Abstract
Traditional risk factors of cardiovascular morbidity and mortality such as hypertension, hypercholesterolemia and obesity are paradoxically associated with better outcomes in dialysis patients, and the few trials of interventions targeting modifiable traditional risk factors have yielded disappointing results in this patient population. Non-traditional risk factors such as inflammation, anemia and abnormalities in bone and mineral metabolism have been proposed as potential explanations for the excess mortality seen in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD), but without clear understanding of what the most important pathophysiologic mechanisms of these risk factors are, which ones might be ideal treatment targets and which therapeutic interventions may be effective and safe in targeting them. Among the novel risk factors, fibroblast growth factor-23 (FGF23) has recently emerged as one of the most powerful predictors of adverse outcomes in patients with CKD and ESRD. FGF23 is a hormone produced by osteoblasts/osteocytes in bone that acts on the kidney to regulate phosphate and vitamin D metabolism through activation of FGF receptor/α-Klotho co-receptor complexes. It is possible that elevated FGF23 may exert its negative impact through distinct mechanisms of action independent from its role as a regulator of phosphorus homeostasis. Elevated circulating FGF23 concentrations have been associated with left ventricular hypertrophy (LVH), and it has been suggested that FGF23 exerts a direct effect on the myocardium. While it is possible that ‘off target’ effects of FGF23 present in very high concentrations could induce LVH, this possibility is controversial, since α-klotho is not expressed in the myocardium. Another possibility is that FGF23's effect on the heart is mediated indirectly, via ‘on target’ activation of other humoral pathways. We will review the physiology and pathophysiology of FGF23, the outcomes associated with elevated FGF23 levels, and describe putative mechanisms of action responsible for its negative effects and potential therapeutic strategies to treat these.
Keywords: chronic kidney disease, FGF23, outcomes, physiology
INTRODUCTION
Mortality is extremely high in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) [1], with a 5-year survival of only ∼35%, largely due to an extremely high rate of cardiovascular (CV) mortality [1]. The burden of CV disease and death related to CKD is possibly even greater in earlier CKD stages in part due to the much larger number of these patients [2]. While the incidence and prevalence of traditional CV risk factors is high in patients with CKD and ESRD [3], decreased kidney function in itself is a strong and independent predictor of CV events and death [4]. Moreover, some of the traditional risk factors of CV morbidity and mortality such as African-American race, hypertension, hypercholesterolemia and obesity are paradoxically associated with better outcomes in CKD and ESRD patients [5–8], and the few trials of interventions targeting modifiable traditional CV risk factors have yielded disappointing results in this patient population [9, 10]. These observations point to the presence of novel CV risk factors in CKD. CKD and ESRD are characterized by a complex metabolic milieu that consists of multiple biochemical and hormonal abnormalities. In particular, abnormalities in bone and mineral metabolism have been associated with worse CV outcomes and mortality independent of traditional risk factors. These and yet to be defined risk factors represent opportunities for interventions to improve outcomes in a population in which traditional risk factors of CV morbidity and mortality appear to be less relevant.
Abnormalities of bone and mineral metabolism associated with increased mortality in CKD include hyperphosphatemia [11, 12], hypo- and hypercalcemia [13], hypo- and hyperparathyroidism [14] and hyperphosphatasemia [15]; these have recently been grouped under the umbrella term of CKD-related mineral and bone disorders (CKD-MBD) [16]. Traditional physiologic theories characterize CKD-MBD as a system in which decreased glomerular filtration rate (GFR) and subsequent pathologic changes linked to changes in serum phosphorus, calcium and vitamin D metabolism lead to maladaptive alterations in parathyroid hormone (PTH) secretion [17]. While hyperparathyroidism is a central adaptive response to loss of renal function, recent discoveries suggest a significantly more complex pathophysiology, in which fibroblast growth factor-23 (FGF23) plays a key role [18]. FGF23 is a regulator of phosphorus and vitamin D homeostasis, with its main bone and mineral-related physiologic actions being to increase renal phosphorus excretion and to decrease 1α-hydroxylation of 25OH vitamin D [19, 20]. FGF23 levels increase early in the course of CKD [21] and elevated FGF23 levels are associated with significantly worse clinical outcomes in both pre-dialysis CKD and in ESRD [22–26]. While it is possible that these associations are merely another manifestation of CKD-MBD's effect on various pathologic processes, there are reasons to believe that FGF23's physiologic roles and its involvement in disease processes may extend beyond the realm of CKD-MBD.
