Abstract
There are several controversies in the surgical management of esophageal cancer including the surgical approach, extent of resection, optimal fields of lymph node dissection, and the ideal location of anastomosis. Optimal surgical treatment strategies must include accurate staging and the selection of an appropriate surgical approach. In addition, other considerations include complete resection, lymph node dissection and evaluation of oncologic and functional outcomes. The objective of this article is to review the literature and discuss our surgical approach to esophageal cancer.
Introduction
The incidence of esophageal carcinoma has increased dramatically over the past three decades and the incidence of adenocarcinoma now surpasses that of squamous cell carcinoma in the United States (1). Surgical resection is the primary curative therapy for patients with resectable esophageal cancer. There are, however, several controversies in the optimal management of esophageal cancer including the surgical approach, extent of resection, optimal fields of lymph node dissection, and the ideal location of anastomosis (2). The objective of this article is to review the literature on surgical resection for esophageal cancer and discuss our approach. This article is not intended to be a comprehensive review of all these issues, but rather give the reader an overview of some of the controversies and describe our preferred surgical approach.
Methods
We reviewed our experience performing esophagectomies and the medical literature on approaches to esophagectomy, including the surgical approach, extent of resection, fields of lymph node dissection, and the location of anastomosis. The medical literature was searched using Pubmed with the following key words: esophageal cancer, esophagectomy, surgical approach, and lymph node dissection. The reference lists from the retrieved articles were also reviewed for additional articles. Since this article is not intended to be a comprehensive review, we selected the most relevant articles for discussion. The search was limited to English language and human studies.
Surgical Approaches
The open approaches used for esophageal resection include transhiatal esophagectomy (THE) and transthoracic approaches, such as Ivor-Lewis esophagectomy, resection with a left thoracotomy or left thoracoabdominal approach and “3-incision” McKeown-type esophagectomy (3-8). The choice of approach depends on various factors including the location of the tumor, preference of the surgeon and choice of conduit for esophageal reconstruction. There are proponents of the transhiatal approach as well as proponents of the transthoracic approach and the ideal approach has not been established.
Transthoracic versus transhiatal approach
The potential advantages of a transthoracic approach include better exposure of and access to the tumor in the chest and improved lymph node dissection in the mediastinum. Proponents of an extended transthoracic esophagectomy (TTE) point to high local recurrence rates that have been noted with THE (9, 10). For example, in the surgery arm of a randomized trial of THE alone vs. THE following neoadjuvant therapy, locoregional recurrence after transhiatal esophagectomy alone was 39% (10). On the other hand, proponents of THE point to its lower morbidity and potentially equivalent survival as compared to TTE.
There have been retrospective studies which have compared the various approaches to esophagectomy and no significant differences in long-term survival have been detected (11, 12). There are, however, very few randomized trials comparing TTE and THE (13-15). Some of these studies were small and comprised primarily of patients with squamous cell neoplasm (13, 14). The largest randomized study comparing TTE and THE was reported by Hulscher and colleagues (15). These authors conducted a prospective randomized trial comparing TTE and THE in patients mid-distal and gastroesophageal junction neoplasm. In this study, comprising of 220 patients, 106 patients were randomized to undergo THE and 114 patients were randomized to undergo TTE. The esophageal cancer stages were similar in both the groups, although there was a non significant trend towards a greater number of Stage IV patients in the transthoracic arm (15% vs. 7%). THE patients had a shorter duration of surgery, less blood loss, and less morbidity. The R0 resection rate was similar. Although, the perioperative morbidity was higher in the TTE group; there was no significant difference in mortality between the two groups. Significantly more lymph nodes were resected in the TTE arm. The median follow-up was 4.7 years. Recurrent disease developed in 58% after THE and 57% after TTE. The median disease-free survival was 1.4 years after THE and 1.7 years after TTE (P=0.15). There was a trend towards a better five-year disease-free survival in the TTE group (39 vs. 27%), although this did not reach statistical significance (Figure 1). The median overall survival was 1.8 years in the THE arm and 2 years in the TTE arm. Similarly, the five-year overall survival showed a nonsignificant trend towards better survival in the TTE arm (39 vs. 29%) (Figure 2). The median quality-adjusted life years after THE was 1.5 years after THE vs. 1.8 years after TTE. In a recently updated follow-up of this series, subgroup analysis of patients with less extensive nodal involvement (1-8 positive nodes) revealed improved locoregional disease-free survival with a transthoracic approach (16).
