance in PFS or OS between the two groups (Figures 1 and 2). The median PFS was 28 months versus 18 months in the early versus late ASCT respectively, with 1, 3, and 5 year PFS of 80, 32, and 25% versus 66, 28, and 23% (p=0.11). The median OS from time of diagnosis was not reached (NR) in the early ASCT versus 75 months in the late ASCT, with 1, 3, and 5 year OS of 96, 90, and 63% versus 100, 82, and 63% (p=0.45) respectively. High risk patients who had early ASCT (N=31, median PFS=25 months) had a statistically significant improved PFS compared to those who had late ASCT (N=20, median PFS=11 months, p=0.049), but no difference in OS (p=0.59) (Figures 3A and 3B). There was no difference in PFS or OS for standard risk patients between the two groups (data not shown).
Figure 1.
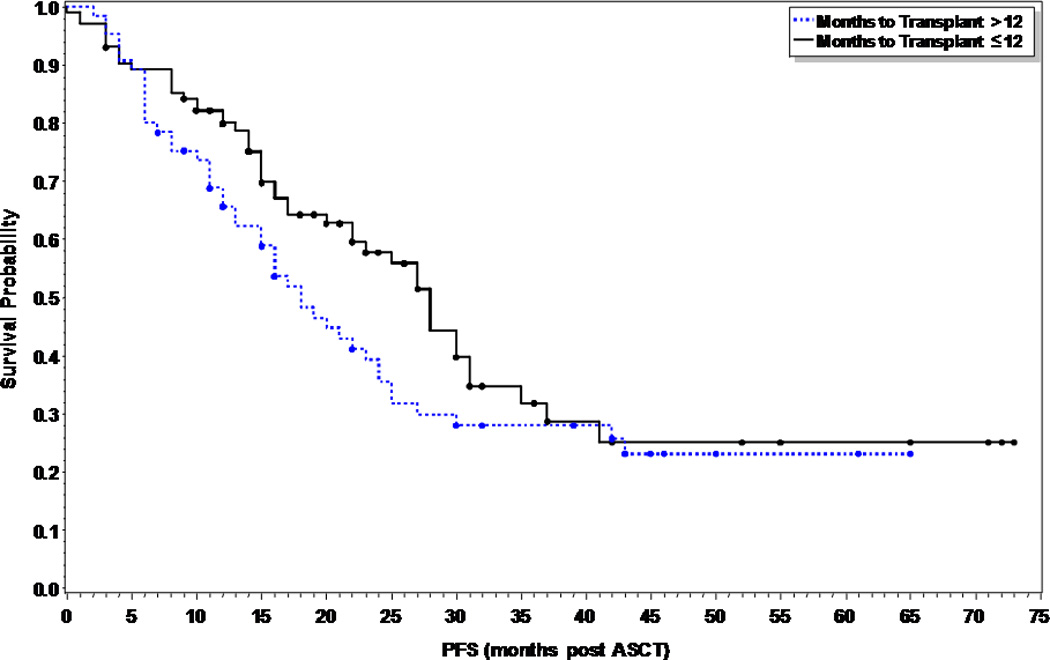
Progression free survival comparing early vs. delayed first ASCT in multiple myeloma patients (p=0.11).
Figure 2.
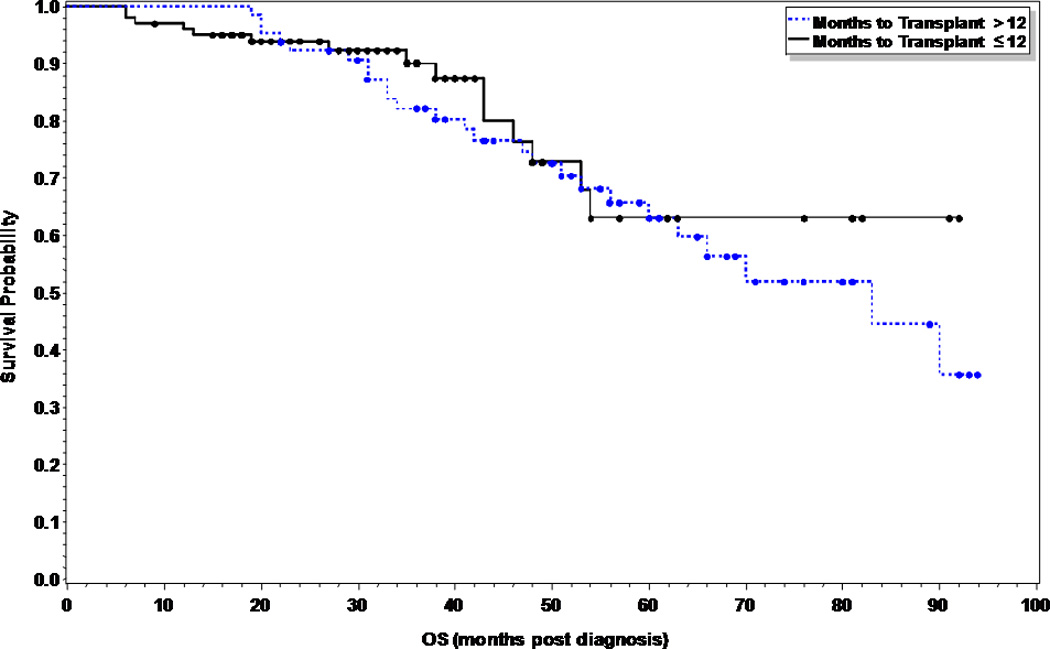
Overall Survival comparing early vs. delayed first ASCT in multiple myeloma patients (p=0.45).
