Abstract
Pulmonary embolism (PE) is common and associated with significant morbidity and mortality. An association between obesity and PE has been suggested, but the nature of the association has not been well defined. We performed a prospective cohort study of 87,226 women in the Nurses’ Health Study (1984–2002) to define the association between BMI and the risk of incident PE. Primary exposure was BMI (<22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, and ≥35.0 kg/m2). Primary outcome was idiopathic PE (medical record confirmed cases of PE not associated with prior surgery, trauma, or malignancy). Secondary analysis of nonidiopathic PE was also performed. Multivariable Cox proportional hazards models were controlled for age, physical activity, caloric intake, smoking, pack-years, race, spouse’s educational attainment, parity, menopause, nonaspirin nonsteroidal anti-inflammatory drugs, warfarin, multivitamin supplements, hypertension, coronary heart disease, and rheumatological disease. There were 157 incident idiopathic PE and 338 nonidiopathic PE. There was a strong positive association between BMI, the risk of idiopathic PE (relative risk (RR) = 1.08 (95% confidence interval (CI), 1.06–1.10) per 1 kg/m2 increase in BMI, P < 0.001) and nonidiopathic PE (RR = 1.08 (95% CI, 1.07–1.10), P < 0.001). The association was linear, and apparent even with modest increases in BMI (22.5–25 kg/m2). The risk increased nearly sixfold among subjects with BMI ≥35 kg/m2, and was present in multiple subgroups. Increasing BMI has a strong, linear association with the development of PE in women. Clinicians should consider BMI when assessing the risk of PE in their patients.
INTRODUCTION
Venous thromboembolism (VTE) represents a spectrum of disease that comprises deep venous thrombosis and pulmonary embolism (PE). The annual incidence of VTE is 1.2/1,000 people, similar to that of stroke (1). The more serious manifestation, PE, is the third most common cause of cardiovascular mortality in the United States and a leading cause of sudden death (2,3).
With the high incidence and mortality associated with PE, primary prevention is of vital public health importance (4–6). In September 2008, the Acting Surgeon General of the United States issued a “Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism” (7) urging “a coordinated, multifaceted plan to reduce the numbers of cases of deep vein thrombosis and pulmonary embolism nationwide” and “more research on the causes, prevention, and treatment of deep vein thrombosis.” Implicit in this message is the fact that our current understanding of modifiable risk factors is limited. Most studies to date focus on acquired risk factors or genetic polymorphisms associated with alterations in coagulation pathways (8,9). These factors are difficult to modify and do not suggest ways to decrease the incidence of PE at a population level.
Obesity, on the other hand, is modifiable and previous studies have suggested a positive association with PE (10–14). Most prior analyses have been cross-sectional however, which limits our ability to establish a temporal relationship. Moreover, studies of obesity tend to disproportionately reflect severely overweight subjects, so the nature of the relationship and the impact of modest increases in body fat on PE risk remain unclear. We hypothesized that, in a prospective cohort of >85,000 women, there would be a positive linear association between BMI and incident PE. We hypothesized that, rather than a threshold effect limited to obese subjects, the effect would be apparent with relatively modest increases in BMI. Finally, we hypothesized that the association would be evident whether the PE was idiopathic or associated with known precipitants, and would be present in multiple subgroups.
METHODS AND PROCEDURES
Participants
The Nurses’ Health Study is a longitudinal cohort established in 1976. At inception, the cohort included 121,700 female nurses between 30 and 55 years old. Every 2 years, participants are mailed a questionnaire assessing risk factors and the interval occurrence of disease. PE diagnoses have been collected since study inception. Detailed data related to lifestyle and dietary intake have been collected since 1984, and physical activity since 1986. Follow-up has been >90% for each questionnaire cycle.
For the current analysis, we began prospective follow-up in 1984 as some risk factors were not collected before then. We excluded women who reported a diagnosis of PE (n = 1,516) or died (n = 1,886) before 1984. We also excluded women missing data required to calculate BMI between 1984 and 2002, and those with BMI <15 kg/m2 or >60 kg/m2 (outliers). The present analysis included 87,226 women.
Primary exposure
Height was obtained from the 1976 questionnaire and presumed to be constant over time. Weight was obtained from the 1984 questionnaire and updated every 2 years thereafter. BMI was calculated according to the standard formula (BMI = weight in kilograms/height in meters, squared). Women were categorized according to BMI (<22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, and ≥35.0 kg/m2). Because only one subject with PE had a BMI <20 kg/m2, this was not considered a separate category. In 1996, subjects were asked to use a tape measure to measure the largest circumference around their hips and their waist (at the navel) while standing. These measurements were used to calculate the waist-to-hip ratio. Other variables related to adiposity, including weight at age 18 and the number of times subjects lost weight, were also compiled.