PHYSIOLOGY OF FGF23
Fibroblast growth factors (FGFs) comprise a family of 22 molecules, which can be grouped into seven subfamilies [27] which have in common the ability to bind to one of the four FGF receptors (FGFR), typically in a paracrine manner [28]. The paracrine FGFs require the presence of heparin sulfate glycoseaminoglycans to allow signal transduction, resulting in a variety of effects including embryonic development, tumor growth, angiogenesis and wound healing [29]. Secreted FGFs belong to the FGF19 family and include FGF19, FGF21 and FGF23. Secreted FGFs appear unique in that the topology of their heparin-binding region diverges from the typical structure seen in the canonical FGFs, which reduces their affinity for heparin sulfate [30], and enables them to avoid capturing in the extracellular matrices and hence allows them to function as endocrine factors [31]. The weak heparin-binding ability of the FGF19 subfamily also reduces their affinity for FGFR [28], which has been shown to be weak even at high concentrations [32]. Rather, the activation of FGFRs by FGF19 family members requires the presence of a different co-factor, namely members of the Klotho family of membrane-bound glucosidases. The expression of α-klotho is limited to certain tissues, most notably to the distal convoluted tubule, and to a lower extent to the parathyroid and pituitary glands, the sinoatrial cells of the heart, placenta, skeletal muscle, urinary bladder, aorta, pancreas, testis, ovary and colon [33, 34]. The limited distribution of the full-length, trans-membrane α-klotho molecule explains the tissue-restricted physiologic actions of FGF23 (vide infra).
FGF23 is a 32 kDa protein expressed in the osteocytes and osteoblasts, which primarily targets the FGF receptor-α-klotho complex in the kidney. Excess FGF23 production results in hypophosphatemia, suppressed 1,25(OH)2 vitamin D levels and elevated PTH levels and impaired bone and cartilage mineralization. FGF23 deficiency, on the other hand, results in hyperphosphatemia, elevated 1,25(OH)2 vitamin D level, suppressed PTH and soft tissue calcification [35].
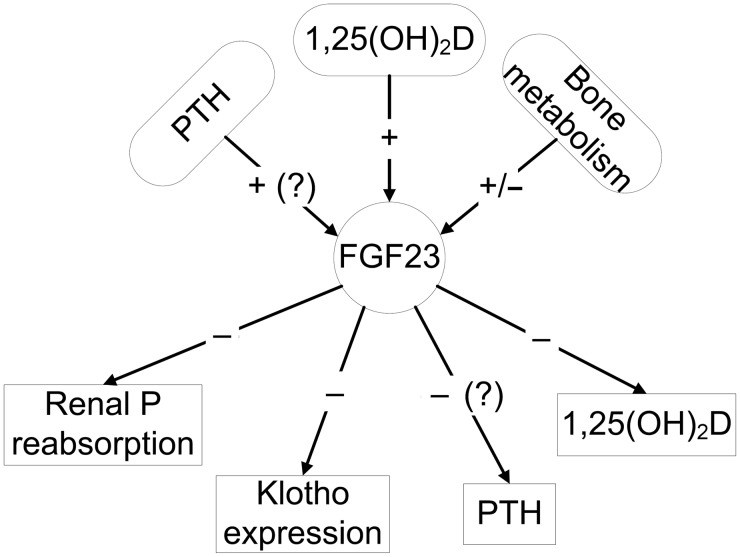
The regulation of FGF23 is complex and incompletely understood. PTH, 1,25(OH)2 vitamin D, secreted klotho, glucocorticoids, calcium and phosphate appear to regulate FGF23 production (Figure 1), but the response is context dependent and the molecular mechanism underlying the transcriptional regulation of FGF23 remain unclear. The principal regulator of FGF23 appears to be 1,25(OH)2 vitamin D, which stimulates FGF23 production in the bone [36]. The role of other regulators of FGF23 remains controversial. Stimulation of FGF23 production by PTH was shown in some [37–40], but not all, studies [36, 41]. Recent studies also suggest that bone mineralization and remodeling may have a direct effect on FGF23 production, and mutations in genes that regulate bone mineralization, such as Phex, Dmp1, Enpp1, as well as FGFR1 and HMW-FGF2 increase FGF23 gene transcription (reviewed in [19]). These local regulators may allow FGF23 to control renal phosphate metabolism according to the actual influx/efflux of calcium and phosphate to and from the bone. In addition, leptin, estrogen and glucocorticoids also regulate FGF23 [42]. Interestingly, although phosphate correlates with FGF23 levels in some settings, such as ESRD, changes in serum phosphorus level do not appear to have an immediate or consistent effect on FGF23 production. Studies that examined the effects of oral phosphate intake on FGF23 levels have detected either no effect [43–45], or described changes in FGF23 production in response to alterations in dietary phosphate intake after a lag time of up to 1 week [46–51]. This suggests that phosphate may affect FGF23 indirectly, either through vitamin D and/or through bone mineralization [36].
