Hyponatremia, serum sodium <135 mEq/L, is the most common electrolyte abnormality affecting ∼30% of hospitalized patients [1]. An infrequent yet serious complication of hyponatremia is hyponatremic encephalopathy. Hyponatremia can result in an influx of water to the intracellular space. This results in cellular swelling that can lead to cerebral edema and encephalopathy. Patients at highest risk of developing hyponatremic encephalopathy in the hospital setting are post-operative patients and patients with SIADH receiving hypotonic fluids [2]. Hyponatremic encephalopathy in the outpatient setting is usually seen as a complication due to medications such as thiazide diuretics or SSRIs, or can result from exercise-associated hyponatremia or psychogenic polydypsia [3]. A significant risk factor for developing hyponatremic encephalopathy is female gender, with the majority of cases of death or permanent neurological injury from hyponatremic encephalopathy reported in females [4]. An increasingly common cause of hyponatremic encephalopathy in the outpatient setting is use of the recreational drug ecstasy (3,4-methylenedioxymethamphetamine [MDMA]) [5]. In this issue of NDT, van Dijken et al. [6] report on the high incidence of hyponatremia in females using ecstasy.
Ecstasy is an illegal synthetic amphetamine that was initially developed as an appetite suppressant but never used for this purpose. It first emerged as a recreational drug in the 1980s popular with young adults at night club parties referred to as ‘rave parties’ [7]. A rave is typically a commercially arranged elaborate all-night dance party that combines electronic music played by disc jockeys with a laser light show. Rave parties are extremely popular in North America and Europe, with thousands of people in attendance at rave events. An annual New Year's Eve rave party in Los Angeles is reported to have almost 50 000 attendants [8]. Ecstasy is frequently taken on such occasions for its mood-enhancement properties. Users experience feelings such as euphoria, increased empathy and sociability, happiness and a sense of well-being, and increased energy [9]. Ecstasy exerts its effect by the release of neuroactive compounds in the central nervous system, including serotonin, dopamine and norepinephrine [10]. With the rise in popularity of ecstasy, medical complications began to be reported including hyponatremia, non-traumatic rhabdomyolysis, seizures, acute kidney injury, hyperthermia, cardiac tachyarrhythmia and sudden death, to name a few [5, 11, 12]. Ecstasy is believed to be the third most commonly used illicit drug, following marijuana and amphetamines, and ahead of cocaine, with an estimated consumption of over 28 million tablets yearly [7]. Emergency medical services are frequently on site at large, commercially organized rave parties [8].
One of the most serious medical complications associated with ecstasy use is hyponatremic encephalopathy [11]. There are over 25 reports of ecstasy-associated hyponatremic encephalopathy in the literature, and over half of them are fatalities. Almost all cases are reported in young females between the ages of 15 and 30 with a serum sodium of ≤130 following the ingestion of just one dose of ecstasy [5]. Symptoms typically develop within 2–12 h of ecstasy ingestion. Presenting symptoms are headache, nausea and vomiting followed by altered mental status, coma, seizure, cardio respiratory arrest, brainstem herniation and death [5]. A common yet frequently unrecognized presenting feature in these patients is neurogenic pulmonary edema, also referred to as Ayus–Arieff syndrome (Figure 1). This complication was first reported in females with post-operative and exercise-associated hyponatremia [11, 13, 14]. This is a particularly dangerous complication as hypoxia impairs brain cell volume regulation, decreases cerebral perfusion and increases the probability of developing neuronal lesions [2, 15].