Prior validation studies have demonstrated that self-reported height, weight, waist and hip measurements, and weight at age 18 are highly correlated with values measured by study technicians or recorded during routine physical examinations (15–17). In particular, self-reported weight is highly correlated (Pearson correlation = 0.97) with weight measured by trained technicians (15).
Confounders
When possible, covariates were obtained from the 1984 questionnaire and updated every 2 years. Variables included: smoking status, pack-years of smoking, physical activity (first obtained in detail in 1986), parity, menopausal status, nonaspirin nonsteroidal anti-inflammatory drug use (first obtained in 1990), warfarin use (first obtained in 1994), multivitamin use, hypertension, coronary heart disease (any history of angina, coronary artery stenosis, or myocardial infarction), and rheumatologic disease (any history of systemic lupus erythematosus or rheumatoid arthritis). Race (white or nonwhite) and spouse’s highest educational attainment (high school, college, or graduate school) were obtained from the 1992 questionnaire. Smoking was categorized as never, exsmoker, or current smoker. Pack-years of smoking were calculated among ever smokers. Physical activity was reported in metabolic equivalent (MET) hours per week. Parity was categorized as nulliparous, 1, 2–3, or ≥4 children. Menopausal status was categorized by pre/post menopausal status and according to whether estrogen or progesterone replacement had or was being used. Medication and vitamin use, hypertension, coronary heart disease, and rheumatological disease were dichotomous.
Primary outcome/diagnosis of PE
On each questionnaire, nurses were asked whether a physician had diagnosed them with PE. Subjects without a prior history of malignancy received a follow-up letter requesting medical records from the facility where the PE was diagnosed. A detailed review of these records was undertaken and cases were coded. Incident cases for which the medical record included imaging diagnostic of PE were considered and confirmed. Imaging was considered diagnostic if a ventilation/perfusion lung scan was read by a radiologist as high probability for PE, if there was a filling defect on contrast enhanced computed tomography of the pulmonary vasculature or on catheter-based pulmonary angiography. Confirmed cases were sub-coded as “idiopathic PE” when medical record review revealed no history of surgery or major trauma within 1 month of PE diagnosis and no history of active malignancy. Cases associated with a history of surgery, major trauma or malignancy were sub-coded as “nonidiopathic PE.” If the nurse died prior to imaging but autopsy records confirmed the cause of death was PE, the nurse was considered to have PE. However, because detailed historical data are not available from autopsy records, these cases could not be confirmed as idiopathic, and were considered nonidiopathic. Thus, we analyzed two case definitions of PE. “Idiopathic PE” was the main outcome measure and refers only to medical record confirmed cases not associated with malignancy, surgery, or trauma. “Nonidiopathic PE” refers to cases that occurred in a subject with known malignancy, cases associated with recent surgery or trauma, and cases confirmed only by autopsy. Using these definitions, over 18 years of follow-up, we identified 157 incident idiopathic PE and 338 nonidiopathic PE (150 in subjects with malignancy, 115 associated with surgery or trauma and 73 confirmed by autopsy), for 495 total PE.
Statistical analysis
We performed age- and multivariable-adjusted Cox proportional hazards analysis to obtain relative risks (RRs) and 95% confidence intervals (CIs) for the risk of PE with increasing BMI. Multivariable models were adjusted for adjusted for age, total physical activity, total caloric intake, smoking, pack-years, race, spouse’s educational attainment, menopausal status, nonaspirin nonsteroidal anti-inflammatory drug use, warfarin use, multivitamin supplement use, hypertension, coronary heart disease, and rheumatological disease. For all BMI analyses, women with BMI <22.5 kg/m2 were the reference group. We performed a test for trend across ordinal categories of interest (i.e., BMI) to determine significant linear associations with PE.
