Abstract
A preclinical model that includes measures of alternative behavior and drug-seeking could improve our understanding of the processes involved in successful recovery; however current preclinical models of relapse do not measure alternative behavior. We assessed the persistence of food-maintained responding and the resumption of ethanol-maintained responding after ethanol-maintained responding was reduced by changing the response requirement for concurrently available food. Ethanol (10% w/v) was always available following 5 responses (FR5). A 16 kHz tone indicating food delivery followed 150 responses (FR150) resulted in ethanol-predominate responding and substantial amounts of ethanol were earned (0.47 g/kg per 30-min session) and consumed. An 8 kHz tone indicating food delivery followed 5 responses (FR5) for 1, 2, 4, or 16 consecutive sessions reduced ethanol-maintained responding despite unchanged ethanol availability. Ethanol-maintained responding resumed upon subsequent presentation of the 16 kHz tone. However, more responses occurred on the food lever before 5 responses occurred on the ethanol lever as the number of preceding FR5 food sessions increased. These results suggest that alternative reinforcement may reduce control by discriminative stimuli that occasion ethanol-seeking and is consistent with the risk of relapse declining with longer periods of recovery because of the strength of alternative behavior.
Keywords: operant, addiction, relapse, resurgence, alcohol, self-administration, rat
1. Introduction
Preventing relapse is essential to the successful maintenance of recovery from addiction (Brownell et al., 1986). Relapse prevention strategies could benefit from a better understanding of the underlying processes and mechanisms of relapse, which animal models may facilitate (Conklin and Tiffany, 2002). The reinstatement procedure (Stretch and Gerber, 1973; de Wit and Stewart, 1981) is currently the most commonly used model of relapse.
In the reinstatement procedure, animals are trained to respond to self-administer alcohol or drug. This responding is then reduced by removing access to alcohol or drug (extinction). Relapse is then assessed by measuring the persistence of resumed responding during extinction after various treatments. For example, after cocaine-maintained responding in monkeys or rats is extinguished, responding during extinction increases following experimenter-administered amphetamine or cocaine (Stretch and Gerber, 1973; de Wit and Stewart, 1981). In addition, de Wit and Stewart also observed modest reinstatement following exposure to stimuli that had been paired with drug delivery (1981). Given that exposure to drug-paired stimuli and drug use (a lapse) often precede relapse, these results were promising.
Other variants of the reinstatement procedure have been developed. These procedures also test for the ability of drug-administration or drug-paired stimuli to reinstate responding during extinction. But, they differ in that drug self-administration is reduced by either extinguishing behavior in the presence of a distinct discriminative stimulus, or by simply not conducting experimental sessions (Crombag et al., 2008). Such designs allow reinstatement to be assessed upon exposure to the discriminative stimulus that had signaled that drug or alcohol was available. This is not possible when responding is established and extinguished in the presence of the the same discriminative stimulus.
In all of these procedures, the experimenter withholds access to reduce responding maintained by the drug. Yet, the use of extinction to model recovery has been questioned (Katz and Higgins, 2003; Tiffany and Conklin, 2002). Tiffany and Conklin (2002) make the point in the following manner: “Lapses by definition occur following some period of abstinence… during this period of abstinence, it is highly unlikely that the addict is exposed to some sort of extinction regimen in which he or she repeatedly engages in drug seeking and drug taking but experiences no drug effect.” Similarly, while abstinence can occur because of suspended access due to incarceration, hospitalization, or voluntary isolation, in these situations problematic drug-seeking behaviors are not extinguished and drug unavailability is unique to these particular situations (Conklin and Tiffany, 2002). This leads to a high likelihood of relapse when familiar stimuli and contingencies are again encountered upon returning home.
Behavioral therapies that encourage replacing substance use with alternative behavior have been shown to be effective. For example, when alternative behavior is reinforced, regardless of the occurrence of the problematic behavior, the problematic behavior declines (Athens and Vollmer, 2010; Iguchi et al., 1997). This strategy has been shown to be effective at reducing drug use and does so without requiring any change in drug availability. Thus, recovery can be achieved by replacing drug use with alternative behavior rather than by extinction or eliminating access.