FIGURE 1:
Physiologic regulation and functions of FGF23.
FGF23 undergoes cleavage into N- and C-terminal fragments that do not activate FGFR/Klotho complexes. The enzymes responsible for FGF23 cleavage have not been identified [52]. Cleavage of the intact FGF23 abrogates its effects not only by removing the binding site to the FGFR-klotho complex, but also by a direct inhibitory effect of the C-terminal peptide fragment [53]. Cleavage of the intact FGF23 molecule may occur in blood samples too and could affect the accuracy of laboratory measurements, depending on the length of time elapsed from obtaining the sample [54] and on the type of laboratory assay (intact, which measures the whole molecule, or C-terminal, which measures whole molecule and C-terminal fragments) [55].
The primary physiologic actions of FGF23 involve regulation of bone and mineral metabolism through bone–kidney endocrine loops (Figure 1). FGF23 inhibits proximal tubular phosphate reabsorption through its action on Na-dependent phosphate transporters, even though its co-factor, α-klotho is expressed in distal, but not in proximal tubular cells; the mechanisms underlying a distal-to-proximal tubular feedback loop regulating the effects of FGF23 are unclear. It is possible that the effect is mediated via soluble klotho acting as a humoral factor in inducing phosphaturia and multiple other physiologic functions (vide infra) [56]. FGF23 also suppresses circulating 1,25(OH)2 vitamin D levels, in part by inhibiting Cyp27b1 (1-α hydroxylase) and in part by activating Cyp24 (24-hydroxylase) [19]. The physiologic effect of FGF23 on PTH secretion remains controversial. In vivo and in vitro studies have indicated that FGF23 suppresses PTH secretion [34], but clinically even extremely elevated FGF23 levels have not prevented the development of secondary hyperparathyroidism in CKD and elevated FGF23 has been associated with refractory secondary hyperparathyroidism [57]. This apparent paradox may be explained by resistance to FGF23 effects in uremia, perhaps due to downregulation of the FGFR in the parathyroid gland and/or to downregulation of klotho [58]. Finally, FGF23 decreases klotho gene transcription in the kidney [59]. Thus, the decreased expression of klotho in CKD could result from both FGF23-mediated suppression as well as loss of parenchyma due to the underlying kidney disease. In addition to being an essential co-factor of FGF23, klotho is released into the circulation by ectodomain shedding of the transmembrane protein or by alternative splicing of a protein that lacks the transmembrane domain and has various paracrine and endocrine functions. These include regulation of tubular, cardiac and smooth muscle calcium, and tubular potassium channels, inhibition of sodium-phosphate co-transporters and induction of FGF23-independent phosphaturia, inhibition of insulin and insulin-like growth factor-1-signaling pathways, increased endothelial nitric oxide production and improved endothelium-dependent vasodilatation, resistance to lipid peroxidation and anti-inflammatory and anti-cancer effects [56]. It is thus possible that by decreasing klotho levels, FGF23 has numerous indirect effects on other organ systems.
FGF23 IN CKD
Physiologic adaptation to loss of renal function
Elevations in FGF23 levels are one of the earliest manifestations of disordered bone-mineral metabolism in CKD [21]. Human cross-sectional studies have indicated an association of FGF23 levels with elevated serum PTH and phosphorus levels and with lower serum 1,25(OH)2 vitamin D levels and GFR [60]. Due to the complex and often still not fully understood interactions between the various humoral components of bone–kidney–parathyroid gland axis described above, there is still debate about whether elevations in FGF23 or PTH represent the initiating step in the sequence of events resulting in this typical constellation of biochemical abnormalities in CKD and ESRD. According to one paradigm, FGF23 is the initial adaptive step after the early decline in GFR, FGF23-mediated reductions in 1,25(OH)2 vitamin D leads to secondary hyperparathyroidism and the combined actions of FGF23 and PTH inhibits phosphate reabsorption in the kidney to maintain phosphate balance in the setting of declining glomerular filtration. On the other hand, PTH stimulates FGF23 in CKD, as evidenced by effects of parathyroidectomy to lower FGF23 levels in patients and animal models with CKD. Thus, elevations in PTH, at least in established CKD, contribute to increased FGF23. Finally, another hypothesis is that FGF23 is increased secondary to primary reductions in α-klotho expression in the diseased kidney leading to end-organ resistance of FGF23 and secondary increments in this hormone. Further work is needed to understand the mechanisms of FGF23 regulation in CKD.