We also performed sensitivity (subgroup) analyses, stratifying according to several factors: (i) whether the nurse had undergone a health screening examination (assessed in 1992, with analysis limited to data from 1992 to 2002) defined as a visit to a physician or clinic, where a physical exam, colonoscopy or sigmoidoscopy, rectal examination, stool occult blood exam, blood pressure check, blood cholesterol check, mammogram, breast exam by a clinician, or eye exam by a doctor were performed for screening purposes, rather than for symptoms; (ii) physical activity (high/low, split at the cohort median of 9.7 METs-hours per week), (iii) age (younger/older, split at 65 years), (iv) lifelong hormone exposure (high/low, high exposure defined as parity ≥2 children, age at menopause >50 and postmenopausal hormone use), and (v) smoking status (never, past, or current smoker). In addition to our primary analysis, we performed additional analyses of nonidiopathic PE to determine whether significant associations carried over to subjects with histories of surgery, trauma, or malignancy. We conducted all analysis using SAS version 9.1 (SAS Institute, Cary, NC).
The Human Research Committee of Partners HealthCare approved this study.
Role of the funding source
The National Institute on Aging, National Institutes of Health (Bethesda, MD), funded the study but the design, conduct, and reporting were entirely the responsibility of the authors.
RESULTS
Table 1 shows baseline characteristics adjusted for age and stratified according to BMI. The median BMI in 1984 was 24.0 kg/m2. At baseline, 27% of women were overweight (BMI 25.0–29.9 kg/m2) and 14% were obese (BMI ≥30.0 kg/m2). Most subjects were white and had given birth to at least two children. Women with higher BMI were more likely to be exsmokers, have a history of hypertension and coronary heart disease, take nonaspirin nonsteroidal anti-inflammatory drugs and warfarin, and were less physically active.
Table 1.
Age-adjusted baseline characteristics according to BMI, Nurses’ Health Study, n = 87,226
| BMI (kg/m2)
|
||||||
|---|---|---|---|---|---|---|
| <22.5 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | ≥35.0 | |
| n | 29,308 | 22,519 | 15,818 | 7,724 | 8,128 | 3,729 |
| Median of BMI (kg/m2) | 20.9 | 23.1 | 25.2 | 27.4 | 30.1 | 36.0 |
| Mean age in 1984 | 56 | 57 | 58 | 58 | 58 | 57 |
| White race/ethnicity, % | 98 | 98 | 98 | 97 | 98 | 97 |
| Smoking, % | ||||||
| Nonsmokers | 43 | 44 | 45 | 45 | 46 | 48 |
| Exsmokers | 36 | 40 | 41 | 42 | 43 | 43 |
| Current smokers | 21 | 16 | 14 | 13 | 11 | 9 |
| Pack-years among ever smokersa,b | 24 | 23 | 23 | 23 | 23 | 23 |
| Spouse’s educational attainment, % | ||||||
| High school | 30 | 34 | 39 | 41 | 43 | 47 |
| College | 25 | 25 | 23 | 22 | 21 | 18 |
| Graduate school | 24 | 21 | 18 | 17 | 15 | 12 |
| Missing | 21 | 20 | 20 | 20 | 21 | 23 |
| Total physical activity (METs/week)c,d | 17 | 15 | 14 | 12 | 11 | 9 |
| Total caloric intake (kcal/day)e | 1,747 | 1,737 | 1,735 | 1,742 | 1,758 | 1,801 |
| Absolute weight change since age 18 (kg)e | 2.3 | 6.4 | 10.2 | 14.0 | 18.9 | 29.8 |
| Relative weight change since age 18, % | 5 | 13 | 19 | 25 | 33 | 48 |
| Waist-to-hip ratio | 0.82 | 0.84 | 0.86 | 0.88 | 0.88 | 0.88 |
| Parity, % | ||||||
| Nulliparous | 7 | 6 | 5 | 5 | 5 | 6 |
| 1 child | 8 | 7 | 7 | 7 | 6 | 8 |
| 2–3 children | 58 | 56 | 55 | 54 | 54 | 54 |
| ≥4 children | 26 | 30 | 32 | 33 | 33 | 31 |
| Missing | 1 | 1 | 1 | 1 | 2 | 1 |
| Menopausal status, % | ||||||
| Pre menopause | 29 | 25 | 22 | 20 | 21 | 24 |
| Post menopause and never HRT use | 23 | 25 | 28 | 31 | 31 | 35 |
| Post menopause and past user for HRT | 15 | 17 | 18 | 18 | 18 | 17 |
| Post menopause and estrogen replacement therapy | 13 | 13 | 13 | 13 | 12 | 10 |
| Post menopause and estrogen-progesterone replacement therapy | 8 | 8 | 7 | 6 | 6 | 4 |
| Missing | 12 | 12 | 12 | 12 | 12 | 10 |
| Nonaspirin NSAID use, % | 27 | 29 | 31 | 33 | 35 | 40 |
| Warfarin use, % | 1.0 | 1.1 | 1.3 | 1.4 | 1.7 | 2.3 |
| Multivitamin supplement use, % | 38 | 37 | 36 | 35 | 33 | 33 |
| Hypertension, % | 12 | 17 | 23 | 28 | 35 | 42 |
| Coronary heart disease, %f | 1.3 | 1.6 | 2.2 | 2.5 | 2.7 | 3.0 |
| Rheumatological disease, %g | 22 | 19 | 17 | 15 | 15 | 16 |
HRT, hormone-replacement therapy; MET, metabolic equivalent; NSAID, nonsteroidal anti-inflammatory drug.