Increasing the strength of alternative behavior could protect individuals from relapse when they are re-exposed to cues that might precipitate relapse. However, if alternative behavior occurs but goes unrewarded, drug-seeking eventually re-emerges, due to spontaneous recovery or resurgence (Conklin and Tiffany, 2002; Podlesnik et al., 2006). If such a lapse results in drug ingestion, drug-seeking quickly regains strength, and relapse may follow (Di Ciano and Everitt, 2002; Crombag and Shaham, 2002). In contrast, robustly maintaining the alternative behavior could keep the occurrence of drug-seeking low, even when cues that had elicited seeking are again encountered. Therefore the occurrence and reinforcement of alternative behaviors likely facilitates recovery, even in environments where drug-seeking had predominated prior to recovery.
Thus, strengthening alternative behavior to drug-seeking could be critical to successful recovery. During extinction training, other behavior undoubtedly increases in frequency and strength, but is not measured in the reinstatement procedure. Concurrent schedules can result in allocation of responding between a lever that produces delivery of drug or ethanol and another lever that provides an alternative. This provides an opportunity to study responding reinforced by drug or ethanol delivery as well as by the alternative (Griffiths et al., 1975). When the response requirement for the alternative is relatively high, responding maintained by drug or ethanol often predominates (Nader and Woolverton, 1992). Conversely, when the response requirement for the alternative is relatively low, responding maintained by the alternative often predominates without any changes in the work requirements for drug or ethanol (Samson et al., 1982). Finally, different stimuli can be associated with each set of work requirements and responding can be brought under stimulus control such that in the presence of one stimulus responding is predominately for food and in the presence of the other, responding is predominately for drug or ethanol: all without any change in the availability of drug or ethanol.
We were interested to see if such a procedure might be useful for studying relapse. Thus, we trained rats in a discrete trial concurrent schedule procedure in which, in the presence of one stimulus, food was delivered following 5 responses and in the presence of another stimulus, food was delivered following 150 responses (fixed-ratio 150, FR150). In the presence of either stimulus, ethanol was presented following 5 responses (FR5). Ethanol-maintained responding predominated in the presence of the stimulus signaling food FR150. Food-maintained responding predominated (and ethanol-maintained responding was almost abolished) in the presence of the stimulus indicating food FR5. We were interested in whether re-exposing rats to the stimulus signaling food FR150 would result in a resumption of ethanol-maintained responding. Additionally, we examined whether longer intervening periods of reduced ethanol-maintained responding (by presenting only food FR5 conditions) affected food and ethanol responding upon re-exposure to food FR150 conditions.
2. Materials and Methods
2.1 Subjects
Male Lewis rats (Harlan, Frederick, MD) served as subjects (n=5). Rats were 6-weeks old upon arrival, singly housed, and allowed one week to habituate with food and water provided ad libitum. Subsequently, food was restricted to 12-15g/day in order to maintain body weights of 280-320 g for the remainder of the study. Water was always available in the rats’ cages. All procedures had prior approval of our Institutional Animal Care and Use and Committee (Protocol 08124x) and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
2.2 Apparatus
Experiments occurred in a commercially available apparatus (ENV-008, Med Associates, St. Albans, VT). On one wall, two levers were arranged horizontally, one on each side of the wall. Equidistant between the levers was a receptacle providing access to 45 mg pellets (Bio-Serve, Frenchtown, NJ) via a pellet dispenser and to a solution via a 0.1-ml retractable dipper. Pink noise was generated in the procedure room to mask ambient noise. Chambers also had a speaker connected to a tone generator (ANL- 926, Med Associates, St. Albans, VT) that produced tones which served as stimuli. Stimuli presentation and reinforcement delivery as well as data collection were accomplished by software written using a commercially available programming language (Med-PC, Med Associates, St. Albans, VT). Ethanol (95%) was obtained from Decon Labs, Inc. (King of Prussia, PA) and mixed with tap water to obtain a 10% (w/v) solution. Solutions were made fresh daily, and allowed to reach room temperature before being provided to the rats.