FIGURE 1:
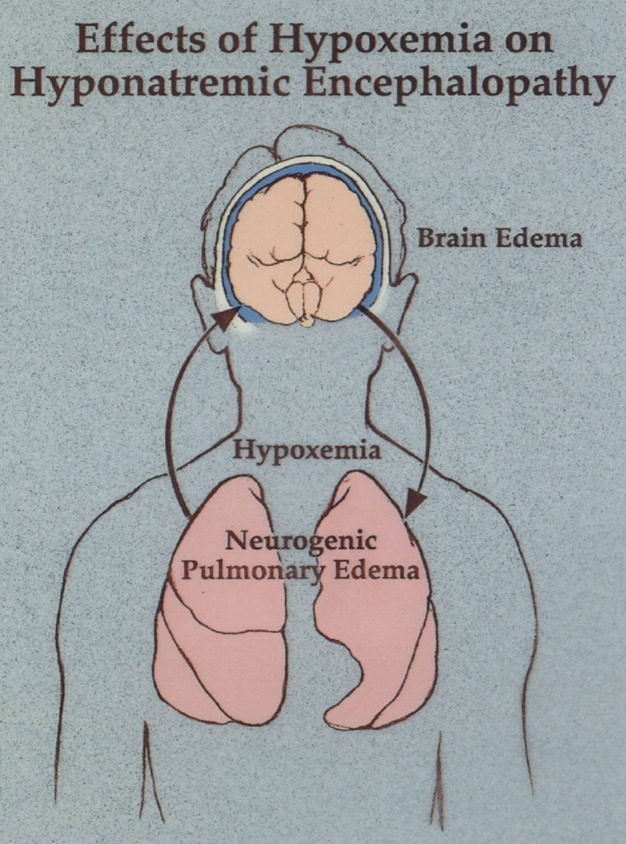
A depiction of the Ayus–Arieff syndrome. Hyponatremia produces cytotoxic cerebral edema, which in turn leads to a neurogenic pulmonary edema. Pulmonary edema leads to hypoxia, which impairs brain cell volume regulation resulting in a vicious cycle of worsening cerebral edema and pulmonary edema. This syndrome can be reversed by the prompt administration of 3% NaCl.
A variety of factors contribute to the development of ecstasy-associated hyponatremia, the two main factors being high fluid intake (associated with ecstasy use) and the inappropriate secretion of arginine vasopressin (AVP), which is induced by ecstasy metabolites. Ecstasy induces transient cardiovascular effects with markedly elevated body temperature, tachycardia, profuse sweating, hot flashes, dry mouth and increased thirst [9]. In response to these side effects, rave parties typically have ‘chill out’ areas where party goers have easy access to fluids in order to hydrate themselves as they feel compelled to do. Ecstasy has been demonstrated both in experimental models and in case reports to stimulate AVP secretion [16–18]. Ecstasy metabolites are known to increase the synaptic concentration of serotonin and dopamine, both of which are involved in the release of AVP and other pituitary hormones. Ecstasy ingestion has been demonstrated to increase the secretion of AVP, oxytocin, prolactin, ACTH and cortisol [17, 19]. The combination of high fluid intake with drug-induced SIADH affects place club goers at high risk of acute symptomatic hyponatremia.
