Abstract
Background
Different phosphate binders exert differing effects on bone mineral metabolism and levels of regulating hormones. The objective of this post hoc evaluation of the CALcium acetate MAGnesium carbonate (CALMAG) study was to compare the effects of calcium acetate/magnesium carbonate (CaMg) and a calcium-free phosphate binder, sevelamer-hydrochloride (HCl), on serum levels of fibroblast growth factor-23 (FGF-23) and markers of bone turnover.
Methods
This secondary analysis of the controlled, randomized CALMAG study, comparing the effect of CaMg and sevelamer-HCl on serum phosphorus (P), aimed to investigate the parameters described above. The analysis included 204 patients who completed the initial study per protocol (CaMg, n = 105; sevelamer-HCl, n = 99).
Results
The study showed that serum levels of FGF-23 were significantly reduced with CaMg and sevelamer-HCl, with no difference between groups at Week 25 [analysis of covariance (ANCOVA); log-intact FGF-23 (iFGF-23), P = 0.1573]. FGF-23 levels strongly correlated with serum P levels at all time points in both groups. The bone turnover parameters alkaline phosphatase (AP), bone AP (BAP), procollagen type 1 amino-terminal propeptide 1 (P1NP), osteoprotegerin (OPG), beta-crosslaps (β-CTX) and tartrate-resistant acid phosphatase 5b (TRAP 5b) increased significantly in the sevelamer-HCl group; they remained almost unchanged in the CaMg group, after the initial phase of P lowering (ANCOVA, P < 0.0001 for all except OPG, P = 0.1718).
Conclusions
CaMg and sevelamer-HCl comparably lower serum levels of iFGF-23. Changes in bone parameters were dependent on characteristics of the phosphate binder; in contrast with sevelamer-HCl, CaMg had no influence on bone turnover markers.
Keywords: bone markers, calcium acetate, fibroblast growth factor-23, haemodialysis, magnesium carbonate, phosphate binder
INTRODUCTION
In patients with chronic kidney disease stage 5 (CKD 5), elevated serum phosphorus (P) levels are associated with an increased risk of morbidity and mortality [1–6]. Therefore, control of serum P levels is a key objective in the management of chronic kidney disease-mineral and bone disorder (CKD–MBD). In addition to optimal dialysis and dietary restrictions, oral phosphate binders such as calcium (Ca) acetate and the non-Ca-containing agent sevelamer-hydrochloride (HCl) constitute key treatment options in hyperphosphataemia. The recent CALcium acetate MAGnesium carbonate (CALMAG) randomized, controlled trial demonstrated that the combination phosphate binder calcium acetate/magnesium carbonate (CaMg) is non-inferior to sevelamer-HCl at controlling serum P levels in patients receiving haemodialysis (HD), thus, presenting another effective treatment option [7].
Hyperphosphataemia is associated with elevated levels of fibroblast growth factor-23 (FGF-23) and parathyroid hormone (PTH) [8]. Increased FGF-23, hyperphosphataemia and elevated PTH are independently associated with increased mortality in CKD [9–14]. FGF-23, a master regulator of P, PTH and 1,25-(OH)2D3 [15–17], is also implicated in cardiovascular disease [10, 14, 18–20] and is becoming increasingly recognized as an important marker for outcome and severity of disease in CKD–MBD. However, unfortunately there are currently very few strategies available to enable a significant reduction in its level in patients with CKD.
Recent studies have reported a greater reduction in FGF-23 with sevelamer-HCl than with Ca acetate [21, 22] and, therefore, there is now an increased interest in investigating how other, newer phosphate-binding agents (such as CaMg) affect FGF-23. Previous studies that have investigated the impact of phosphate binders on bone metabolism include the comparison between sevelamer-HCl and Ca acetate [21] and between sevelamer-HCl and Ca carbonate [23]; however, no comparisons of sevelamer-HCl and CaMg have yet been reported. Moreover, as an emerging role of FGF-23 in the bone–kidney axis becomes apparent [24], it is also important to compare the concomitant changes in bone markers in response to treatment.
In order to evaluate the effects of different phosphate binders on FGF-23 and markers of bone turnover, we performed a secondary analysis of data from the CALMAG study comparing the effect of CaMg versus sevelamer-HCl on these parameters in patients receiving HD.
SUBJECTS AND METHODS
Study population and design
Full details of the CALMAG study, which compared the tolerability and efficacy of two different oral phosphate binder treatments (CaMg and sevelamer-HCl) for 24 weeks in HD or online haemodiafiltration (HDF) patients, have been described previously [7]. The primary endpoint of the original trial was to determine the efficacy of CaMg compared with sevelamer-HCl as an active control in lowering serum P [7].
This secondary analysis was performed with the objective of comparing the effects of both phosphate binders on FGF-23 and different bone turnover markers.
Patients aged 18–85 years, in stable condition, without additional serious illness, received one of two study medications: 435 mg of Ca acetate (containing 110 mg elemental Ca) combined with 235 mg of magnesium (Mg) carbonate (containing 60 mg elemental Mg) (OsvaRen®; Fresenius Medical Care Nephrologica Deutschland GmbH, Bad Homburg, Germany) or sevelamer-HCl 800 mg (Renagel®; Genzyme Corporation, Cambridge, MA) for 24 weeks. A standardized titration scheme was used in order to reduce serum P levels <1.78 mmol/L (5.5 mg/dL).
Study parameters
In addition to parameters reported previously, serum samples from baseline, Weeks 9 and 25 were also analysed for FGF-23. Markers of bone formation including alkaline phosphatase (AP), bone AP (BAP), procollagen type 1 amino-terminal propeptide 1 (P1NP) and osteoprotegerin (OPG) were determined as well as a marker of bone resorption, beta-crosslaps (β-CTx), and a marker of osteoclast activity, tartrate-resistant acid phosphatase 5b (TRAP 5b).