Regardless, FGF23 levels increase significantly with advancing stages of CKD and can reach levels that are up to 1000-fold higher than normal in patients with ESRD. Some studies in ESRD have found increased accumulation of inactive C-terminal fragments [61], but others suggested that most circulating FGF23 may be functionally intact [62], indicating that impaired degradation may be a major contributor to the high levels seen in CKD and ESRD. Furthermore, increased production of FGF23 by the bone (via translational and post-translational regulation) may also contribute to elevated levels in CKD and ESRD [63].
Association of FGF23 with adverse outcomes
While physiologically FGF23 represents an adaptive mechanism meant to maintain normal bone-mineral homeostasis, elevated FGF23 levels have been associated with a significant increase in adverse outcomes, such as increased mortality in patients with ESRD, with non-dialysis-dependent CKD and with kidney transplantation [22–26, 64] and even in patients with normal kidney function [65, 66]. Furthermore, elevated FGF23 levels are associated with CV events [65], with increased progression of CKD [22, 24, 25, 64], with vascular calcification [67], with left ventricular hypertrophy (LVH) [68–75], with arterial stiffness and endothelial dysfunction [76] and with increased levels of inflammatory markers [77]. These associations appeared to be robust and independent of other concomitant bone-mineral abnormalities, and many have been described in patients with normal kidney function in whom the typical constellation of abnormal bone-mineral metabolism seen in CKD and ESRD is not present.
Possible mechanisms underlying the association of FGF23 with poor outcomes
The causality of the association between elevated circulating levels of FGF23 and adverse outcomes remains to be established. To this end, it is important to determine what mechanisms may underlie these associations, and to test interventions that could reverse the responsible pathophysiologic processes and their outcomes; otherwise, the association between FGF23 and adverse outcomes could represent an epiphenomenon of the uremic state. Thus, at present, FGF23 may be a surrogate marker for other concomitant bone-mineral abnormalities such as hyperphosphatemia, hyperparathyroidism, hyperphosphatasemia or abnormal calcium homeostasis that are causative of the adverse events. The association of FGF23 with adverse outcomes in observational studies, however, has been extremely robust and resistant to adjustment for other biomarkers of bone mineral disorders, which suggests an independent effect.
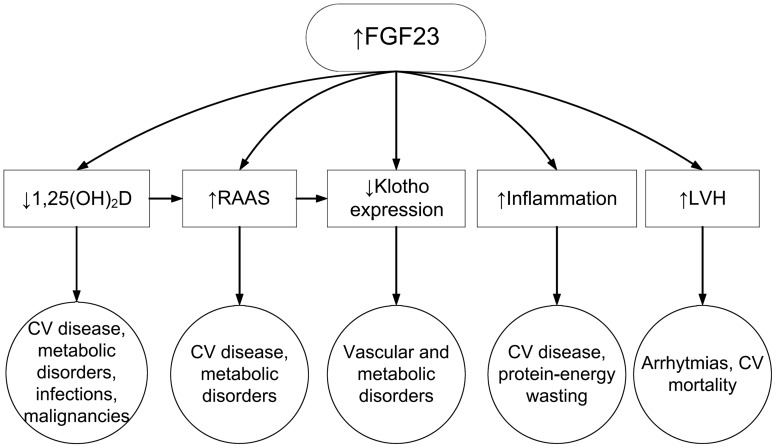
Figure 2 lists several possible explanations for FGF23 having adverse clinical outcomes. One mechanism could be through FGF23 effects to suppress vitamin D metabolism (Figure 2). As detailed above, FGF23 lowers circulating 1,25(OH)2 vitamin D levels, and through its actions on Cyp24 it may also contribute to the low 25(OH) vitamin D levels that are very common in patients with CKD and ESRD. Low vitamin D levels have shown a strong association with adverse outcomes in the general population and in CKD and ESRD [78–80], and treatment of patients with CKD and ESRD with active vitamin D has also been associated with significantly lower mortality [81]. It is thus possible that, by lowering vitamin D levels, FGF23 could be instrumental in engendering adverse consequences, which would under this paradigm be related to the multiple and complex end-organ effects of low vitamin D, which have themselves been linked to activation of the renin–angiotensin–aldosterone system (RAAS) [82], higher blood pressure, vascular calcification, inflammation and infections and malignancies [83]. Most or all of the studies supporting the argument that FGF23 exerts its negative effects by affecting vitamin D metabolism is derived from observational studies; hence, further examination of this hypothesis will be required.