Figure 1.
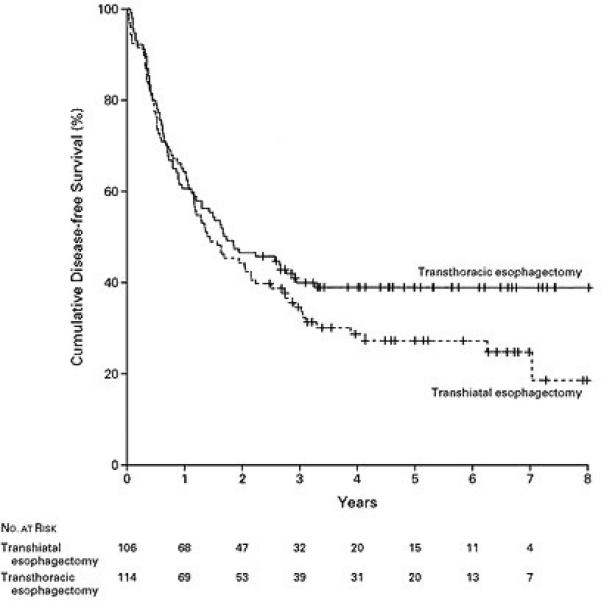
Kaplan-Meier Curves Showing Disease-free Survival among Patients Randomly Assigned to Transhiatal Esophagectomy or Transthoracic Esophagectomy with Extended en Bloc Lymphadenectomy (Reprinted from [15] by permission of the Massachusetts Medical Society.)
Figure 2.
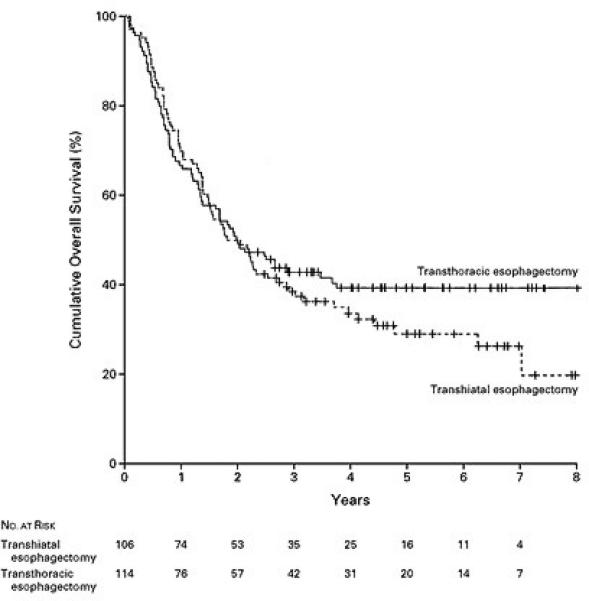
Kaplan-Meier Curves Showing Overall Survival among Patients Randomly Assigned to Transhiatal Esophagectomy or Transthoracic Esophagectomy (Reprinted from [15] by permission of the Massachusetts Medical Society.)
Meta-analysis of transthoracic versus transhiatal esophagectomy
Meta-analyses of transthoracic versus transhiatal esophagectomy have also been reported (11, 12). Rindani and colleagues reviewed 44 studies, with 33 papers on 2675 patients undergoing THE and 29 papers on 2808 patients undergoing an Ivor Lewis esophagectomy (11). There were no significant differences in patient characteristics including stage. There were no differences in pulmonary or cardiovascular complications; however, there appeared to be an increased risk of anastomotic complications (leaks and strictures) and recurrent laryngeal nerve (RLN) injuries with a THE approach. The mortality was 6.3% for THE and 9.5% for Ivor Lewis esophagectomy. The overall survival at 5 years was similar (24% for THE, 26% for Ivor Lewis esophagectomy).
Hulscher and colleagues reported another meta-analysis of 50 articles, comprising more than 7500 patients (12). This analysis showed an increase in pulmonary complications and mortality with TTE. Similar to the other meta-analysis, there was an increase in anastomotic leaks and RLN injury with THE. There was no significant difference in overall survival during long-term follow-up between the groups.