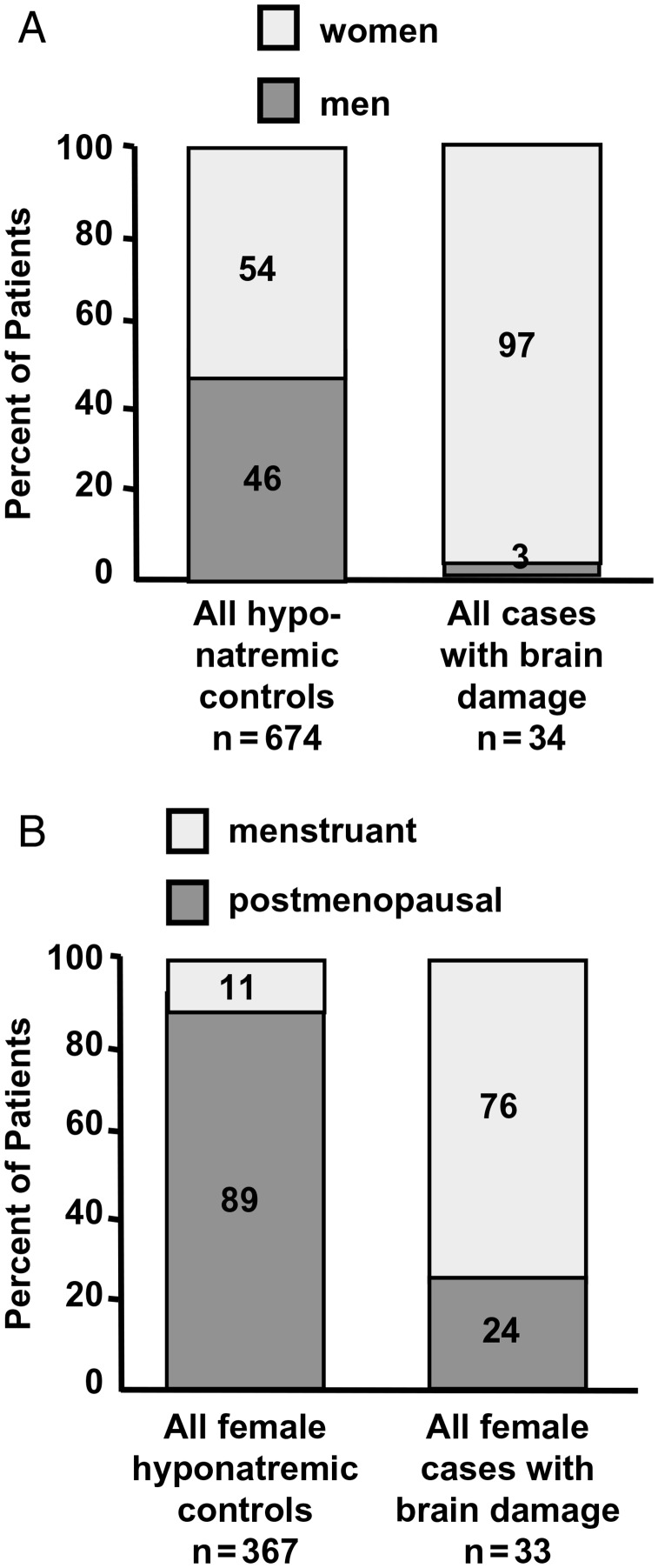
In 1992 Ayus and Arieff made the seminal observation that young hospitalized females were at particularly high risk of developing as well as dying from symptomatic hyponatremia, despite having a similar incidence and magnitude of hyponatremia to their male counterparts (Figure 2) [4]. This association has subsequently been confirmed in a variety of settings (Table 1) [14, 20]. This disparity appears to be the result of two separate mechanisms: (i) estrogens appear to impair brain cell volume regulation by reducing Na+-K+-ATPase pump activity and thereby inhibit sodium extrusion from brain astrocytes and (ii) the vasoconstrictive effects of AVP are more pronounced in the female brain, which results in increased cerebral vasoconstriction with corresponding decreased oxygen delivery [21]. Therefore, it is not surprising that majority of reported cases of deaths from ecstasy-associated hyponatremia have occurred in females.
FIGURE 2:
Effects of gender and menstrual status on brain damage from hyponatremic encephalopathy. (A) The relative risk of dying or developing permanent brain damage is 28 times higher for women than for men. (B) The relative risk of dying or developing permanent brain damage is 26 times higher for menstruant women than for menopausal woman. (Reproduced from Annals of Internal Medicine. 1992;117:891–897.).
Table 1.