FIGURE 2:
Putative mechanisms of action underlying the pathologic effects of elevated FGF23 levels.
Another possible explanation for the association of FGF23 with mortality and other adverse outcomes is an effect that is independent of its main physiologic (bone-mineral related) actions. As detailed above, FGF23 belongs to a larger family of growth factors, which have multiple and complex physiologic roles. It is conceivable that FGF23 may be able to induce deleterious effects on various organ systems that are unrelated to phosphaturia or vitamin D metabolism, especially at very high concentrations such as those seen in ESRD. Animal experiments suggest that besides effects mediated indirectly through vitamin D (vide supra), FGF23 also directly stimulates the RAAS by suppressing angiotensin-converting enzyme-2 (ACE2) expression in the kidneys, independent of other abnormalities typical of bone-mineral disorders [59]. Activation of the RAAS has been linked to numerous adverse consequences such as hypertension, baroreceptor dysfunction, sympathetic activation, diabetic nephropathy, progression of atherosclerosis, endothelial dysfunction, the inhibition of the fibrinolytic system [84], and a reduction in renal klotho expression [82], and hence an effect of FGF23 on the RAAS could be a potential link explaining its association with untoward outcomes. Activation of the RAAS by FGF23 could also explain the association of the latter with LVH [68–70], since activated RAAS is a known risk factor for pathologic myocardial hypertrophy [85]. A potential effect of bone-mineral metabolism on the RAAS was also suggested by a recent secondary analysis of the Ramipril Efficacy in Nephropathy (REIN) trial, which showed that the renoprotective effects of ACE-inhibitor therapy were only present in individuals with lower serum phosphorus levels [86]. This study did not examine FGF23 levels; hence it is unclear to what extent elevated FGF23 levels might have played a role in the observed effect modification by serum phosphorus.
A link between inflammation and FGF23 could also explain many of the adverse effects associated with elevated FGF23 levels. Inflammation is common in CKD/ESRD and it is associated with significantly worse outcomes [87, 88]. Experimental data suggest that FGF23 increases the production of inflammatory markers such as lipocalin-2, transforming growth factor-beta and tumor necrosis factor-alpha [59]. Furthermore, elevated FGF23 levels were associated with markers of inflammation in a cross-sectional study [77]. The clinical relevance of the effects of FGF23 on inflammation will need to be tested in the future.
The limited tissue distribution of klotho (vide supra), which is necessary for FGF23's activation of the FGFR, theoretically limits the actions of FGF23 to organs expressing it. However, the effects of FGF23 on organ systems that do not express klotho could occur by virtue of its suppressing effects on klotho production, and consequently its lowering of circulating levels of klotho (Figure 2), which in itself has various metabolic effects [19] and could thus act as a mediator of FGF23's effects on other organ systems. Another intriguing hypothesis that has been promoted recently suggests that FGF23 may in fact be able to exert direct effects on organs that do not express α-klotho. In a series of experiments from a single group of investigators, FGF23 was shown to induce LVH in vitro and in experimental animals by inducing molecular mechanisms typical of pathological LVH [70]. Since the myocardial cells do not express klotho, these experiments challenge the prevailing paradigm that FGF23's effects on the FGFR are weak without the concomitant presence of klotho, even at high concentrations of FGF23 [32]. Testing and confirmation of these unorthodox hypotheses by independent groups are awaited.
Practical implications
Clarification of the pathomechanism underlying the association of FGF23 with adverse outcomes is paramount in determining the best therapeutic strategies aimed at alleviating the untoward effects of FGF23 excess. If FGF23's main effects are accounted for by the actions of abnormalities of bone-mineral metabolism, then correcting abnormalities such as hyperphosphatemia, hyperparathyroidism or especially hypovitaminosis D should be sufficient to abrogate FGF23's negative effects. There are to date no clinical trials that examined the effects of intervention aimed at correcting bone-mineral abnormalities on hard clinical endpoints in CKD or ESRD, but it appears that the administration of some of the standard medication classes used to treat bone-mineral abnormalities may have significant effects on serum FGF23 levels. As mentioned above, changes in dietary phosphorus intake may or may not affect serum FGF23 levels depending on the duration of the change. Dietary habits may thus be important, given the large amounts of rapidly absorbable inorganic phosphorus in modern Western diets containing various food additives and preservatives [89]. Conversely, dietary and pharmacologic interventions could affect serum FGF23 levels, but their mechanisms may be unrelated to changes in serum phosphorus levels. For example, prolonged administration of sevelamer, a noncalcium-containing phosphate binder, to ESRD and to CKD patients has been shown to result in lowering of FGF23 levels; interestingly, a similar effect was not present with calcium-containing phosphate binders [90–92], suggesting that the effects of binders may not be solely mediated by their effects on serum phosphorus and that other effects (e.g. such as bone turnover) may also play a role. Supporting this hypothesis were studies showing that long term [93], but not short term administration of lanthanum carbonate [45] significantly decreased FGF23 levels, in spite of both strategies equally decreasing urine phosphorus levels.