In summary, based on meta-analyses (with their limitations), it appears that there is an increase in mortality with open TTE for cancer when compared to open THE. Anastomotic leaks and RLN injury appear to be decreased with a transthoracic approach. There is no significant difference in long-term survival; however, the largest randomized study shows a nonsignificant trend, favoring the transthoracic approach for disease free and overall survival (15). We prefer a transthoracic approach and, as described below, have adopted a minimally invasive approach in an effort to decrease the morbidity of esophagectomy.
Extent of Lymph Node Dissection
The extent of lymph node dissection required in patients with esophageal cancer is also controversial (17). There are proponents of 3-field lymphadenectomy, which includes the abdomen, chest and neck, and proponents of 2-field lymphadenectomy, which includes the abdomen and chest. Proponents of THE typically dissect the abdominal lymph node field alone, and thoracic dissection of lymph nodes is limited. Proponents of an extended 3-field lymph node dissection point out low recurrence rates and the benefits of accurate staging. The 3-field lymphadenectomy was initiated in Japan, after a high neck recurrence rate was observed in patients with esophageal squamous cell carcinoma (17). Nishihara and associates conducted a randomized trial of 62 patients with squamous cell cancer comparing 2-field lymphadenectomy (thoracic and abdominal) with 3-field lymphadenectomy (including cervical and superior mediastinal lymphadenectomy) (18). There was a significant increase in complications noted in the 3-field (extended) lymphadenectomy group including a 53% tracheostomy rate and 13% incidence of phrenic nerve palsy. During follow-up, the recurrence rate was 19.9% in the 3-field field lymph node dissection group and 24.1% in the 2-field group, which was not significantly different. However, a nonsignificant trend towards better survival was noted on long-term follow-up in the extended lymphadenectomy group.
Three- field lymph node dissection is primarily practiced in Japan and in the west, and there have been few proponents of the 3-field approach (19, 20). Altorki reported the results in 80 patients, 60% of whom had adenocarcinoma of the esophagus treated with 3-field lymph node dissection. These authors’ reported a 37% incidence of cervical lymph node metastases, an estimated 5-year overall survival of 50%, and an estimated disease-free survival of 46%. RLN injuries occurred in only 6% of patients in this series. Another large, single institution study of three-field lymphadenectomy was reported by Lerut and colleagues who analyzed their results in 174 patients (55% adenocarcinoma). The operative mortality was 1.4%, 5-year overall survival was 41.9 %, and 5-year disease-free survival was 46.3 %. Although these results appear promising, they are at odds with the randomized controlled trial detailed above (18).
The extent of lymph node dissection required for patients with esophageal cancer remains controversial. It is unclear whether the survival results after 3-field dissection may be, in part, because of stage migration, due to improved staging, rather than indicating a true therapeutic benefit. However, it is becoming clear that for more accurate staging, adequate lymph node sampling is required (21). One of the potential advantages of the transthoracic approach is better exposure and improved lymph node dissection in the mediastinum. We prefer a two-field approach for the lymph node dissection for the treatment of esophageal cancer.
Location of anastomosis
Another controversy surrounds the optimal location for the anastomosis, in the neck or in the chest. Proponents of the cervical anastomosis point out the benefits of a more proximal resection margin and the potentially lower mortality associated with a cervical anastomotic leak. Proponents of an intrathoracic anastomosis point to reduced tension at the anastomosis and a lower risk of anastomotic complications such as leaks and strictures. Potential causes for an increased risk of leak of a cervical anastomosis includes the tenuous blood supply of the fundus, potentially more tension at the anastomosis, and extrinsic compression of the conduit at the thoracic inlet (22).
The blood supply of the fundus is tenuous because the fundal tip is supplied via collaterals from the mucosal and submucosal collaterals (23). An anastomosis in the chest has potentially less tension and allows for better gastric margins for gastroesophageal junction and cardia tumors. The leak rate after an intrathoracic anastomosis is lower, as shown in large, single-institution studies and meta-analyses (8, 11, 12).
There have been few randomized trials that have evaluated outcomes based on the site of anastomosis (cervical vs. intrathoracic) (24). Walther reported the results of a prospective randomized trial with 83 patients; 41 were randomized to have a neck anastomosis and 42 who were randomized to have the anastomosis performed in the chest (24). There was an overall 1.8% leak rate with no differences based on site of anastomosis. There were no differences in the rate of positive margins, anastomotic diameter and overall survival in between the groups.