Measurements were performed at the central laboratory using the following assays: inorganic P, molybdate reaction without deproteinization; total Ca, photometric colour test; ionized Ca (iCa), OPTI® R blood gas analysis (OPTI Medical); total Mg, xylidyl blue; intact PTH (iPTH), human intact PTH electrochemiluminescence immune assay (ECLIA); intact FGF-23 (iFGF-23), human intact FGF-23 enzyme-linked immunosorbent assay (ELISA) (Immutopics); AP, according to International Federation of Clinical Chemistry and Laboratory Medicine, 37°C; BAP and TRAP 5b, enzyme immunoassay (EIA) (Microvue Bonehealth, Quidel Corporation, Biosource); β-CTX and P1NP, ECLIA for Cobas/Elecsys (Roche); human OPG, ELISA (Biovendor).
Reference values: P, 0.87–1.45 mmol/L; total Ca, 2.20–2.60 mmol/L; iCa, 0.95–1.35 mmol/L; total Mg, 0.65–1.05 mmol/L; iPTH, 15–65 pg/mL; iFGF-23, 7.0–29.3 pg/mL; AP, female 35–104 U/L, male 40–129 U/L; BAP, female median 25.0 U/L (90% CI 14.2–42.7 U/L), male median 23.2 U/L (90% CI 15.0–41.3 U/L); P1NP, female median 37.09 µg/L (5–95th percentile, 16.27–73.87 µg/L); β-CTx, female 556 ± 226 pg/mL, male 304 ± 200 pg/mL; TRAP 5b, female 4.3 ± 1.5 U/L, male 4.0 ± 1.4 U/L; OPG, mean 4.1 ± 2.3 pmol/L.
Statistical analysis
The differences in biochemical serum parameters between treatment groups were tested using an analysis of covariance (ANCOVA) model for repeated measures. The factors for study treatment, centre (pooled), dialysate Ca concentration, dialysis method (HD or online-HDF) and use of vitamin D and cinacalcet were included in the model. The respective baseline value was added as a covariate.
Differences between groups in mean baseline biochemical serum parameters were tested using the Mann–Whitney test. Wilcoxon tests were applied to describe the statistical significance of changes over time within a treatment group. All tests were carried out at a two-sided significance level α of 5%.
In order to dissect the acute effect of phosphate lowering after the wash-out and the long-term effect of different phosphate binders, in addition to the overall comparison, we split the analysis into two phases: the initial phase of phosphate lowering, Weeks 1–9, and the maintenance phase, Weeks 9–25.
All analyses were based on the per protocol set (PPS), which included all patients who were randomized and completed the study without major protocol violations. Data and figures are presented as median and interquartile range (Q3–Q1). Pearson's correlation coefficients and the respective two-sided P values were calculated between iFGF-23, iPTH, actual bicarbonate and P, iCa, total Ca, Mg, AP, BAP, P1NP, OPG, TRAP 5b and β-CTx over the whole treatment period and at all measured time points.
RESULTS
Patients and baseline characteristics
Of the 326 patients who were included in the CALMAG study, 255 were randomized and 204 [105 patients (CaMg) and 99 patients (sevelamer-HCl)] completed the study as per protocol. There were no significant differences in baseline characteristics between groups in terms of underlying disease, comorbidities or disposition of covariates, including the use of any vitamin D (reported previously) [7] or in biochemical serum parameters (Table 1). In both of the groups, the minority of patients (CaMg: 38.1%, n = 40; sevelamer-HCl: 29.3%, n = 29) were treated with any form of vitamin D, either with cholecalciferol, calcitriol or another vitamin D receptor activator. As written in the protocol and as confirmed by further analysis of co-medication, baseline medication was not changed during the course of the study.
Table 1.
Baseline biochemical serum parameters (median; Q1–Q3) of the two study groups (PPS)
| Parameter | CaMg | Sevelamer-HCl | Significance Mann– Whitney test (P) |
|---|---|---|---|
| Phosphorus (mmol/L), n = 105/99 | 2.41; 2.05–2.73 | 2.42; 2.12–2.78 | 0.6762 |
| Total calcium (mmol/L), n = 105/99 | 2.20; 2.03–2.30 | 2.17; 2.09–2.31 | 0.6418 |
| Ionized calcium (mmol/L), n = 99/91 | 1.09; 1.01–1.16 | 1.09; 1.03–1.16 | 0.6147 |
| Magnesium (mmol/L), n = 105/99 | 0.98; 0.89–1.09 | 0.99; 0.89–1.09 | 0.9046 |
| Actual bicarbonate (mmol/L), n = 93/84 | 21.3; 19.1–23.2 | 20.9; 18.8–22.9 | 0.6884 |
| iPTH (pg/mL), n = 102/96 | 390; 251–607 | 422; 286–557 | 0.9486 |
| iFGF-23 (pg/mL), n = 100/96 | 570; 251–1741 | 779; 278–1769 | 0.3334 |
| Log iFGF-23 (pg/mL), n = 100/96 | 6.3; 5.5–7.5 | 6.7; 5.6–7.5 | 0.3334 |
| AP (U/L), n = 105/99 | 90; 69–110 | 88; 66–115 | 0.6598 |
| Bone AP (U/L), n = 99/95 | 25.4; 16.5–33.5 | 23.1; 17.9–32.3 | 0.4866 |
| Total P1NP (µg/L), n = 99/95 | 403.9; 289.5–606.5 | 425.5; 271.5–670.6 | 0.9470 |
| OPG (pmol/L), n = 96/95 | 19.47; 12.99–24.24 | 15.78; 12.43–21.68 | 0.0568 |
| β-CTx (pg/mL), n = 99/95 | 2552; 1806–3357 | 2818; 1726–3544 | 0.3491 |
| TRAP 5b (U/L), n = 99/95 | 3.3; 2.1–5.3 | 3.3; 2.2–5.5 | 0.8050 |
| 25-(OH) vitamin D3 (ng/mL), n = 99/95 | 38; 20–50 | 33; 24–60 | 0.6571 |
| 1,25-(OH)2 vitamin D3 (pmol/L), n = 99/95 | 16.2; 10.2–22.9 | 14.1; 10.7–20.3 | 0.3964 |
AP, alkaline phosphatase; β-CTx, beta-crosslaps; CaMg; calcium acetate/magnesium carbonate; HCI, hydrochloride; iFGF-23, intact fibroblast growth factor-23; iPTH, intact parathyroid hormone; OPG, osteoprotegerin; P1NP, procollagen type 1 amino-terminal propeptide; PPS, per protocol set; TRAP 5b, tartrate-resistant acid phosphatase 5b.