Administration of active vitamin D leads to stimulation of FGF23 production and higher FGF23 levels [94]; such an effect may seem undesirable, and would counter the apparent benefit of active vitamin D therapy that was described in numerous observational studies [95]. A potential explanation for this apparent paradox could be that the main pathologic effects of FGF23 are mediated through the lowering of 1,25(OH)2 vitamin D levels, in which case the pharmacologic correction of these levels could render the elevation in FGF23 levels irrelevant. Another agent used to treat secondary hyperparathyroidism in ESRD is cinacalcet hydrochloride, a calcium receptor sensitizer agent. Administration of cinacalcet has been shown to decrease FGF23 levels [94, 96, 97], suggesting a possible additional biochemical benefit from cinacalcet besides the lowering of PTH and phosphorus levels. Whether or not such biochemical benefits translate to better clinical outcomes in patients treated with cinacalcet versus active vitamin D remains unclear, since a recent large, randomized, controlled, clinical trial comparing the two regimens in ESRD patients yielded inconclusive results [98]. The FGF23-lowering effects of cinacalcet in CKD are also accompanied by a concomitant rise in serum phosphorus levels [94], complicating the prediction of downstream clinical outcomes. Furthermore, clinical trials will be needed to clarify the effects of the various therapeutic interventions on FGF23 levels, and to determine how these changes impact clinical outcomes.
The discovery that FGF23 may have either direct and/or indirect effects outside of the realm of bone-mineral metabolism suggests additional possibilities for the treatment of the adverse effects associated with elevated FGF23 levels. If activation of the RAAS or the induction of inflammation are indeed relevant pathomechanisms, then medications such as ACE inhibitors, angiotensin-receptor blockers, aldosterone receptor antagonists or a variety of agents with anti-inflammatory effects [99] could be tested towards this goal. The advantage of such an approach would be the use of approved medications with a known therapeutic profile, which would obviate the need to develop novel agents, with obvious cost benefits and with a lesser potential for adverse outcomes associated with their use.
It is, however, possible that FGF23 may have a direct effect on organs such as the myocardium (vide supra), which would mean that in order to alleviate its adverse effects one would have to either lower its production, or to directly block its end-organ effects. The application of agents blocking the effects of FGF23 are enticing, as such agents have been shown to reverse the skeletal and biochemical abnormalities attributed to excess FGF23 in experimental settings of X-linked hypophosphatemic rickets [100, 101]. Furthermore, the use of an FGF23 monoclonal antibody in rats with CKD has effectively neutralized FGF23, and resulted in the correction of both secondary hyperparathyroidism and low vitamin D level and also in the normalization of bone structure and turnover rate [102]. However, this intervention also resulted in the development of hyperphosphatemia, in a significant increase in aortic calcification and in increased mortality [102], suggesting that FGF23 may in fact represent an essential regulatory pathway and completely abolishing it may prove to be deleterious. A potential solution to this dilemma could be the development of agents that selectively block only certain subtypes of the FGFR, in hopes of blocking the deleterious effects of FGF23 while maintaining its essential physiological functions.
Finally, elevated FGF23 is associated with reductions in α-klotho in CKD [59]. Since klotho is also a hormone whose deficiency results in an accelerated aging phenotype, it is possible that klotho deficiency may contribute to the poor outcomes associated with CKD and replacement of this hormone might have salutary effects on morbidity and mortality in CKD. Further research in all four of these areas is needed.
CONCLUSIONS
FGF23 has emerged as a novel risk factor for adverse outcomes in patients with CKD and ESRD. While its physiologic role is primarily the regulation of bone-mineral metabolism, FGF23 has been implicated in numerous other physiologic processes that may explain its strong association with mortality and other adverse outcomes independent of abnormalities of bone-mineral metabolism. Clarification of FGF23's physiological pathways could lead to the development of new therapeutic paradigms aimed at improving outcomes in patients with CKD and ESRD.