Relationship between female gender and risk of developing hyponatremia or hyponatremic encephalopathy
| Setting | Hyponatremia | Hyponatremic encephalopathy in females |
|---|---|---|
| Post-operative | − | ++ |
| Ecstasy | ++ | ++ |
| Exercise | +/− | ++ |
| Desmopressin | + | ++ |
| SSRI | + | ++ |
| Thiazide | − | ++ |
Van Dijken et al. [6] have made the novel finding that females are at significantly increased risk of developing ecstasy-associated hyponatremia compared with males. They prospectively measured serum sodium values in partygoers at a rave event who did and in those who did not use ecstasy. They found the incidence of hyponatremia, sodium <136 mEq/L, in females using ecstasy to be 26.7% compared with 3% for males, with no cases of hyponatremia in those who did not take ecstasy. An additional interesting finding was that even among non-ecstasy users, serum sodium was significantly lower in females compared with males. No other factors could be identified to explain this increased incidence of hyponatremia in females using ecstasy, such as fluid intake or amount of ecstasy taken.
This study of van Dijken et al. lends support to other reports suggesting that the incidence of outpatient hyponatremia may in fact be greater in females [22, 23]. The authors put forward a variety of plausible reasons why females may be at increased risk of developing ecstasy-associated hyponatremia. Females appear to be more susceptible to the effects of ecstasy than males due to a stronger serotoninenergic response [24]. It has been demonstrated that ecstasy increased levels of circulating copeptin, a marker of AVP secretions, in females but not in males [25]. Females may also be more prone to the development of hyponatremia as (i) estrogen, but not progesterone, stimulates AVP secretion [26] and (ii) females may have greater sensitivity to ADH with increased expression of renal vasopressin receptors [22].
There are a variety of measures that can be taken to prevent hyponatremia in both the inpatient and outpatient setting, but it is unlikely that any of these measures will be successful for ecstasy-associated hyponatremia. The most important measure to prevent hospital-acquired hyponatremia is the avoidance of hypotonic intravenous fluids [27, 28], but this clearly does not apply in the outpatient setting. The condition that is most similar to ecstasy-associated hyponatremia is exercise-associated hyponatremia. Both conditions disproportionately affect young females and are usually connected with a commercially staged event. Two measures have been taken to prevent complications from exercise-associated hyponatremia: (i) educating high-endurance athletes on the dangers of overhydrating and (ii) educating and equipping emergency personnel on the recognition and treatment of exercise-associated hyponatremia [29, 30]. Clearly, the best method of avoiding ecstasy-associated hyponatremia would be to curtail the use of ecstasy at rave events, but this is unlikely to happen. Educating ecstasy users on the dangers of overhydrating also may not be practical as ecstasy raises body temperature and stimulates thirst, and the users are in a euphoric state that impairs appropriate judgment.
The only practical measure that can be taken to prevent complication from ecstasy-associated hyponatremia is to educate and equip on-site medical personnel and emergency rooms in the recognition and treatment of this condition. We propose that a protocol be followed by emergency personnel for the management of ecstasy-associated hyponatremia that is similar to that put forward by the Second International Exercise-Associated Hyponatremia Consensus Development Conference [30], as the two conditions are very similar. First, on-site medical personnel should be available at any large rave event. Second, medical personal should be equipped to do on-site analysis of [Na+] in any partygoer with symptoms suggestive of ecstasy-associated hyponatremia, such as headache, nausea, vomiting, lethargy, confusion, altered mental status or seizure. Any partygoer with ecstasy-associated hyponatremic encephalopathy, with either mild or advanced symptoms, should be immediately treated with a 100 mL bolus infusion of 3% NaCl [31, 32]. A single bolus would result in at most a 2 mEq/L acute rise in serum sodium, which would quickly reduce brain edema. The bolus could be repeated 1–2 times if symptoms persist. No harm could come from using this approach as cerebral demyelination is not a complication of acute symptomatic hyponatremia (48 h duration). The use of the 100 mL NaCl bolus provides a safe, effective and simple method of treating ecstasy-associated hyponatremia when it develops. It results in an acute and controlled rise in serum sodium without the need for complex formulas or special equipment and minimizes the risk of overcorrection. This approach was initially proposed by us in 2005 [31], and has subsequently been used successfully in marathons and should prove to be equally successful at rave events. With the growing popularity of rave events and the increasing use of ecstasy, otherwise healthy young females are at risk of a potentially fatal complication that could easily go unrecognized and untreated. Efforts should be made to disseminate this information to emergency personnel at the time of a rave event as has successfully been done for high-endurance sporting events.