Figure 3.
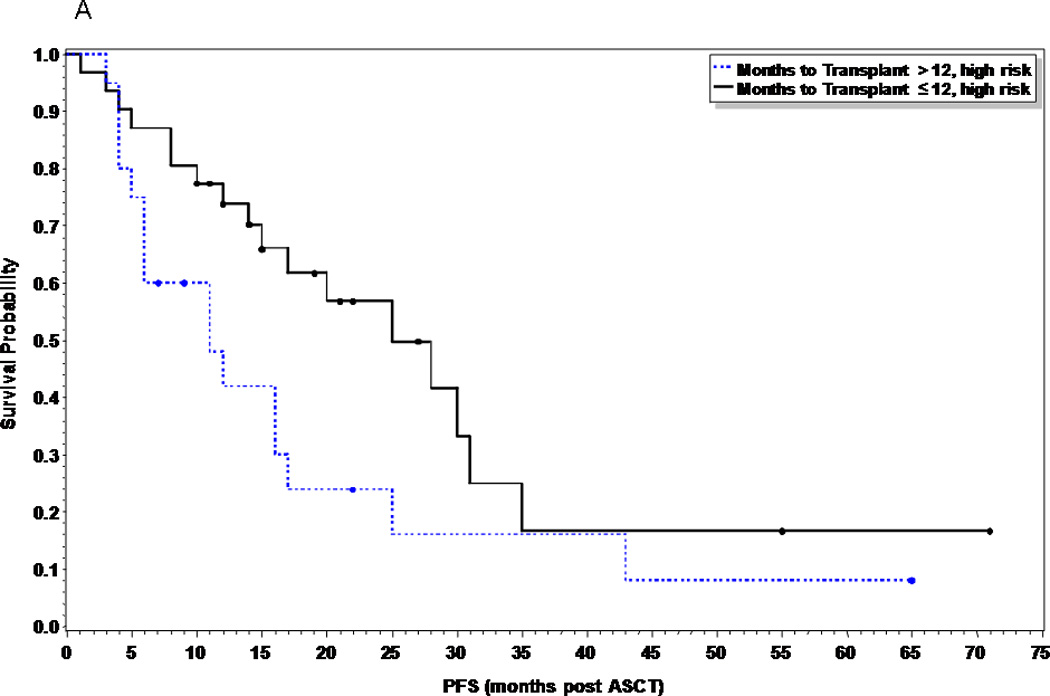
Progression free survival comparing early vs. delayed first ASCT in high risk multiple myeloma patients (p=0.049).
B. Overall Survival comparing early vs. delayed first ASCT in high risk multiple myeloma patients (p=0.59)
Prognostic Factors
On univariate analysis, factors predictive of increased risk of progression post transplant were: 2 or more previous therapies before ASCT, pre and post transplant response less than VGPR, DS and ISS stage III, deletion 13 by karyotype, and high risk cytogenetics (del p53, complex karyotype, t(4;14), t(14;16)) (Table III). There was no statistical difference in PFS with regards to sex (M vs. F), race, age (≤60 vs.>60), smoking history (≤10 pk yr vs.>10), conditioning regimen (melphalan 200 vs.140), initial creatinine level (≤2 vs.>2), histological type, beta 2 microglobulin (≤3.5 vs.>3.5), ejection fraction (≤50 vs.>50), comorbidity index score (≤2 vs.>2), or hemodialysis status. In the overall survival analysis, factors predictive of increased mortality were deletion 13 by karyotype and hemodialysis (Table III). On multivariable analysis, factors predictive of increased risk for progression were ISS stage III (p=0.007), and < VGPR post-ASCT (p<0.001), and factors predictive of worst outcomes for OS were observed in patients on hemodialysis(p=0.037). There was no superiority of one novel agent over another.