Number of packs smoked per day.
Number of years smoked, among past and current smokers.
MET-hour/week, sum of the average time per week spent in each activity.
MET value of each activity.
Age-adjusted mean (all such values).
Coronary heart disease includes any history of myocardial infarction, coronary artery stenosis, or angina.
Rheumatological disease includes any history of systemic lupus erythematosus or rheumatoid arthritis.
We found a strong, independent, positive linear association between BMI and the risk of idiopathic PE (Table 2). After multivariable adjustment, the risk of idiopathic PE increased by 8% for every 1 kg/m2 increase in BMI: RR 1.08 (95% CI, 1.06–1.10), P < 0.001. There was a similar increase in the risk of nonidiopathic PE: RR 1.08 (95% CI, 1.07–1.10). With early events removed (PE from 1984 to 1990, n = 34 for idiopathic PE), the BMI–PE association remained: 1.00 (reference) for BMI <22.5 kg/m2, 1.52 (95% CI, 0.73–3.13) for BMI 22.5–24.9 kg/m2, 2.11 (95% CI, 1.04–4.26) for BMI 25.0–27.4 kg/m2, 3.86 (95% CI, 1.93–7.73) for BMI 27.5–29.9 kg/m2, 3.46 (95% CI, 1.71–6.99) for BMI 30.0–34.9 kg/m2, and 5.05 (95% CI, 2.31–11.04) for BMI >35 kg/m2, P for trend <0.001. Results were similar for nonidiopathic PE (data not shown).
Table 2.
Association between BMI and newly diagnosed PE, Nurses’ Health Study (1984–2002, n = 87,226)
| BMI (kg/m2)
|
P for trend | ||||||
|---|---|---|---|---|---|---|---|
| <22.5 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | 35.0 | ||
| Median of BMI, kg/m2 | 20.9 | 23.1 | 25.2 | 27.4 | 30.1 | 36.0 | |
| Idiopathic PE | |||||||
| No. of cases/person-years | 17/146,188 | 23/132,746 | 29/107,645 | 31/60,155 | 34/64,756 | 23/30,205 | |
| Age-adjusted RR (95% CI) | 1.00 | 1.38 (0.74–2.59) | 2.03 (1.12–3.70) | 3.74 (2.06–6.76) | 3.80 (2.12–6.83) | 5.77 (3.07–10.9) | <0.001 |
| Multivariable RR (95% CI)a | 1.00 | 1.37 (0.73–2.57) | 2.07 (1.13–3.79) | 3.72 (2.04–6.81) | 3.75 (2.06–6.85) | 5.79 (2.97–11.27) | <0.001 |
| Nonidiopathic PE | |||||||
| No. of cases/person-years | 36/146,207 | 52/132,775 | 75/107,691 | 61/60,185 | 61/64,783 | 53/30,235 | |
| Age-adjusted RR (95% CI) | 1.00 | 1.52 (0.99–2.33) | 2.56 (1.72–3.82) | 3.65 (2.41–5.52) | 3.44 (2.28–5.21) | 6.96 (4.55–10.66) | <0.001 |
| Multivariable RR (95% CI)a | 1.00 | 1.48 (0.97–2.27) | 2.45 (1.64–3.65) | 3.37 (2.22–5.12) | 3.01 (1.97–4.59) | 5.42 (3.46–8.48) | <0.001 |
| Total PE | |||||||
| No. of cases/person-years | 53/146,224 | 75/132,798 | 104/107,720 | 92/60,216 | 95/64,817 | 76/30,258 | |
| Age-adjusted RR (95% CI) | 1.00 | 1.48 (1.04–2.10) | 2.39 (1.72–3.33) | 3.68 (2.62–5.17) | 3.57 (2.55–5.00) | 6.56 (4.61–9.34) | <0.001 |
| Multivariable RR (95% CI)a | 1.00 | 1.45 (1.02–2.06) | 2.32 (1.66–3.25) | 3.49 (2.47–4.92) | 3.24 (2.29–4.58) | 5.53 (3.81–8.01) | <0.001 |
PE, pulmonary embolism; RR, relative risk; CI, confidence interval.