Mineral metabolism and intact parathyroid hormone
Parameters of mineral metabolism and iPTH at Weeks 1, 9 and 25 are given in Table 2. Data analysed from the PPS confirmed the results of the primary analysis involving the full analysis set regarding mineral metabolism in both of the groups [7]. Major changes in P, actual bicarbonate and total Ca (only in the CaMg group) and iPTH occurred during the first 9 weeks—the phosphate-lowering phase of the study. Changes in serum P from Weeks 1–25 for both of the groups are shown in Figure 1a. Changes in iPTH from Weeks 1–9 correlated significantly with changes in serum P, for both treatment arms (r = 0.44, P < 0.0001 for CaMg and r = 0.37, P = 0.0002 for sevelamer-HCl, respectively). In addition, in the CaMg group they were also inversely related to changes in total (r = −0.35, P = 0.0003) and ionized serum Ca (r = −0.27, P = 0.0065), Mg (r = −0.34, P = 0.0005) and actual bicarbonate (r = −0.21, P = 0.0458) (Supplementary data, Table S1).
Table 2.
Median (Q1–Q3) serum minerals, actual bicarbonate and intact parathyroid hormone parameters of the two study groups (PPS) at baseline, Weeks 9 and 25 (for the statistical analyses between treatment groups, values were adjusted for the baseline value, centre and covariates)
| Laboratory parameter | CaMg |
Sevelamer-HCl |
Significance (between groups) ANCOVA (repeated measures) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 9 | Wilcoxon test (W1 versus W9) | Week 25 | Wilcoxon test (W9 versus W25) | Week 1 | Week 9 | Wilcoxon test (W1 versus W9) | Week 25 | Wilcoxon test (W9 versus W25) | ||
| Phosphorus (mmol/L) | 2.41; 2.05–2.73 | 1.65; 1.35–2.04 | P < 0.0001 | 1.66; 1.30–1.98 | P = 0.4802 | 2.42; 2.12–2.78 | 1.78; 1.45–2.10 | P < 0.0001 | 1.69; 1.32–2.02 | P = 0.7013 | P = 0.1635 |
| Total calcium (mmol/L) | 2.20; 2.03–2.30 | 2.22; 2.13–2.36 | P = 0.0001 | 2.24; 2.14–2.34 | P = 0.8032 | 2.17; 2.09–2.31 | 2.22; 2.11–2.31 | P = 0.3285 | 2.20; 2.06–2.30 | P = 0.0565 | P = 0.0053 |
| Ionized calcium (mmol/L) | 1.09; 1.01–1.16 | 1.13; 1.08–1.18 | P < 0.0001 | 1.13; 1.07–1.19 | P = 0.9442 | 1.09; 1.03–1.16 | 1.13; 1.09–1.19 | P < 0.0001 | 1.13; 1.07–1.18 | P = 0.0588 | P = 0.7356 |
| Magnesium (mmol/L) | 0.98; 0.89–1.09 | 1.26; 1.15–1.41 | P < 0.0001 | 1.28; 1.15–1.46 | P = 0.2868 | 0.99; 0.89–1.09 | 1.07; 0.96–1.17 | P < 0.0001 | 1.04; 0.95–1.15 | P = 0.0537 | P < 0.0001 |
| Actual bicarbonate (mmol/L) | 21.3; 19.1–23.2 | 23.3; 21.2–25.3 | P < 0.0001 | 23.0; 20.3–25.2 | P = 0.8501 | 20.9; 18.8–22.9 | 20.2; 18.1–22.9 | P = 0.0442 | 20.2; 17.8–23.6 | P = 0.4566 | P = 0.0002 |
| iPTH (pg/mL) | 390; 251–607 | 283; 187–478 | P < 0.0001 | 312; 159–436 | P = 0.1159 | 422; 286–557 | 345; 217–468 | P < 0.0001 | 373; 227–521 | P = 0.0896 | P = 0.0553 |
ANCOVA, analysis of covariance; CaMg; calcium acetate/magnesium carbonate; HCI, hydrochloride; iPTH, intact parathyroid hormone; PPS, per protocol set.