2.3 Training
Sessions were conducted on weekdays and were 30-min in duration. Initially, rats were trained to respond on the left lever for 8% sucrose solution in the presence of a 16 kHz tone presented at 80 dB. Except during test sessions (see below), responses on the appropriate lever in the presence of the 16 kHz tone always resulted in presentation of 0.1 mL of the solution and a changed the tone in the chamber to white noise, also presented at 80 dB. The dipper remained accessible for 30-s, at which point it returned to the inaccessible position, and white noise was replaced with the 16kHz tone. During this 30-s period, responses had no programmed consequences. Responses on the right lever had no programmed consequences during this portion of training. Once rats earned over 80 sucrose deliveries in a 30-min session (typically 2-7 sessions), the fixed-ratio (FR) was increased over a few sessions until rats were required to respond five times for a sucrose delivery. Subsequently, ethanol was introduced into the solution at 10% w/v, then sucrose was gradually removed from the solution over the next 10-25 sessions so rats responded for 10% (w/v) ethanol solution in tap water (Samson, 1986).
Food-maintained responding was trained in a subsequent 30-min session during presentation of a 8 kHz tone at 80dB. Under this stimulus condition, responses on the right lever always resulted in the delivery of a 45 mg food pellet, and the tone changed to 0.1 kHz. Over the next several sessions, the FR was increased to 25, then rats were introduced to the multiple concurrent schedule. The discriminative stimulus frequencies (8kHz and 16 kHz) were selected based on a published audiogram which indicates that in Sprague-Dawley rats (Lewis rat progenitors), the threshold (in dB) for detection is similar across a range of frequencies from 8 – 32 kHz (Kelly and Masterton, 1977).
Under the multiple concurrent schedule, responses on either lever were reinforced during each component, and components alternated between the two different stimulus conditions (8 and 16 kHz) and associated contingencies. When the 16 kHz tone was present, the ethanol contingency was FR5 and the food contingency was FR150. When the 8 kHz tone was present, the ethanol contingency remained FR5, but the food contingency was also FR5. Completion of the FR was based on total responses (no reset) on either lever and no change-over delay was imposed. The order of components (8 and 16 kHz)was randomized within each block of two components. Training under these conditions continued until each rat responded >80% on the ethanol lever when the food FR was 150 and >80% on the food lever when the food FR was 5. Training took 121 ± 12 sessions (mean ± S.E.M).
2.4 Testing
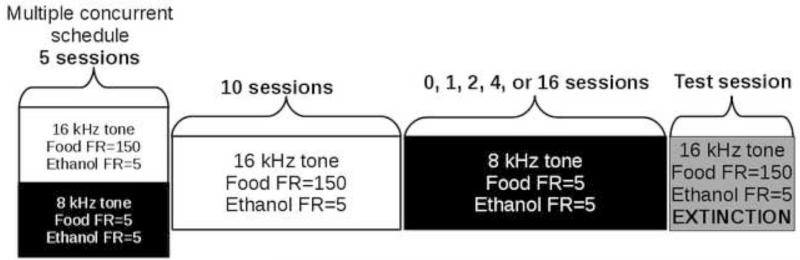
After this training, testing commenced. Testing occurred in each rat during five separate blocks comprised of the four phases shown in Figure 1. The order of intervention period was mixed across subjects. In each block, rats were first exposed to at least five consecutive sessions under the multiple concurrent schedule. This allowed training performance to be re-established after each test session. Subsequently, rats were exposed to 10 consecutive sessions in which the only condition presented was 16 kHz tone, Food FR150, Ethanol FR5. Following these 10 sessions, rats were exposed to an intervention period of 0, 1, 2, 4, or 16 consecutive sessions in which the only condition presented was 8 kHz tone, Food FR5, Ethanol FR5.