Another important complication to avoid in esophageal resections is RLN palsy (22, 25, 26). Patients with RLN injury not only complain of hoarseness of voice, but RLN injury also affects swallowing and the ability to generate a good cough and protect the airway. This may lead to an increase in pulmonary complications. Not unexpectedly, a 10-fold increase of pulmonary complications, from 2.4% to 24%, has been reported in patients with RLN injury (25).
Rindani conducted a meta-analysis of transhiatal versus Ivor Lewis esophagectomy (11) and, as described above, there were no differences in pulmonary or cardiovascular complications. However, there appeared to be an increased risk of anastomotic complications (leaks and strictures) and RLN injuries with a transhiatal approach. In this analysis, the risk of RLN injury was 11% with THE and 4.2% with an Ivor Lewis approach (11). Hulscher, in their meta-analysis of THE versus TTE, also reported a significant decrease in anastomotic leaks and RLN injury with a transthoracic approach (12). The relative risk reduction of anastomotic leak was 0.53 and of RLN injury was 0.36.
In summary, an Ivor Lewis approach with an intrathoracic anastomosis appears to be associated with lower rates of anastomotic leak and RLN injury with similar long-term survival. In our reported experience with minimally invasive Ivor Lewis esophagectomy (27), there were no RLN injuries and few anastomotic leaks, and this has become our procedure of choice (27).
Minimally invasive approach to esophagectomy
One of the main concerns for recommendation of esophagectomy is the risk associated with surgery (28). Birkmeyer reported that the mortality of esophagectomy ranged from 8% to 23% (28). In an effort to decrease the morbidity and mortality from open esophagectomy, we have adopted a minimally invasive approach. We have described the technique and our experience at the University of Pittsburgh in detail elsewhere (29). We reported our results of a series of 222 consecutive minimally invasive esophagectomies (MIE) (29). MIE was successfully completed in 206 (92.8%) patients. The median ICU stay was 1 day, and hospital stay 7 days. The operative mortality was 1.4%. The oncologic results, per stage, were similar to historical series of open esophagectomy.
Recently, the initial results of a Phase II multi-institutional study (Eastern Cooperative Oncology Group, ECOG 2202) to evaluate the results of MIE in a multi-institutional setting were reported (30). In this multicenter cooperative group trial, a total of 106 patients were enrolled. This study showed a low morbidity and mortality of less than 2% (30). Median ICU stay was 2 days. At a mean follow-up of 19 months, the estimated 3-year overall survival for the entire cohort was 50% (95% Confidence interval 35-65%). Stage specific survival was similar to open series.
In addition to the mortality and morbidity from esophagectomy, the quality of life (QOL) after esophagectomy is also an important consideration for patients. In our study of MIE, the general QOL was measured with the Short-Form 36 (SF 36) (29). There were no significant differences between pre- and postoperative scores indicating preservation of QOL. We also administered the Gastro-Esophageal Reflux Disease-Health Related Quality of Life Scale (GERD-HRQOL) to assess reflux-related QOL. The mean GERD-HRQOL score was 4.6, a normal (no reflux) score. Thus, the QOL after MIE appears to be preserved.
Comparative Studies
There are few comparative studies comparing minimally invasive to open esophagectomy (31-34). There have been no randomized studies comparing open esophagectomy versus MIE with a thoracoscopic and laparoscopic approach. Nguyen and colleagues, in a retrospective study, compared minimally invasive (n=18) and open (n=16) esophagectomy (31). The mean operative time, blood loss, and length of intensive care unit stay were decreased with the minimally invasive approach compared with an open transthoracic esophagectomy or transhiatal esophagectomy. The incidence of respiratory complications was similar between the groups. This retrospective study, however, had limitations, and the patients who underwent open esophagectomy had more advanced cancers. In addition, the open esophagectomies were performed by a group of four surgeons, whereas the MIE procedures were performed by a single surgeon with expertise in minimally invasive esophageal surgery. In addition, these surgeries were performed in different time periods and there may have been differences in practice patterns with different lengths of stay.