Multivariable RRs have been adjusted for age, total physical activity, total caloric intake, smoking, pack-years, race, spouse’s educational attainment, parity, menopausal status, nonaspirin nonsteroidal anti-inflammatory drug use, warfarin use, multivitamin supplement use, hypertension, coronary heart disease, and rheumatological disease.
Although waist-to-hip ratio was associated with idiopathic PE in an age-adjusted model (RR 1.11 (95% CI, 1.02–1.22)), it was not associated in a fully adjusted model that included BMI (RR 1.03 (95% CI, 0.94–1.14)). The multivariable RRs for increasing quintiles of waist-to-hip ratio were 1.00 (reference), 1.28 (95% CI, 0.62–2.62), 1.08 (95% CI, 0.52–2.24), 1.66 (95% CI, 0.84–3.27), and 1.10 (95% CI, 0.54–2.26), P for trend = 0.51. However, the power of this analysis may have been limited by the fact that waist and hip measurements were only requested from a portion of the cohort in 1996 and therefore not available for a large number of subjects (n = 32,982). That notwithstanding, in this multivariable model, the association between BMI and idiopathic PE and nonidiopathic PE remained unchanged (data not shown). We therefore removed waist-to-hip ratio from the model.
We applied a similar approach to evaluating weight change since age 18. On age-adjusted analysis, absolute weight change (assessed as a continuous variable) and relative weight change (as a percentage of weight at age 18) were associated with the risk of idiopathic PE. For every 1 kg of weight gained since age 18, the risk of idiopathic PE increased by 4%: RR 1.04 (95% CI, 1.02–1.05), P < 0.001. For every 10% increase in weight since age 18 the risk of idiopathic PE increased (RR 1.21 (95% CI, 1.13–1.28), P < 0.001). However, in the fully adjusted model that included BMI, neither absolute weight change: (RR 1.01 (95% CI, 0.995–1.03) P for trend = 0.17), nor relative weight change (RR 1.06 (95% CI, 0.97–1.16) P for trend = 0.16) were associated with idiopathic PE. Results were similar for nonidiopathic PE (data not shown). We therefore removed weight change since age 18 from the model.
To address potential detection bias, we reexamined the BMI–PE association according to whether the nurse had undergone a health screening examination (67% of the cohort). A health screening examination was defined as a visit to a doctor or clinic, for a physical exam or testing, not prompted by symptoms. In women who exhibited this health oriented behavior, the strong linear association between BMI and PE was similar to that in the cohort as a whole (P for interaction = 0.69). The multivariable-adjusted RR (95% CI) for idiopathic PE were, by BMI group: 1.00 (reference), 1.47 (95% CI, 0.60–3.61), 2.15 (95% CI, 0.91–5.12), 4.89 (95% CI, 2.13–11.23), 4.22 (95% CI, 1.81–9.87), and 4.39 (95% CI, 1.61–11.97); P for trend <0.001.
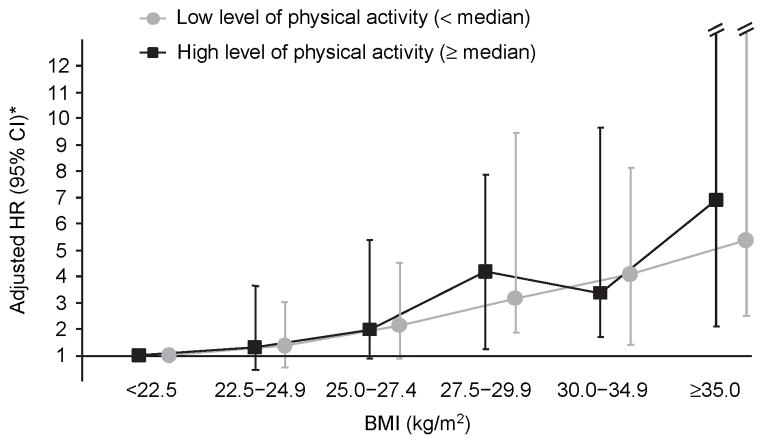
We also assessed whether the BMI–PE association varied according to whether the subjects’ physical activity levels were above or below the cohort median of 9.7 METs-hours per week. Figure 1 shows that the association between BMI and idiopathic PE is similar among women below (mean = 0.82 METs-hour per week) and above the cohort’s median level of physical activity (mean = 3.20 METs-hour per week). The P for interaction was 0.54.