CONFLICT OF INTEREST STATEMENT
K.K.-Z. has received honoraria and/or grants from Abbott, Amgen, DaVita, Fresenius, Genzyme, Otsuka, Shire and Vifor, the manufacturers of drugs or devices and/or providers of services for CKD patients. M.L.M. has served as a consultant for Otsuka.
(See related article by van Dijken et al. High incidence of mild hyponatraemia in females using ecstasy at a rave party. Nephrol Dial Transplant 2013; 28: 2277–2283.)
ACKNOWLEDGEMENTS
Supported by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health R01-DK078106, R21-DK077341 and K24-DK091419 and a philanthropic grant from Mr Harold C. Simmons. JAT is professor-in-residence of medicine at UCLA David Geffen School of Medicine and attending physician at Harbor-UCLA Medical Center.
REFERENCES
- 1.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119:S30–S35. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Moritz ML, Ayus JC. New aspects in the pathogenesis, prevention, and treatment of hyponatremic encephalopathy in children. Pediatr Nephrol. 2010;25:1225–1238. doi: 10.1007/s00467-009-1323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moritz ML, Ayus JC. The pathophysiology and treatment of hyponatraemic encephalopathy: an update. Nephrol Dial Transplant. 2003;18:2486–2491. doi: 10.1093/ndt/gfg394. [DOI] [PubMed] [Google Scholar]
- 4.Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med. 1992;117:891–897. doi: 10.7326/0003-4819-117-11-891. [DOI] [PubMed] [Google Scholar]
- 5.Campbell GA, Rosner MH. The agony of ecstasy: MDMA (3,4-methylenedioxymethamphetamine) and the kidney. Clin J Am Soc Nephrol. 2008;3:1852–1860. doi: 10.2215/CJN.02080508. [DOI] [PubMed] [Google Scholar]
- 6.van Dijken GD, Blom RE, Hene RJ, et al. High incidence of mild hyponatremia in females using ecstasy at a rave party. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft023. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Soo Hoo GW. The agony with ecstasy: lessons from a recent rave. J Intensive Care Med. 2012 doi: 10.1177/0885066612457331. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Ecstasy overdoses at a New Year's Eve rave—Los Angeles, California, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:677–681. [PubMed] [Google Scholar]
- 9.de la Torre R, Farre M, Roset PN, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Nguyen MK, Chang R, et al. Fatal hyponatremia in a young woman after ecstasy ingestion. Nat Clin Pract Nephrol. 2006;2:283–288. doi: 10.1038/ncpneph0167. quiz 9. [DOI] [PubMed] [Google Scholar]
- 12.Armenian P, Mamantov TM, Tsutaoka BT, et al. Multiple MDMA (ecstasy) overdoses at a rave event: a case series. J Intensive Care Med. 2012 doi: 10.1177/0885066612445982. [DOI] [PubMed] [Google Scholar]
- 13.Ayus JC, Arieff AI. Pulmonary complications of hyponatremic encephalopathy. Noncardiogenic pulmonary edema and hypercapnic respiratory failure. Chest. 1995;107:517–521. doi: 10.1378/chest.107.2.517. [DOI] [PubMed] [Google Scholar]
- 14.Ayus JC, Varon J, Arieff AI. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann Intern Med. 2000;132:711–714. doi: 10.7326/0003-4819-132-9-200005020-00005. [DOI] [PubMed] [Google Scholar]
- 15.Ayus JC, Armstrong D, Arieff AI. Hyponatremia with hypoxia: effects on brain adaptation, perfusion, and histology in rodents. Kidney Int. 2006;69:1319–1325. doi: 10.1038/sj.ki.5000187. [DOI] [PubMed] [Google Scholar]