CONFLICT OF INTEREST STATEMENT
C.P.K. has received honoraria from Amgen. L.D.Q. has received honoraria and research support from Amgen. This manuscript has not been published elsewhere in whole or in part.
ACKNOWLEDGEMENTS
This work was supported in part by Grant RO1 AR 045955 from the National Institutes of Health to L.D.Q.
REFERENCES
- 1.U.S. Renal Data System. Bethesda, MD: 2010. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 2.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 3.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 4.Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol. 2007;18:304–311. doi: 10.1681/ASN.2006060674. [DOI] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, et al. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 9.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 10.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Outcomes associated with serum phosphorus level in males with non-dialysis dependent chronic kidney disease. Clin Nephrol. 2010;73:268–275. doi: 10.5414/cnp73268. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz S, Trivedi BK, Kalantar-Zadeh K, et al. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:825–831. doi: 10.2215/CJN.02101205. [DOI] [PubMed] [Google Scholar]
- 13.Kovesdy CP, Kuchmak O, Lu JL, et al. Outcomes associated with serum calcium level in men with non-dialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:468–476. doi: 10.2215/CJN.06040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Ureche V, Lu JL, et al. Outcome predictability of serum alkaline phosphatase in men with pre-dialysis CKD. Nephrol Dial Transplant. 2010;25:3003–3011. doi: 10.1093/ndt/gfq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moe SM, Drueke T, Lameire N, et al. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Bricker NS. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N Engl J Med. 1972;286:1093–1099. doi: 10.1056/NEJM197205182862009. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 Associates with Death, Cardiovascular Events, and Initiation of Chronic Dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 27.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 30.Harmer NJ, Pellegrini L, Chirgadze D, et al. The crystal structure of fibroblast growth factor (FGF) 19 reveals novel features of the FGF family and offers a structural basis for its unusual receptor affinity. Biochemistry. 2004;43:629–640. doi: 10.1021/bi035320k. [DOI] [PubMed] [Google Scholar]
- 31.Goetz R, Ohnishi M, Kir S, et al. Conversion of a paracrine fibroblast growth factor into an endocrine fibroblast growth factor. J Biol Chem. 2012;287:29134–29146. doi: 10.1074/jbc.M112.342980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 37.Kawata T, Imanishi Y, Kobayashi K, et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 38.Lavi-Moshayoff V, Wasserman G, Meir T, et al. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 39.Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–643. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T, Tominaga Y, Ueki T, et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:481–487. [PubMed] [Google Scholar]
- 41.Saji F, Shigematsu T, Sakaguchi T, et al. Fibroblast growth factor 23 production in bone is directly regulated by 1{alpha},25-dihydroxyvitamin D, but not PTH. Am J Physiol Renal Physiol. 2010;299:F1212–F1217. doi: 10.1152/ajprenal.00169.2010. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji K, Maeda T, Kawane T, et al. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25:1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 43.Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 44.Nishida Y, Taketani Y, Yamanaka-Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 45.Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 47.Burnett SM, Gunawardene SC, Bringhurst FR, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 48.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 49.Perwad F, Azam N, Zhang MY, et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 50.Vervloet MG, van Ittersum FJ, Buttler RM, et al. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011;6:383–389. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Guo R, Simpson LG, et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 53.Goetz R, Nakada Y, Hu MC, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith ER, Ford ML, Tomlinson LA, et al. Instability of fibroblast growth factor-23 (FGF-23): implications for clinical studies. Clin Chim Acta. 2011;412:1008–1011. doi: 10.1016/j.cca.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Jonsson KB. The role of fibroblast growth factor 23 in renal disease. Nephrol Dial Transplant. 2005;20:479–482. doi: 10.1093/ndt/gfh701. [DOI] [PubMed] [Google Scholar]
- 56.Kuro-o M. Klotho in health and disease. Curr Opin Nephrol Hypertens. 2012;21:362–368. doi: 10.1097/MNH.0b013e32835422ad. [DOI] [PubMed] [Google Scholar]
- 57.Nakanishi S, Kazama JJ, Nii-Kono T, et al. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 58.Galitzer H, Ben-Dov IZ, Silver J, et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 59.Dai B, David V, Martin A, et al. A Comparative Transcriptome Analysis Identifying FGF23 Regulated Genes in the Kidney of a Mouse CKD Model. PLoS One. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 61.Weber TJ, Liu S, Indridason OS, et al. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 62.Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pereira RC, Juppner H, Azucena-Serrano CE, et al. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnlov J, Carlsson AC, Sundstrom J, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83:160–166. doi: 10.1038/ki.2012.327. [DOI] [PubMed] [Google Scholar]