Figure 1.
Association between idiopathic PE and BMI according to the level of physical activity, Nurses’ Health Study (1984–2002, n = 87,226). RR, relative risk; CI, confidence interval. *Adjusted for age, total caloric intake, smoking, pack-years, race, spouse’s educational attainment, parity, menopausal status, nonaspirin nonsteroidal anti-inflammatory drug use, warfarin use, multivitamin supplement use, hypertension, coronary heart disease, and rheumatological disease. Among women with the lowest level of physical activity, the number of cases according to BMI were: 10, 10, 19, 17, 17, and 15 (<22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, and ≥35.0 kg/m2, respectively). Among women with the highest level of physical activity, the number of cases according to BMI were: 7, 13, 10, 14, 17, and 8 (<22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–34.9, and ≥35.0 kg/m2, respectively).
We stratified according to age (<65 or ≥65 years old), and found no significant difference in the effect of BMI on idiopathic PE (P for interaction = 0.64). For younger women the results, by BMI group, were RR = 1.00 (reference), 1.02 (95% CI, 0.42–2.47), 2.42 (95% CI, 1.12–5.27), 3.76 (95% CI, 1.69–8.36), 3.65 (95% CI, 1.62–8.21), and 6.36 (95% CI, 2.69–15.02); P for trend <0.001. For older women, the results were: RR = 1.00 (reference) for BMI <22.5 kg/m2, 1.77 (95% CI, 0.70–4.45), 1.50 (95% CI, 0.56–3.96), 3.40 (95% CI, 1.35–8.53), 3.77 (95% CI, 1.52–9.34), and 4.68 (95% CI, 1.61–13.61); P for trend <0.001.
Studies suggest that obesity and endogenous estrogen exposure may have a synergistic effect on the risk of PE (18,19). We reanalyzed the BMI–PE relationship according to lifelong estrogen exposure. High exposure was defined as parity ≥2 children, age at menopause >50 and postmenopausal hormone use. There were relatively few cases of PE among women with high lifelong hormone exposure, so we collapsed BMI into three categories (<25.0, 25.0–29.9, ≥30.0). The risk of PE, by BMI group, was similar in women with low (RR = 1.00 (reference), 2.27 (95% CI, 1.45–3.56), 3.16 (95% CI, 1.96–5.09); P for trend <0.001) and high hormone exposure (RR = 1.00 (reference), 1.92 (95% CI, 0.74–4.96), 4.75 (95% CI, 1.82–12.36); P for trend <0.001). The P for interaction was 0.49.
Finally, we restricted our analysis to never smokers (n = 38,187)—as opposed to current or past smokers. As above, we collapsed BMI into three categories (<25.0, 25.0–29.9, ≥30.0). The risk of PE was still highly related to BMI: 1.00 (reference), 1.78 (95% CI, 0.95–3.33) and 3.32 (95% CI, 1.75–6.32); P for trend <0.001.
DISCUSSION
We found a strong, independent, positive linear association between BMI and incident PE in this large cohort of female nurses. The risk of PE increased even with modest increases in BMI. In the most overweight subjects, the risk increased nearly sixfold. The association was present for idiopathic PE and PE associated with surgery, trauma, and malignancy. The association was strong and consistent across several subgroups. BMI was more strongly associated with incident PE than were other obesity-related measures such as the distribution of adiposity and change in adiposity over time.