Narumiya and colleagues reported the results of a prospective study of 40 patients assigned to an open approach with traditional laparotomy and thoracotomy or a less invasive approach to esophagectomy with a “mini”-thoracotomy and “mini”-laparotomy (32). Patients assigned to the less invasive approach had lower narcotic requirements, decreased release of the inflammatory mediator IL-6, and a shorter hospital stay. The recovery of vital capacity was faster in the minimally invasive group. It is reasonable to expect these benefits to be present or improved with a complete minimally invasive approach (laparoscopy and thoracoscopy). Taguchi and colleagues also demonstrated better preservation of pulmonary function and QOL with a thoracoscopic approach (33). In a recent systematic review of more than 1100 patients evaluating open esophagectomy and MIE, MIE was associated with a decreased blood loss, less morbidity, and a shorter hospital stay (35).
Conclusions
Surgical resection is the primary curative modality in patients with resectable esophageal neoplasm. There are several controversies regarding the ideal approach to esophageal resection. The type of surgical approach is typically based on surgeon and institutional preference with potential advantages and disadvantages to each approach. Based on our experience and review, our current, preferred approach is to perform a minimally invasive Ivor Lewis esophagectomy with 2-field lymph node dissection in patients with resectable esophageal cancer. The advantages of this approach include better exposure and access in the chest, the potential for an improved rate of complete resection, the potential for better gastric margins, improved lymph node dissection in the mediastinum, and lower rates of anastomotic and RLN complications. Further prospective studies are required to further evaluate the various approaches to esophageal cancer and optimize the outcomes.
Acknowledgement
This work was supported in part by NIH grant R01 CA090665 (PI. Dr. Luketich)
Footnotes
Presented at the 2nd International Bi-Annual Minimally Invasive Thoracic Surgery Summit, Boston, MA, October 9-10, 2009.
References
- 1.Enzinger PC, Mayer RJ. Esophageal Cancer. N Engl J Med. 2003;349(23):2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Luketich JD. Resection for esophageal cancer: strategies for optimal management. Ann Thorac Surg. 2008 Feb;85(2):S751–6. doi: 10.1016/j.athoracsur.2007.11.078. [DOI] [PubMed] [Google Scholar]
- 3.Hagen JA, DeMeester SR, Peters JH, Chandrasoma P, DeMeester TR. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg. 2001 Oct;234(4):520–30. doi: 10.1097/00000658-200110000-00011. discussion 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999 Sep;230(3):392–400. doi: 10.1097/00000658-199909000-00012. discussion 400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001 Nov;234(5):581–7. doi: 10.1097/00000658-200111000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg. 2001 Dec;72(6):1918–24. doi: 10.1016/s0003-4975(01)03203-9. discussion 1924-5. [DOI] [PubMed] [Google Scholar]
- 7.Visbal AL, Allen MS, Miller DL, Deschamps C, Trastek VF, Pairolero PC. Ivor Lewis esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2001 Jun;71(6):1803–8. doi: 10.1016/s0003-4975(01)02601-7. [DOI] [PubMed] [Google Scholar]
- 8.Mathisen DJ, Grillo HC, Wilkins E, Jr, et al. Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg. 1988;45:137–143. doi: 10.1016/s0003-4975(10)62424-1. [DOI] [PubMed] [Google Scholar]
- 9.Altorki N. En-bloc esophagectomy--the three-field dissection. Surg Clin North Am. 2005;85(3):611–9. doi: 10.1016/j.suc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–13. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 11.Rindani R, Martin CJ, Cox MR. Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? Aust N Z J Surg. 1999 Mar;69(3):187–94. doi: 10.1046/j.1440-1622.1999.01520.x. [DOI] [PubMed] [Google Scholar]
- 12.Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg. 2001 Jul;72(1):306–13. doi: 10.1016/s0003-4975(00)02570-4. [DOI] [PubMed] [Google Scholar]
- 13.Chu KM, Law SY, Fok M, Wong J. A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg. 1997 Sep;174(3):320–4. doi: 10.1016/s0002-9610(97)00105-0. [DOI] [PubMed] [Google Scholar]
- 14.Goldminc M, Maddern G, Le Prise E, Meunier B, Campion JP, Launois B. Oesophagectomy by a transhiatal approach or thoracotomy: a prospective randomized trial. Br J Surg. 1993 Mar;80(3):367–70. doi: 10.1002/bjs.1800800335. [DOI] [PubMed] [Google Scholar]