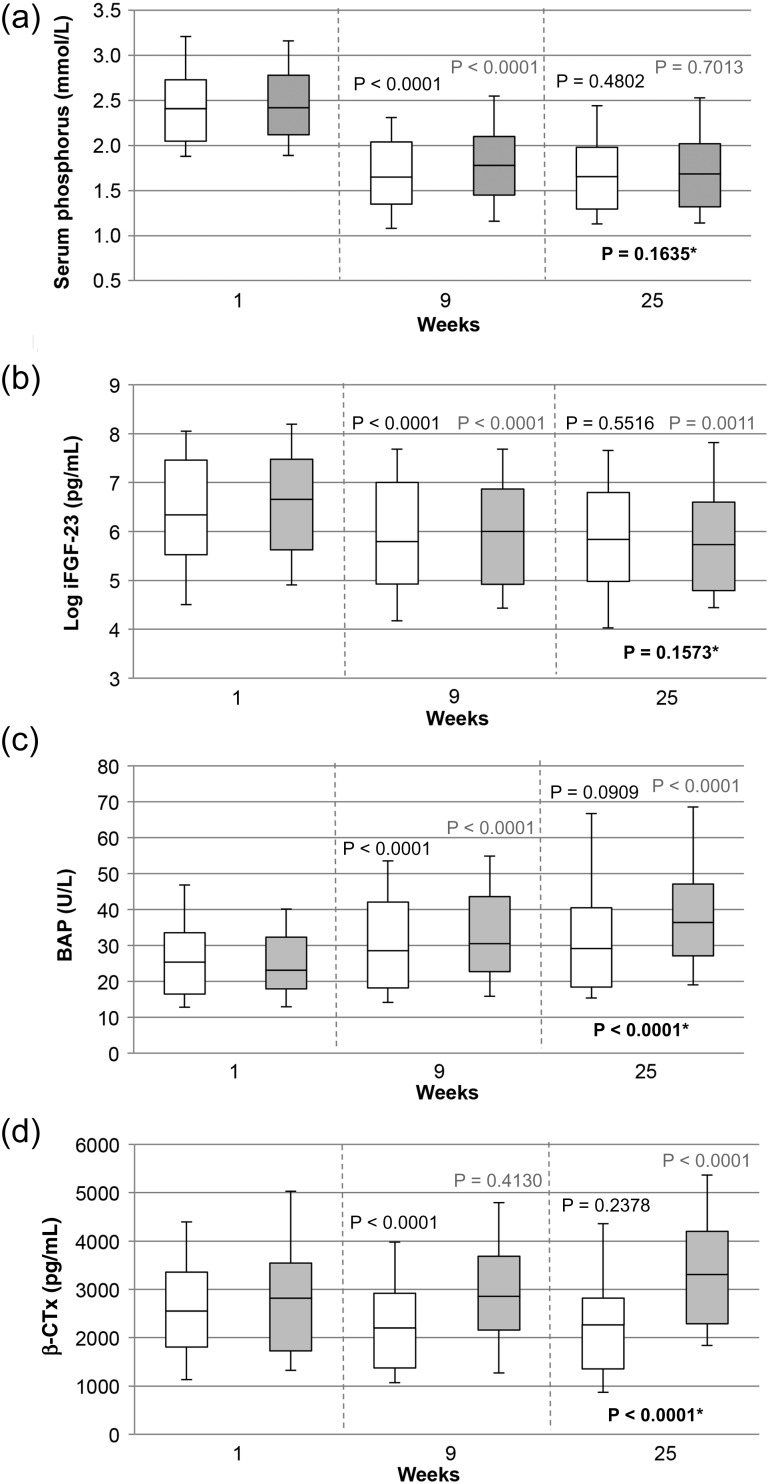
FIGURE 1:
View of selected parameters from Tables 2 and 3 to illustrate the impact of phosphate binders on serum levels of (a) phosphorus, (b) log-intact FGF-23, (c) bone AP and (d) beta-crosslaps. Time course of values at Weeks 9 and 25 of the calcium acetate/magnesium carbonate group (n = 105) and of the sevelamer-HCl group (n = 99) (PPS) is displayed in white and grey, respectively. Probability values are the result of the Wilcoxon test for within-group significance (Week 9 versus Week 1 and Week 25 versus Week 9, respectively) and repeated measures ANCOVA for between-group evaluation (indicated by asterisks). The plotted values indicate medians and interquartile range, with 10th and 90th percentiles as the error bars.
Intact fibroblast growth factor-23
Intact FGF-23 (iFGF-23) and log iFGF-23 declined significantly during treatment with both phosphate binders, specifically during the first 9 weeks of treatment, with no difference between groups after 25 weeks of treatment (ANCOVA, P = 0.1573) (Table 3 and Figure 1b). iFGF-23 strongly correlated with serum P (Week 25 all patients: r = 0.34, P < 0.0001) at all time points in both of the groups. However, the change in iFGF-23 correlated with the change in serum P in the CaMg group (r = 0.36, P = 0.0002), but not in the sevelamer-HCl group (r = 0.11, P = 0.3047). In addition, the change in iFGF-23 correlated inversely with the change in serum Mg (r = −0.28, P = 0.0047) in the CaMg group. No correlations of the change in iFGF-23 with changes in ionized or total Ca were observed in either group. The change in iFGF-23 was found to correlate weakly with a change in iPTH, but only in the sevelamer-HCl group and only in the first part of the study (Supplementary data, Table S1).
Table 3.