Our study is novel in that it demonstrates that the BMI–PE association is linear, and not limited to severely obese subjects. It is the most detailed exploration of the relationship between BMI and PE to date. Prior epidemiological data on the relationship between adiposity and VTE/PE are suggestive, but prospective data are limited. Studies using cross-sectional or case–control designs demonstrate that individuals with VTE have higher BMI than those without (10,11). Although these studies’ results generally agree with ours, they do not share the benefits of our longitudinal cohort, and their design makes it difficult to determine whether obesity preceded or followed the PE. Because PE may lead to weight gain as a result of activity limitations, demonstrating the temporal nature of the relationship, as we have, is critical. A few prospective studies have demonstrated an association between obesity and VTE, but these have generally limited their analysis to severely overweight subjects (10,13,14,20,21). One study of a smaller cohort than ours, focusing on VTE rather than PE, found the RR of VTE was: 1.0 for BMI <25, 1.5 for BMI 25 to <30, 2.2 for BMI 30 to <35, 1.5 for BMI 35 to <40, and 2.7 for BMI ≥40 (20). Another study, of men, found that increasing BMI was an independent risk factor for VTE (OR 1.1/1 kg/m2), and that high BMI was a greater risk factor for VTE than for either coronary heart disease or stroke (22). We therefore believe that the totality of the evidence is sufficient to support the observed association.
Although mechanistic research is understandably limited, there are several explanations for how adiposity might increase PE risk. Leptin may mediate the relationship between obesity and PE. In mice, leptin inhibition has been shown to decrease the rate of fatal PE associated with the injection of thrombogenic material (23). Leptin also induces tissue factor activity in vitro, and obese human subjects have elevated levels of both leptin and tissue factor (24). Another possibility is that the relationship is mediated by estrogen and progesterone, which have established links to obesity and the risk of PE in women (18,25). In our study, we found no evidence that high lifelong estrogen exposure modified the association between BMI and PE. However, the relationship between obesity and sex hormones is complex, and we did not analyze progesterone. Finally, it is possible that subjects with higher BMI are at greater risk of PE once a deep venous thrombosis forms (11).
Regardless of the pathophysiological mechanism, the public health implications of our findings are potentially large. Nationally representative surveys indicate more than one-third of Americans are overweight and that the prevalence of obesity is increasing (from 25% in 1974 to 33% in 2004) (26,27). Moreover, an Italian study found 50% of women and 35% of men have at least one risk factor for PE other than obesity (28). The rising prevalence of obesity may help explain the high prevalence of PE, and public health campaigns that lead to weight loss in the general population may also reduce the incidence of PE.
Limitations
One limitation of our study involves the nature of the cohort: female nurses, >95% of whom are white. This represents the demographics of nurses in 1976, but limits the generalizability of our results to other racial groups and to men. The mean age of our cohort was >55 years old at inception, so our results may not reflect younger women. In elderly individuals however, who for various reasons tend to gain weight with age, (29–31) and find weight loss difficult, (32) the BMI–PE association may be particularly important.
In the early phases of any long-term study, participants tend to be healthier than nonparticipants, and women enrolled in the Nurses’ Health Study are different from the general population. Their education, access to medical care, and above-average standard of living all might limit generalizability. This may explain the Nurses’ Health Study cohort’s lower mean adiposity level compared to the general US population. Nonetheless, there is little biological basis for suspecting that the relationship we observed between BMI and PE would be materially different in other groups of women. Furthermore, the group’s relative homogeneity and good baseline health help minimize confounding by socioeconomic factors, access to health care, and health literacy.
Finally, we considered that our results could have been influenced by measurement bias, workup bias, or inaccuracies arising from self-reported measures of adiposity. The BMI–PE association we found was consistent across various subgroups however, and the association was not previously so well established that clinicians would likely work up individuals for PE simply on the basis of BMI. Furthermore, prior validation studies have found weight to be highly correlated with measured values (15,17).
Conclusions
This large prospective cohort study demonstrates that increased BMI is associated with increased risk of PE. The effect is linear and apparent even with modest increases in BMI. The magnitude of the risk is larger than that of many putative PE risk factors and, when combined with the high prevalence of obesity in the United States, suggests that obesity may explain a significant proportion of PE cases. These data add to the evidence that excess body fat is a major cause of human disease. Confirmation of our findings is warranted in other populations.