- 67.Khan AM, Chirinos JA, Litt H, et al. FGF-23 and the Progression of Coronary Arterial Calcification in Patients New to Dialysis. Clin J Am Soc Nephrol. 2012;7:2017–2022. doi: 10.2215/CJN.02160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirza MA, Larsson A, Melhus H, et al. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337:116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 72.Seiler S, Cremers B, Rebling NM, et al. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011;32:2688–2696. doi: 10.1093/eurheartj/ehr215. [DOI] [PubMed] [Google Scholar]
- 73.Kirkpantur A, Balci M, Gurbuz OA, et al. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant. 2011;26:1346–1354. doi: 10.1093/ndt/gfq539. [DOI] [PubMed] [Google Scholar]
- 74.Canziani ME, Tomiyama C, Higa A, et al. Fibroblast growth factor 23 in chronic kidney disease: bridging the gap between bone mineral metabolism and left ventricular hypertrophy. Blood Purif. 2011;31:26–32. doi: 10.1159/000321368. [DOI] [PubMed] [Google Scholar]
- 75.Stevens KK, McQuarrie EP, Sands W, et al. Fibroblast growth factor 23 predicts left ventricular mass and induces cell adhesion molecule formation. Int J Nephrol. 2011;2011:297070. doi: 10.4061/2011/297070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirza MA, Larsson A, Lind L, et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Mendoza JM, Isakova T, Ricardo AC, et al. Fibroblast Growth Factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melamed ML, Michos ED, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehrotra R, Kermah DA, Salusky IB, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977–983. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 81.Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 82.de Borst MH, Vervloet MG, ter Wee PM, et al. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603–1609. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008;73:1355–1363. doi: 10.1038/ki.2008.35. [DOI] [PubMed] [Google Scholar]
- 84.Perazella MA, Setaro JF. Renin-angiotensin-aldosterone system: fundamental aspects and clinical implications in renal and cardiovascular disorders. J Nucl Cardiol. 2003;10:184–196. doi: 10.1067/mnc.2003.392. [DOI] [PubMed] [Google Scholar]
- 85.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 86.Zoccali C, Ruggenenti P, Perna A, et al. Phosphate may promote ckd progression and attenuate renoprotective effect of ace inhibition. J Am Soc Nephrol. 2011;22:1923–1930. doi: 10.1681/ASN.2011020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalantar-Zadeh K, Kopple JD, Humphreys MH, et al. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1507–1519. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- 88.Stenvinkel P, Lindholm B, Heimburger M, et al. Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: association with malnutrition, inflammation, and cardiovascular disease. Nephrol Dial Transplant. 2000;15:1624–1630. doi: 10.1093/ndt/15.10.1624. [DOI] [PubMed] [Google Scholar]
- 89.Noori N, Sims JJ, Kopple JD, et al. Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran J Kidney Dis. 2010;4:89–100. [PubMed] [Google Scholar]
- 90.Koiwa F, Kazama JJ, Tokumoto A, et al. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial. 2005;9:336–339. doi: 10.1111/j.1744-9987.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 91.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis. 2012;59:177–185. doi: 10.1053/j.ajkd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant. 2011;26:2567–2571. doi: 10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 94.Finch JL, Tokumoto M, Nakamura H, et al. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1315–F1322. doi: 10.1152/ajprenal.00552.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kovesdy CP. Survival benefits with vitamin D receptor activation: new insights since 2003. Clin J Am Soc Nephrol. 2010;5:1704–1709. doi: 10.2215/CJN.02590310. [DOI] [PubMed] [Google Scholar]
- 96.Wetmore JB, Liu S, Krebill R, et al. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5:110–116. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koizumi M, Komaba H, Nakanishi S, et al. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27:784–790. doi: 10.1093/ndt/gfr384. [DOI] [PubMed] [Google Scholar]
- 98.Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 99.Kovesdy CP, Kalantar-Zadeh K. Novel targets and new potential: developments in the treatment of inflammation in chronic kidney disease. Expert Opin Investig Drugs. 2008;17:451–467. doi: 10.1517/13543784.17.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wohrle S, Henninger C, Bonny O, et al. Pharmacological inhibition of FGFR signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1810. doi:10.1002/jbmr.1810. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 101.Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 102.Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. [DOI] [PMC free article] [PubMed] [Google Scholar]