Median (Q1–Q3) serum bone regulating factors and markers of the two study groups (PPS) at baseline, Weeks 9 and 25 (for the statistical analyses between treatment groups, values were adjusted for the baseline value, centre and covariates)
| Laboratory parameter | CaMg |
Sevelamer-HCl |
Significance (between groups) ANCOVA (repeated measures) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 9 | Wilcoxon test (W1 versus W9) | Week 25 | Wilcoxon test (W9 versus W25) | Week 1 | Week 9 | Wilcoxon test (W1 versus W9) | Week 25 | Wilcoxon test (W9 versus W25) | ||
| iFGF-23 (pg/mL) | 570; 251–1741 | 327; 138–1104 | P < 0.0001 | 343; 145–894 | P = 0.4881 | 779; 278–1769 | 404; 137–962 | P < 0.0001 | 309; 121–736 | P = 0.0047 | P = 0.0705 |
| Log iFGF-23 (pg/mL) | 6.3; 5.5–7.5 | 5.8; 4.9–7.0 | P < 0.0001 | 5.8; 5.0–6.8 | P = 0.5516 | 6.7; 5.6–7.5 | 6.0; 4.9–6.9 | P < 0.0001 | 5.7; 4.8–6.6 | P = 0.0011 | P = 0.1573 |
| AP (U/L) | 90; 69–110 | 94; 74–132 | P < 0.0001 | 87; 71–130 | P = 0.3955 | 88; 66–115 | 109; 83–146 | P < 0.0001 | 117; 94–145 | P = 0.0315 | P < 0.0001 |
| Bone AP (U/L) | 25.4; 16.5–33.5 | 28.5; 18.2–42.1 | P < 0.0001 | 29.2; 18.5–40.5 | P = 0.0909 | 23.1; 17.9–32.3 | 30.5; 22.7–43.6 | P < 0.0001 | 36.4; 27.1–47.1 | P < 0.0001 | P < 0.0001 |
| Total P1NP (µg/L) | 403.9; 289.5–606.5 | 377.4; 264.1–640.4 | P = 0.1041 | 385.4; 249.0–613.2 | P = 0.7522 | 425.5; 271.5–670.6 | 460.1; 337.2–742.4 | P = 0.0014 | 518.3; 331.9–803.5 | P = 0.0006 | P < 0.0001 |
| Osteoprotegerin (pmol/L) | 19.47; 12.99–24.24 | 20.13; 13.90–24.58 | P = 0.1674 | 18.74; 13.43–25.18 | P = 0.9779 | 15.78; 12.43–21.68 | 16.75; 12.68–23.14 | P = 0.0888 | 16.80; 14.16–23.32 | P = 0.3097 | P = 0.1718 |
| β-CTx (pg/mL) | 2552; 1806–3357 | 2200; 1373–2921 | P < 0.0001 | 2264; 1354–2821 | P = 0.2378 | 2818; 1726–3544 | 2855; 2158–3685 | P = 0.4130 | 3308; 2288–4200 | P < 0.0001 | P < 0.0001 |
| TRAP 5b (U/L) | 3.3; 2.1–5.3 | 3.3; 2.1–4.9 | P = 0.1201 | 3.6; 2.3–5.5 | P = 0.0013 | 3.3; 2.2–5.5 | 3.9; 2.8–5.8 | P < 0.0001 | 4.7; 3.2–6.5 | P = 0.0008 | P < 0.0001 |
ANCOVA, analysis of covariance; AP, alkaline phosphatase; β-CTx, beta-crosslaps; CaMg; calcium acetate/magnesium carbonate; HCI, hydrochloride; iFGF-23, intact fibroblast growth factor 23; P1NP, procollagen type 1 amino-terminal propeptide; PPS, per protocol set; TRAP 5b, tartrate-resistant acid phosphatase 5b.
Bone parameters
Over the entire treatment period, all bone markers increased significantly in the sevelamer-HCl group (P < 0.0001 for all, except OPG, P = 0.0100 and β-CTx, P = 0.0003). In the CaMg group, bone markers showed a more differentiated behaviour. BAP and AP also increased (P < 0.0001 and P = 0.0018, respectively), but to a lower degree than in the sevelamer group (ANCOVA, both P < 0.0001). P1NP (P = 0.1228), OPG (P = 0.0906) and TRAP 5b (P = 0.3930) remained unchanged. β-CTx decreased significantly over time (P = 0.0004), although the decrease occurred only during the first period (Weeks 1–9) (Table 3).
When analysing the data separately for the two investigation periods (Weeks 1–9 and 9–25), more pronounced changes were observed from Weeks 1–9 in both of the groups. In the CaMg group, TRAP 5b was the only marker that increased significantly from Weeks 9 to 25. However, in the sevelamer-HCl group, significant increases were seen for BAP, P1NP, β-CTx and TRAP 5b from Weeks 9–25 (Table 3, Figure 1c and d).
Changes in AP and BAP did not correlate with changes in iPTH or iFGF-23 in either group. However, from Weeks 1 to 25 changes in other bone markers correlated with changes in iPTH: for sevelamer-HCl, with P1NP, β-CTx, TRAP 5b and inversely with OPG; for the CaMg group, only with P1NP and TRAP 5b (Supplementary data, Table S1).
DISCUSSION
The aim of this post hoc analysis of the randomized, controlled CALMAG study was to determine the impact of phosphate binder therapy on serum levels of FGF-23 and bone markers and to explore whether a difference exists between combined Ca and Mg salts and a non-Ca-containing phosphate binder.
Decrease in serum levels of fibroblast growth factor-23
The study confirmed that serum levels of FGF-23 decreased significantly with both CaMg and sevelamer-HCl during the study period, with no difference between groups at Week 25. Furthermore, FGF-23 levels strongly correlated with serum P levels at all time points in both of the groups; however, changes over the whole study period in serum P correlated with changes in iFGF-23 only in the CaMg group. Similarly, in this group changes in serum Mg over time inversely correlated with changes in iFGF-23.
While a greater reduction in iFGF-23 with sevelamer-HCl versus Ca acetate has been reported in other studies [21, 22], our investigation clearly shows that the phosphate binder CaMg leads to similar effects. Differences between Weeks 1 and 9, and Weeks 9 and 25, point to the important role of P on levels of hormones such as FGF-23 and iPTH and on bone turnover. However, it is not clear whether the reason underlying the greater reduction in FGF-23 in both of the groups in the first phase is attributable to the effect of the phosphate binder on serum P alone or whether the binder itself has an intrinsic effect, especially since no correlation could be established between any parameter in this phase (with the exception of a weak correlation between iFGF-23 and iPTH in the sevelamer-HCl group).
Vitamin D strongly influences iFGF-23 levels [25] and administration of vitamin D in patients on dialysis leads to an increase of iFGF-23 levels [26]. In our study, the dose of vitamin D (independent of whether it was prescribed as a supplementation or as active vitamin D) remained constant and there was no difference between treatment arms; thus, we could not observe any relationship to iFGF-23 levels (data not shown).