Acknowledgments
The Nurses’ Health Study is coordinated at the Channing Laboratory, Brigham and Women’s Hospital, Boston, Massachusetts. This work was supported by: NIH R21AG031079 (National Institute of Aging, National Institutes of Health, Bethesda, Maryland).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 3.Kurkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med. 2000;160:1529–1535. doi: 10.1001/archinte.160.10.1529. [DOI] [PubMed] [Google Scholar]
- 4.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130:172–175. doi: 10.1378/chest.130.1.172. [DOI] [PubMed] [Google Scholar]
- 5.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 6.Tapson VF, Humbert M. Incidence and prevalence of chronic thromboembolic pulmonary hypertension: from acute to chronic pulmonary embolism. Proc Am Thorac Soc. 2006;3:564–567. doi: 10.1513/pats.200605-112LR. [DOI] [PubMed] [Google Scholar]
- 7.Acting Surgeon General Issues ‘Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism’. Office of the Surgeon General, U.S. Department of Health and Human Sciences; < http://www.surgeongeneral.gov/news/pressreleases/pr20080915.html>. [PubMed] [Google Scholar]
- 8.Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost. 2002;28(Suppl 2):3–13. doi: 10.1055/s-2002-32312. [DOI] [PubMed] [Google Scholar]
- 9.Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost. 2001;86:452–463. [PubMed] [Google Scholar]
- 10.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Kucher N, Tapson VF, Goldhaber SZ. Risk factors associated with symptomatic pulmonary embolism in a large cohort of deep vein thrombosis patients. Thromb Haemost. 2005;93:494–498. doi: 10.1160/TH04-09-0587. [DOI] [PubMed] [Google Scholar]
- 12.Linnemann B, Zgouras D, Schindewolf M, et al. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis. 2008;19:159–165. doi: 10.1097/MBC.0b013e3282f54558. [DOI] [PubMed] [Google Scholar]
- 13.Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA. 1997;277:642–645. [PubMed] [Google Scholar]
- 14.Goldhaber SZ, Savage DD, Garrison RJ, et al. Risk factors for pulmonary embolism. The Framingham Study. Am J Med. 1983;74:1023–1028. doi: 10.1016/0002-9343(83)90805-7. [DOI] [PubMed] [Google Scholar]
- 15.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 17.Troy LM, Hunter DJ, Manson JE, et al. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 18.Simon T, Beau Yon de Jonage-Canonico M, Oger E, et al. Indicators of lifetime endogenous estrogen exposure and risk of venous thromboembolism. J Thromb Haemost. 2006;4:71–76. doi: 10.1111/j.1538-7836.2005.01693.x. [DOI] [PubMed] [Google Scholar]
- 19.Canonico M, Oger E, Conard J, et al. Obesity and risk of venous thromboembolism among postmenopausal women: differential impact of hormone therapy by route of estrogen administration. The ESTHER Study. J Thromb Haemost. 2006;4:1259–1265. doi: 10.1111/j.1538-7836.2006.01933.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 21.Hansson PO, Eriksson H, Welin L, Svardsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the study of men born in 1913. Arch Intern Med. 1999;159:1886–1890. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 22.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162:975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinides S, Schafer K, Neels JG, Dellas C, Loskutoff DJ. Inhibition of endogenous leptin protects mice from arterial and venous thrombosis. Arterioscler Thromb Vasc Biol. 2004;24:2196–2201. doi: 10.1161/01.ATV.0000146531.79402.9a. [DOI] [PubMed] [Google Scholar]
- 24.Napoleone E, ADIS, Amore C, et al. Leptin induces tissue factor expression in human peripheral blood mononuclear cells: a possible link between obesity and cardiovascular risk? J Thromb Haemost. 2007;5:1462–8. doi: 10.1111/j.1538-7836.2007.02578.x. [DOI] [PubMed] [Google Scholar]
- 25.Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292:1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, McDowell MA, Flegal KM. NCHS data brief no 1. Hyattsville, MD: National Center for Health Statistics; 2007. Obesity among adults in the United States—no statistically significant change since 2003–2004. [PubMed] [Google Scholar]
- 28.Di Minno G, Mannucci PM, Tufano A, et al. The first ambulatory screening on thromboembolism: a multicentre, cross-sectional, observational study on risk factors for venous thromboembolism. J Thromb Haemost. 2005;3:1459–1466. doi: 10.1111/j.1538-7836.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 29.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–1912. doi: 10.1111/j.1532-5415.2004.52517.x. [DOI] [PubMed] [Google Scholar]
- 30.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan MS, Huguet N, Newsom JT, McFarland BH, Lindsay J. Prevalence and correlates of overweight and obesity among older adults: findings from the Canadian National Population Health Survey. J Gerontol A Biol Sci Med Sci. 2003;58:1018–1030. doi: 10.1093/gerona/58.11.m1018. [DOI] [PubMed] [Google Scholar]
- 32.Gostic CL. The crucial role of exercise and physical activity in weight management and functional improvement for seniors. Clin Geriatr Med. 2005;21:747–756. vii. doi: 10.1016/j.cger.2005.06.002. [DOI] [PubMed] [Google Scholar]