Table III.
Univariate Analysis for Progression Free Survival and Overall Survival
| PFS variable | HR | p-value |
|---|---|---|
| Number of prev therapy >1 vs 1 |
1.60 | 0.02 |
| Pre-SCT response Others vs. CR+VGPR |
1.67 | 0.018 |
| Post-SCT response Others vs. CR+VGPR |
2.79 | <0.001 |
| Pre-SCT response Others vs. CR |
2.49 | 0.015 |
| Post-SCT response Others vs. CR |
3.63 | <0.001 |
| Durie salmon 3 vs.1 or 2 |
1.80 | 0.033 |
| Complex Karyotype No vs. yes |
0.63 | 0.042 |
| Del 13 Karyotype No. vs. yes |
0.56 | 0.035 |
| High risk FISH/Karyotype No vs. yes |
0.65 | 0.041 |
| ISS 1 vs. 2/3 |
1 vs. 2: 0.89 1vs. 3: 0.49 2 vs. 3: 0.56 |
0.038 |
| OS Variable | ||
| Pre-SCT response Others vs. CR |
1.74 | 0.35 |
| Post-SCT response Others vs. CR |
3.10 | 0.0043 |
| Del 13 Karyotype No vs. yes |
0.44 | 0.045 |
| HD No vs. yes |
0.36 | 0.018 |
HR stands for hazard ratio; prev previous; SCT stem cell transplant; CR complete response; VGPR very good partial response; PR partial response; SD stable disease; NRM non relapse mortality; OS overall survival; PFS progression free survival; FISH florescent in-situ hybridization; ISS international scoring system; HD hemodialysis.
DISCUSSION
In our retrospective analysis, we chose 12 months from diagnosis as a cut-off for early ASCT since older studies and current on-going trials specify first ASCT within 12 months from diagnosis (e.g. BMT-CTN 0702 trial). All our patients received only novel agent based regimens and none received consolidation or maintenance treatment post ASCT to avoid biases between the two groups. Although not statistically significant, probably due to the number of patients, there was a difference in PFS of a median of 10 months between the 2 groups, with the early ASCT having a longer PFS than the late gp (28 mos vs. 18 mos), however, no statistically significant differences in OS between the two groups was seen. This correlated with findings by Kumar et al22. Using the same ASCT criteria as ours in a retrospective analysis of patients who received initial therapy with novel agents, they reported a 4 year OS from diagnosis of 67.8% in the early ASCT compared to 63.6% in the late ASCT (p=0.5). Our 3 year OS were 90 and 82% respectively(P=0.45). Their study however did not report if any patient received consolidation or maintenance treatment post ASCT. The only randomized study presented as an abstract reported an PFS advantage for early tandem ASCT but no OS benefit when compared to combination melphalan, prednisone and lenalidomide(MPR)23. In this study, after receiving 4 cycles of lenalidomide and dexamethasone, patients were randomized to MPR versus tandem ASCT using melphalan 200 mg/m2. They reported an 18 months PFS of 78% in the tandem arm versus 68% in the MPR arm (p=0.006), but no OS benefit (95% vs. 91%) and increased grade 3–4 toxicity in the tandem arm (p<0.001). In contrast, in a post-hoc analysis of the ECOG E403 study comparing lenalidomide with high-dose dexamethasone vs. lenalidomide with low-dose dexamethasone, Siegal et al reported an increased survival probability for patients less than 65 years old who elected for early ASCT after 4 cycles of induction treatment with a 3-year OS of 94% vs. 78% for those who continued protocol therapy24. However, it was unclear what circumstances led to the decision for delay transplantation in the other group and they admitted that differences in OS seen between the groups may have been influenced by factors such as performance status, comorbidities, and initial response to therapy.
Although there were no statistically significant differences in overall PFS and OS, several features were in favor of early ASCT. Early ASCT patients obtained a statistically significant better response rate post SCT (77% ≥ VGPR) as compared to late ASCT patients (56%, p=0.0066). This may have translated into a better quality of life although this was not assessed in our retrospective study. Our results support findings of other studies showing that first ASCT improves upon the responses seen with induction using the novel agents16,25. The study by Harousseau et al showed a 52% VGPR or better with induction treatment with bortezomib plus dexamethasone which improved to 89% post first ASCT as compared to 21.5% and 55.6% respectively with vincristine, adriamycin and dexamethasone25. Our study also showed that patients who went to ASCT within 12 months of diagnosis with a ≥ VGPR with induction treatment, had the best disease free benefit post transplant (p=0.035). Our results are comparable to prior studies demonstrating that pre-ASCT response is an important prognostic factor to post-ASCT response, PFS and OS even in patients without exposure to novel agents26–28. It has also been shown that the depth of response obtained after ASCT is one of the most robust predictor of PFS and OS28–30. Our study confirms that for patients who obtained less than a VGPR post ASCT, the risk of progression was three times higher than those who had a VGPR or better. In particular, patients with CR post ASCT had statistically improved PFS (p <0.001) and OS (p=0.0043). The role of a second ASCT or consolidation may play a significant impact in these patients.