Additional factors that may influence the levels of fibroblast growth factor-23
As previously described [21], other factors that determine serum Ca and/or Ca balance (oral intake, vitamin D administration, dialysate Ca concentration) may also have an impact on the extent of FGF-23 decrease. In our study, the slight increase in serum iCa in both groups and the very small increase in total serum Ca for CaMg did not play a role in attenuating the decrease in FGF-23 (Supplementary data, Table S1) [27]. Similar results were reported in an acute study in HD patients with elevated PTH levels investigating the effect of high and low serum Ca with the use of different dialysate Ca concentrations. In the same study, no direct relationship between Ca levels and iFGF-23 could be established [28]. In contrast, an association between serum Ca changes and log iFGF-23 was reported in a separate long-term study [29].
Our data suggest that an increase in Mg might be another factor that can influence the decrease in iFGF-23. A possible and hitherto unknown direct or indirect physiological interaction remains to be elucidated. FGF-23 lowering might also be modulated via the Ca-sensing receptor [29], and perhaps the reason that no correlation was found between the decline of iPTH and iFGF-23 in the CaMg group may be attributable to the effects of Mg on the Ca-sensing receptor. A similar finding was described in the secondary analysis of a study investigating the effect of cinacalcet on FGF-23 [29]. A direct relationship between iPTH and iFGF-23 was not found; however, the authors discussed a direct effect or an indirect mechanism via the Ca-sensing receptor and/or phosphate lowering as a possible explanation [29]. In our study, a direct, albeit weak, correlation is evident between iFGF-23 and the decrease in iPTH from Weeks 1 to 9 in the sevelamer-HCl group, which is not surprising in patients with CKD, in whom a direct effect of PTH on FGF-23 mRNA has previously been described [20].
Differing effects of phosphate binders on bone parameters
In general, there was a more significant increase in markers of bone turnover in the sevelamer-HCl group compared with the CaMg group, especially after the initial phase of P lowering. This suggests that while control of P levels with CaMg is associated with a similar reduction in serum levels of FGF-23 as with sevelamer-HCl, the influence of CaMg on bone turnover markers is less pronounced.
Interestingly, while iPTH levels dropped significantly during the first 9 weeks in both of the groups, bone parameters such as BAP increased. While the changes in the levels of BAP and iPTH frequently tend to correlate positively with each other [30, 31], a lack of correlation between these two parameters has also been documented [32]. In our longitudinal study, the initial lowering of iPTH is most probably caused by the direct effect of P on the parathyroid gland [33, 34], while the increase in membrane-bound enzyme BAP probably reflects the normalization in osteoblast activity following the same reduction in serum P levels (that is, the effects on bone-remodelling and BAP levels are independent of the effect on the parathyroid gland).
In line with other studies [23, 35–38], a more pronounced increase in bone markers with sevelamer-HCl in comparison to CaMg was evident in our study. This may represent some clinical benefit, for example, in patients with low bone turnover disease [36], or it may reflect an aggravation of secondary hyperparathyroidism [31]. Furthermore, an increased level of AP is associated with increased mortality, as recently reported in epidemiological studies [39, 40]. However, whether an increase in markers associated with bone mineral loss [41–43], such as TRAP 5b and OPG seen in the patients on sevelamer-HCl, can lead to an apparent clinical effect is an issue that requires further investigation.
Although the ideal levels of bone markers in patients receiving dialysis remain to be established, the use of CaMg as a phosphate binder appears to moderately correct levels towards the normal range and prevents a continuous increase. The lack of a placebo group in this study limits the extent to which we can detail relative effects of phosphate binders on bone turnover. Nevertheless, a recent investigation of several different phosphate binders, which also included a placebo group, in pre-dialysis patients similarly showed differential changes in bone mineral density, depending on the phosphate binder or placebo use, further supporting our findings [44]. However, the reason for the different effects of both phosphate binders on bone parameters in the current study remains to be elucidated. Possibilities include an effect of Ca and Mg, as well as an effect of acid–base status on the bone turnover [45, 46], which improved with CaMg but remained unchanged in the sevelamer-HCl group [7].
There is a concern that increased serum Mg levels may potentially have a harmful effect on bone, such as mineralization defects and adynamic bone disease, in dialysis patients [47]. In contrast, Mg levels in the upper normal range in the general population and mildly elevated levels in patients with CKD 5 have in fact been associated with benefits, including reduced cardiovascular calcification, reduced hypertension and reduced mortality [47–49].
In our study, we did not perform bone biopsies, which is a major limitation, and therefore we cannot exclude the possibility of development of adynamic bone disease. However, the observed lack of decrease in bone formation markers such as BAP, known for its high specificity and sensitivity in identifying bone remodelling when compared with bone biopsies [30, 31], supports the absence of adynamic bone disease. In addition, a large bone biopsy study in 100 patients did not find Mg bone content to be correlated with osteomalacia or any other type of bone disease, even though the Mg/Ca ratio was elevated in dialysis patients compared with healthy controls [50]. Furthermore, in 35 patients in the same study who were diagnosed with adynamic bone disease, the Mg content of bone biopsies did not differ significantly from other types of renal osteodystrophy.
In summary, in contrast to the sevelamer group, bone marker changes over time indicate that CaMg helps maintain bone health, causing neither an overstimulation nor a suppression of bone turnover.
CONCLUSION
This study provides an intriguing insight into the therapeutic effects of the phosphate binders CaMg and sevelamer-HCl on FGF-23 and bone markers. The differing effects of phosphate binders on bone metabolism reinforce the need for studying these effects for each respective phosphate binder separately [44]. Limitations of the study are that it did not include a placebo control and we did not conduct any bone biopsies. Further studies are required to understand better the reason(s) underlying the different effects of CaMg and sevelamer-HCl on FGF-23 and bone parameters.