- 15.Hulscher JBF, van Sandick J, de Boer AGEM, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 16.Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared to limited transhiatal resection for adenocarcinoma of mid/distal esophagus. Five-year survival of a randomized clinical trial. Ann Surg. 2007 Dec;246(6):992–1000. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 17.Altorki NK. Lymph node dissection for carcinoma of the esophagus. In: Ferguson MK, editor. Difficult decisions in thoracic surgery. Springer; pp. 225–233. [Google Scholar]
- 18.Nishihara T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg. 1998;175:47–51. doi: 10.1016/s0002-9610(97)00227-4. [DOI] [PubMed] [Google Scholar]
- 19.Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002 Aug;236(2):177–83. doi: 10.1097/00000658-200208000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerut T, Nafteux P, Moons J, Coosemans W, Decker G, De Leyn P, Van Raemdonck D, Ectors N. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg. 2004 Dec;240(6):962–72. doi: 10.1097/01.sla.0000145925.70409.d7. discussion 972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizk N, Venkatraman E, Park B, Flores R, Bains MS, Rusch V, American Joint Committee on Cancer staging system The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006 Dec;132(6):1374–81. doi: 10.1016/j.jtcvs.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Pennathur A, Luketich JD. Minimally Invasive Esophagectomy: Avoidance and Treatment of Complications. In: Little AG, Merrill WH, editors. Complications in Cardiothoracic Surgery 2nd Edition: Avoidance and Treatment. Wiley-Blackwell; 2009. pp. 247–265. [Google Scholar]
- 23.Liebermann-Meffert DMI, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54:1110–1115. doi: 10.1016/0003-4975(92)90077-h. [DOI] [PubMed] [Google Scholar]
- 24.Walther B, Johansson J, Johnsson F, Von Holstein CS, Zilling T. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg. 2003 Dec;238(6):803–12. doi: 10.1097/01.sla.0000098624.04100.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright CD, Zeitels SM. Recurrent laryngeal nerve injuries after esophagectomy. Thorac Surg Clin. 2006;16(1):23–33. doi: 10.1016/j.thorsurg.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Hulscher JB, van Sanick JW, Devriese PP, et al. Vocal cord paralysis after subtotal oesophagectomy. Br J Surg. 1999;86:1583–1587. doi: 10.1046/j.1365-2168.1999.01333.x. [DOI] [PubMed] [Google Scholar]
- 27.Bizekis C, Kent MS, Luketich JD, et al. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2006 Aug;82(2):402–6. doi: 10.1016/j.athoracsur.2006.02.052. discussion 406-7. [DOI] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 29.Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003 Oct;238(4):486–94. doi: 10.1097/01.sla.0000089858.40725.68. discussion 494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luketich JD, Pennathur A, Catalano PJ, Swanson SJ, de Hoyos AL, Maddaus MA, Nguyen N, Benson AB, Fernando HC. Results of a phase II multicenter study of MIE (Eastern Cooperative Oncology Group Study E2202). J Clin Oncol. 2009;27(suppl):15s. abstr 4516. [Google Scholar]
- 31.Nguyen NT, Follette DM, Wolfe BM, et al. Comparison of minimally-invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg. 2000;135:920–5. doi: 10.1001/archsurg.135.8.920. [DOI] [PubMed] [Google Scholar]
- 32.Narumiya K, Nakamura T, Ide H, Takasaki K. Comparison of extended esophagectomy through mini-thoracotomy/laparotomy with conventional thoracotomy/laparotomy for esophageal cancer. Jpn J Thorac Cardiovasc Surg. 2005;53(8):413–9. doi: 10.1007/s11748-005-0076-9. [DOI] [PubMed] [Google Scholar]
- 33.Taguchi S, Osugi H, Higashino M, et al. Comparison of three-field esophagectomy for esophageal cancer incorporating open or thoracoscopic thoracotomy. Surg Endosc. 2003 Sep;17(9):1445–50. doi: 10.1007/s00464-002-9232-9. [DOI] [PubMed] [Google Scholar]
- 34.Osugi H, Takemura M, Higashino M, Takada N, Lee S, Kinoshita H. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg. 2003 Jan;90(1):108–13. doi: 10.1002/bjs.4022. Erratum in: Br J Surg. 2003 Jan;90(6):764.
- 35.Verhage RJ, Hazebroek EJ, Boone J, Van Hillegersberg R. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir. 2009 Apr;64(2):135–46. [PubMed] [Google Scholar]