In conclusion, we found no statistically significant differences in PFS and OS between the early versus late ASCT groups. . A major limitation of this study is that we do not know the reason(s) for the late referral for patients in the late ASCT. Reasons could be patient initial refusal, physician preference because patients were doing well with induction or initial feeling that patients may not be good candidates for ASCT. Also, this study is a retrospective analysis and likely the number of patients may not be powered to attain statistical significance. We excluded patients who received maintenance treatment post ASCT as this was not balanced between the two groups. Although retrospective, these data support the ongoing prospective randomized trial examining this important question (NCT01191060, NCT 1208662, and NCT00551928).
ACKNOWLEDGEMENTS
Support includes NCI K12, Multiple Myeloma Opportunities for Research and Education (MMORE), Leukemia and Lymphoma Society.
Footnotes
AUTHORSHIP
Contribution: N.C.D., P. E., and Y.E. collected data; N.C.D., L.W., G.P. and Y.E. analyzed results and made the figures; JCB, SD, CH, and DB were involved with treatment of patients on this study, reviewed versions of the paper and approved the final version. NH performed the cytogenetics reported, reviewed the manuscript and approved the final version; N.C.D. and Y.E. designed the research and wrote the paper.
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1.Group USCSW. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2010. [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 4.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 5.Blade J, Rosinol L, Sureda A, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–3759. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 6.Koreth J, Cutler C, Djulbegovic B, et al. High-dose Therapy with Single Autologous Transplantation versus Chemotherapy for Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biology of Blood and Marrow Transplantation. 2007;13:183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 8.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harousseau JL, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- 12.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 13.Cavallo F, Boccadoro M, Palumbo A. Review of thalidomide in the treatment of newly diagnosed multiple myeloma. Ther Clin Risk Manag. 2007;3:543–552. [PMC free article] [PubMed] [Google Scholar]
- 14.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA--Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459–4465. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 15.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 16.Lokhorst HM, Schmidt-Wolf I, Sonneveld P, et al. Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93:124–127. doi: 10.3324/haematol.11644. [DOI] [PubMed] [Google Scholar]
- 17.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson KC, Alsina M, Bensinger W, et al. Multiple Myeloma Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2009;7:908–942. doi: 10.6004/jnccn.2009.0061. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu JC, editor. Multiple comparisons: theory and methods. ed First. Chapman and Hall/CRC; 1996. [Google Scholar]
- 22.Kumar SK, Lacy MQ, Dispenzieri A, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–1592. doi: 10.1002/cncr.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M Boccadoro FC, Nagler A, et al. Melphalan/prednisone/lenalidomide (MPR) versus high-dose melphalan and autologous transplantation (MEL200) in newly diagnosed multiple myeloma (MM) patients: A phase III trial. JClin Oncology. 2011;29 (suppl; abstr 8020) [Google Scholar]
- 24.Siegel DSd, Jacobus S, Rajkumar SV, et al. Outcome with Lenalidomide Plus Dexamethasone Followed by Early Autologous Stem Cell Transplantation In the ECOG E4A03 Randomized Clinical Trial. ASH Annual Meeting Abstracts. 2010;116:38. doi: 10.1038/bcj.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 26.Nadal E, Gine E, Blade J, et al. High-dose therapy/autologous stem cell transplantation in patients with chemosensitive multiple myeloma: predictors of complete remission. Bone Marrow Transplant. 2004;33:61–64. doi: 10.1038/sj.bmt.1704313. [DOI] [PubMed] [Google Scholar]
- 27.Dingli D, Pacheco JM, Dispenzieri A, et al. Serum M-spike and transplant outcome in patients with multiple myeloma. Cancer Sci. 2007;98:1035–1040. doi: 10.1111/j.1349-7006.2007.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahuerta JJ, Mateos MV, Martinez-Lopez J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 29.Alexanian R, Weber D, Giralt S, et al. Impact of complete remission with intensive therapy in patients with responsive multiple myeloma. Bone Marrow Transplant. 2001;27:1037–1043. doi: 10.1038/sj.bmt.1703035. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Delasalle K, Feng L, et al. CR represents an early index of potential long survival in multiple myeloma. Bone Marrow Transplant. 45:498–504. doi: 10.1038/bmt.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]