In conclusion, CaMg lowered serum levels of iFGF-23 to the same extent as sevelamer-HCl in this controlled, randomized study. Changes in bone parameters were dependent on the characteristics of each phosphate binder. CaMg resulted in an increase in serum Mg but did not have a distinct effect on bone turnover markers.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
This study was presented as an abstract at the meeting of the European Renal Association-European Dialysis and Transplant Association; Prague, Czech Republic; 23–26 June 2011; otherwise, the results presented in this paper have not been published previously in whole or part.
A.C. is an adviser to FMC, Affymax and has received speakers' honoraria from Abbott, Amgen and Vifor. J.P.-D is a consultant to Fresenius Medical Care. M.K. serves as the responsible nephrologist at a Fresenius NephroCare dialysis centre. B.B.-S. states no conflict of interest. A.G. serves as the responsible nephrologist at a Fresenius NephroCare dialysis centre. P.P. serves as the Portuguese Country Medical Director at a Fresenius NephroCare dialysis centre. B.M. is an employee of Fresenius Medical Care Deutschland GmbH, Bad Homburg. A.L.M. de F. is an adviser to Amgen and has received speakers' honoraria from Abbott, Amgen, Fresenius and Gambro.
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing assistance was provided by Shilpa Aggarwal, ApotheCom ScopeMedical Ltd, funded by Fresenius Medical Care. This study was funded by Fresenius Medical Care.
REFERENCES
- 1.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. doi:10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 2.Covic A, Kothawala P, Bernal M, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24:1506–1523. doi: 10.1093/ndt/gfn613. doi:10.1093/ndt/gfn613. [DOI] [PubMed] [Google Scholar]
- 3.Lezaic V, Tirmenstajn-Jankovic B, Bukvic D, et al. Efficacy of hyperphosphatemia control in the progression of chronic renal failure and the prevalence of cardiovascular calcification. Clin Nephrol. 2009;71:21–29. doi: 10.5414/cnp71021. [DOI] [PubMed] [Google Scholar]
- 4.Plantinga LC, Fink NE, Melamed ML, et al. Serum phosphate levels and risk of infection in incident dialysis patients. Clin J Am Soc Nephrol. 2008;3:1398–1406. doi: 10.2215/CJN.00420108. doi:10.2215/CJN.00420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. doi:10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Wald R, Sarnak MJ, Tighiouart H, et al. Disordered mineral metabolism in hemodialysis patients: an analysis of cumulative effects in the hemodialysis (HEMO) study. Am J Kidney Dis. 2008;52:531–540. doi: 10.1053/j.ajkd.2008.05.020. doi:10.1053/j.ajkd.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 7.de Francisco AL, Leidig M, Covic AC, et al. Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: a controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol Dial Transplant. 2010;25:3707–3717. doi: 10.1093/ndt/gfq292. doi:10.1093/ndt/gfq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evenepoel P, Meijers B, Viaene L, et al. Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010;5:1268–1276. doi: 10.2215/CJN.08241109. doi:10.2215/CJN.08241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. doi:10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 10.Cozzolino M, Galassi A, Apetrii M, et al. What would we like to know, and what do we not know about fibroblast growth factor 23? J Nephrol. 2011;24:696–706. doi: 10.5301/jn.5000003. doi:10.5301/jn.5000003. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. doi:10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. doi:10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–2796. doi: 10.1093/ndt/gfp191. doi:10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 14.Larsson TE. The role of FGF-23 in CKD-MBD and cardiovascular disease: friend or foe? Nephrol Dial Transplant. 2010;25:1376–1381. doi: 10.1093/ndt/gfp784. doi:10.1093/ndt/gfp784. [DOI] [PubMed] [Google Scholar]
- 15.Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–298. doi: 10.1038/ki.2009.466. doi:10.1038/ki.2009.466. [DOI] [PubMed] [Google Scholar]
- 16.Krajisnik T, Bjorklund P, Marsell R, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. doi:10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 17.Nakai K, Komaba H, Fukagawa M. New insights into the role of fibroblast growth factor 23 in chronic kidney disease. J Nephrol. 2010;23:619–625. [PubMed] [Google Scholar]
- 18.Larsson TE. FGF23 beyond mineral metabolism: a bridge to cardiovascular disease. Clin J Am Soc Nephrol. 2011;6:2735–2737. doi: 10.2215/CJN.10711011. doi:10.2215/CJN.10711011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiler S, Reichart B, Roth D, et al. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;25:3983–3989. doi: 10.1093/ndt/gfq309. doi:10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 20.Silver J, Rodriguez M, Slatopolsky E. FGF23 and PTH—double agents at the heart of CKD. Nephrol Dial Transplant. 2012;27:1715–1720. doi: 10.1093/ndt/gfs050. doi:10.1093/ndt/gfs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancela AL, Oliveira RB, Graciolli FG, et al. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract. 2011;117:c74–c82. doi: 10.1159/000319650. doi:10.1159/000319650. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD–MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. doi:10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira A, Frazão JM, Monier-Faugere MC, et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19:405–412. doi: 10.1681/ASN.2006101089. doi:10.1681/ASN.2006101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Gupta A, Quarles LD. Emerging role of fibroblast growth factor 23 in a bone–kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr Opin Nephrol Hypertens. 2007;16:329–335. doi: 10.1097/MNH.0b013e3281ca6ffd. doi:10.1097/MNH.0b013e3281ca6ffd. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. doi:10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 26.Nishi H, Nii-Kono T, Nakanishi S, et al. Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract. 2005;101:c94–c99. doi: 10.1159/000086347. doi:10.1159/000086347. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Ortiz ME, Lopez I, Muñoz-Castaneda JR, et al. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012;23:1190–1197. doi: 10.1681/ASN.2011101006. doi:10.1681/ASN.2011101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wetmore JB, Santos PW, Mahnken JD, et al. Elevated FGF23 levels are associated with impaired calcium-mediated suppression of PTH in ESRD. J Clin Endocrinol Metab. 2011;96:E57–E64. doi: 10.1210/jc.2010-1277. doi:10.1210/jc.2010-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koizumi M, Komaba H, Nakanishi S, et al. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27:784–790. doi: 10.1093/ndt/gfr384. doi:10.1093/ndt/gfr384. [DOI] [PubMed] [Google Scholar]
- 30.Couttenye MM, D'Haese PC, Van Hoof VO, et al. Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant. 1996;11:1065–1072. doi:10.1093/oxfordjournals.ndt.a027457. [PubMed] [Google Scholar]
- 31.Ureña P, Hruby M, Ferreira A, et al. Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol. 1996;7:506–512. doi: 10.1681/ASN.V73506. [DOI] [PubMed] [Google Scholar]
- 32.Jorge C, Gil C, Possante M, et al. Bone alkaline phosphatase besides intact parathyroid hormone in hemodialysis patients—any advantage? Nephron Clin Pract. 2005;101:c122–c127. doi: 10.1159/000086682. doi:10.1159/000086682. [DOI] [PubMed] [Google Scholar]
- 33.Almaden Y, Hernandez A, Torregrosa V, et al. High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol. 1998;9:1845–1852. doi: 10.1681/ASN.V9101845. [DOI] [PubMed] [Google Scholar]
- 34.Canalejo A, Canalejo R, Rodriguez ME, et al. Development of parathyroid gland hyperplasia without uremia: role of dietary calcium and phosphate. Nephrol Dial Transplant. 2010;25:1087–1097. doi: 10.1093/ndt/gfp616. doi:10.1093/ndt/gfp616. [DOI] [PubMed] [Google Scholar]
- 35.Barreto DV, Barreto Fde C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification–results from the BRiC study. Nephron Clin Pract. 2008;110:c273–c283. doi: 10.1159/000170783. doi:10.1159/000170783. [DOI] [PubMed] [Google Scholar]
- 36.Iwata Y, Wada T, Yokoyama H, et al. Effect of sevelamer hydrochloride on markers of bone turnover in Japanese dialysis patients with low biointact PTH levels. Intern Med. 2007;46:447–452. doi: 10.2169/internalmedicine.46.6338. doi:10.2169/internalmedicine.46.6338. [DOI] [PubMed] [Google Scholar]
- 37.Liu YL, Lin HH, Yu CC, et al. A comparison of sevelamer hydrochloride with calcium acetate on biomarkers of bone turnover in hemodialysis patients. Ren Fail. 2006;28:701–707. doi: 10.1080/08860220600925388. doi:10.1080/08860220600925388. [DOI] [PubMed] [Google Scholar]
- 38.Wesseling-Perry K, Harkins GC, Wang HJ, et al. Response of different PTH assays to therapy with sevelamer or CaCO3 and active vitamin D sterols. Pediatr Nephrol. 2009;24:1355–1361. doi: 10.1007/s00467-009-1143-8. doi:10.1007/s00467-009-1143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blayney MJ, Pisoni RL, Bragg-Gresham JL, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–663. doi: 10.1038/ki.2008.248. doi:10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]
- 40.Regidor DL, Kovesdy CP, Mehrotra R, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. doi:10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doumouchtsis KK, Kostakis AI, Doumouchtsis SK, et al. Associations between osteoprotegerin and femoral neck BMD in hemodialysis patients. J Bone Miner Metab. 2008;26:66–72. doi: 10.1007/s00774-007-0785-5. doi:10.1007/s00774-007-0785-5. [DOI] [PubMed] [Google Scholar]
- 42.Fujimori A, Okada S, Sakai M, et al. Relationship between biochemical markers and radial cortical bone changes in hemodialysis patients. Nephron Clin Pract. 2011;118:c375–c379. doi: 10.1159/000323669. doi:10.1159/000323669. [DOI] [PubMed] [Google Scholar]
- 43.Shidara K, Inaba M, Okuno S, et al. Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, as a useful marker of cortical bone loss in hemodialysis patients. Calcif Tissue Int. 2008;82:278–287. doi: 10.1007/s00223-008-9127-4. doi:10.1007/s00223-008-9127-4. [DOI] [PubMed] [Google Scholar]
- 44.Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. doi:10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bushinsky DA, Wolbach W, Sessler NE, et al. Physicochemical effects of acidosis on bone calcium flux and surface ion composition. J Bone Miner Res. 1993;8:93–102. doi: 10.1002/jbmr.5650080112. doi:10.1002/jbmr.5650080112. [DOI] [PubMed] [Google Scholar]
- 46.Bichara M, Mercier O, Borensztein P, et al. Acute metabolic acidosis enhances circulating parathyroid hormone, which contributes to the renal response against acidosis in the rat. J Clin Invest. 1990;86:430–443. doi: 10.1172/JCI114729. doi:10.1172/JCI114729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro-González JF, Mora-Fernández C, Garcia-Pérez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009;22:37–44. doi: 10.1111/j.1525-139X.2008.00530.x. doi:10.1111/j.1525-139X.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 48.Kanbay M, Goldsmith D, Uyar ME, et al. Magnesium in chronic kidney disease: challenges and opportunities. Blood Purif. 2010;29:280–292. doi: 10.1159/000276665. doi:10.1159/000276665. [DOI] [PubMed] [Google Scholar]
- 49.Van Laecke S, Van Biesen W, Vanholder R. Hypomagnesaemia, the kidney and the vessels. Nephrol Dial Transplant. 2012;27:4003–4010. doi: 10.1093/ndt/gfs126. doi:10.1093/ndt/gfs126. [DOI] [PubMed] [Google Scholar]
- 50.D'Haese PC, Couttenye MM, Lamberts LV, et al. Aluminum, iron, lead, cadmium, copper, zinc, chromium, magnesium, strontium, and calcium content in bone of end-stage renal failure patients. Clin Chem. 1999;45:1548–1556. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.