- 16.Fallon JK, Shah D, Kicman AT, et al. Action of MDMA (ecstasy) and its metabolites on arginine vasopressin release. Ann N Y Acad Sci. 2002;965:399–409. doi: 10.1111/j.1749-6632.2002.tb04181.x. [DOI] [PubMed] [Google Scholar]
- 17.Forsling ML, Fallon JK, Shah D, et al. The effect of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and its metabolites on neurohypophysial hormone release from the isolated rat hypothalamus. Br J Pharmacol. 2002;135:649–656. doi: 10.1038/sj.bjp.0704502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff K, Tsapakis EM, Winstock AR, et al. Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. J Psychopharmacol. 2006;20:400–410. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- 19.Parrott AC. Cortisol and 3,4-methylenedioxymethamphetamine: neurohormonal aspects of bioenergetic stress in ecstasy users. Neuropsychobiology. 2009;60:148–158. doi: 10.1159/000253551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayus JC, Arieff AI. Chronic hyponatremic encephalopathy in postmenopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281:2299–2304. doi: 10.1001/jama.281.24.2299. [DOI] [PubMed] [Google Scholar]
- 21.Ayus JC, Achinger SG, Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol. 2008;295:F619–F624. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 22.Juul KV, Klein BM, Sandstrom R, et al. Gender difference in antidiuretic response to desmopressin. Am J Physiol Renal Physiol. 2011;300:F1116–F1122. doi: 10.1152/ajprenal.00741.2010. [DOI] [PubMed] [Google Scholar]
- 23.Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618–1622. doi: 10.1345/aph.1G293. [DOI] [PubMed] [Google Scholar]
- 24.Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 2001;154:161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- 25.Simmler LD, Hysek CM, Liechti ME. Sex differences in the effects of MDMA (ecstasy) on plasma copeptin in healthy subjects. J Clin Endocrinol Metab. 2011;96:2844–2850. doi: 10.1210/jc.2011-1143. [DOI] [PubMed] [Google Scholar]
- 26.Forsling ML, Stromberg P, Akerlund M. Effect of ovarian steroids on vasopressin secretion. J Endocrinol. 1982;95:147–151. doi: 10.1677/joe.0.0950147. [DOI] [PubMed] [Google Scholar]
- 27.Moritz ML, Ayus JC. Prevention of hospital-acquired hyponatremia: a case for using isotonic saline. Pediatrics. 2003;111:227–230. doi: 10.1542/peds.111.2.227. [DOI] [PubMed] [Google Scholar]
- 28.Moritz ML, Carlos Ayus J. Hospital-acquired hyponatremia—why are hypotonic parenteral fluids still being used? Nat Clin Pract Nephrol. 2007;3:374–382. doi: 10.1038/ncpneph0526. [DOI] [PubMed] [Google Scholar]
- 29.Hew-Butler T, Almond C, Ayus JC, et al. Consensus statement of the 1st International Exercise-Associated Hyponatremia Consensus Development Conference, Cape Town, South Africa 2005. Clin J Sport Med. 2005;15:208–213. doi: 10.1097/01.jsm.0000174702.23983.41. [DOI] [PubMed] [Google Scholar]
- 30.Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18:111–121. doi: 10.1097/JSM.0b013e318168ff31. [DOI] [PubMed] [Google Scholar]
- 31.Ayus JC, Arieff A, Moritz ML. Hyponatremia in marathon runners. N Engl J Med. 2005;353:427–428. [PubMed] [Google Scholar]
- 32.Moritz ML, Ayus JC. 100 cc 3% sodium chloride bolus: a novel treatment for hyponatremic encephalopathy. Metab Brain Dis. 2010;25:91–96. doi: 10.1007/s11011-010-9173-2. [DOI] [PubMed] [Google Scholar]