Figure 1.
Experimental design. The design of the study included at least 5 days of training under the multiple concurrent schedule in which trials alternated within the session between a response requirement for food of 150 lever presses (FR150) and the associated stimulus tone (16 kHz) and a response requirement for food of 5 lever presses (FR5) and the associated stimulus tone (8 kHz). The response requirement for ethanol was always 5 lever presses (FR5). The training period was followed by 10 consecutive sessions in which the only tone and contingencies presented were 16 kHz and FR150 for food and FR5 for ethanol. This was followed by various periods of exposure to only the 8 kHz tone and matched (FR5) contingencies for food and ethanol. During the next session, a test was conducted in the presence of the 16 kHz tone. During this session, responses on the food or ethanol lever did not result in delivery of either reinforcer. Each subject was tested under each condition (0,1,2,4,16 days under the 8kHz tone and FR5 contingencies for food and ethanol) in mixed order.
The following session was a test session in which the stimulus and contingencies were the 16 kHz tone, Food FR150, and ethanol FR5 (the same as those which occasion ethanol-predominate responding), but food and ethanol were not delivered (though the ethanol solution was present but inaccessible in the chamber). Thus, tones changed (white noise or 0.1 kHz following FR5 on the ethanol-associated or FR150 on the food-associated lever, respectively), but no food or ethanol was delivered.
2.5 Analysis
All analyses were performed using the R Statistical Programming Language (R Development Core Team, 2011). The method of Benjamini and Hochberg (1995) was used to correct p-values for multiple comparisons. Results were considered significant when p-values < 0.05.
2.5.1 Behavior preceding the test session
The total number of food and ethanol deliveries earned under each condition (16 kHz tone, food FR150, ethanol FR5 or 8kHz tone, food FR5, ethanol FR5) during the sessions that preceded each test session were analyzed using repeated measures ANOVA with day as a factor. In addition, the percentage of responses on the ethanol lever (as percent total responses) under each condition was calculated and analyzed in the same manner. Finally, as a basis for comparison with responding prior to first fixed-ratio completion during test sessions, responding prior to first fixed-ratio completion during the sessions preceding the test session was assessed.
2.5.2 Responding during the test session
The first question of interest was whether ethanol-maintained responding resumed upon presentation of the stimulus that had previously been associated with ethanol-predominant responding, similar to cue-induced reinstatement. To address this question, the total number of ethanol responses completed in the test session was compared with the number completed in the preceding session. A difference score was calculated for each condition. Positive difference scores were considered indicative of resumed responding. Difference scores for each of the condition lengths (1, 2, 4, and 16 sessions) were compared with 0 condition using a Student’s t-test.
The second question of interest was whether longer periods of reduced ethanol-maintained responding due to alternative reinforcement would modify behavior allocation in the test session. Before the first fixed-ratio was completed, responding was guided entirely by the stimulus present and the context of the test chamber. After the first fixed ratio was completed, subsequent responding was also influenced by the delivery (or non-delivery in the case of the test session) of reinforcement. Thus, our primary measure was the number of food responses completed prior to completion of the first fixed-ratio. These results were analyzed using a repeated-measures ANOVA with intervention duration as the factor.
In addition, the effect of intervention duration on the total number of responses for ethanol during the test session was determined using a repeated measures ANOVA. This characterized whether the intervention period affected the persistence of ethanol-maintained responding under extinction. Finally, the total number of responses for food after the completion of the first fixed-ratio was analyzed as a function of the preceding intervention to allow assessment of whether the intervention length affected the persistence of food-maintained responding once extinction contingencies had been encountered.
2.5.3 Order of treatments
The order of intervention conditions was mixed across subjects, and potential order effects on responding during the test session were assessed with a repeated-measures ANOVA with order as a covariate.
2.5.4 Ethanol Consumption
Blood ethanol concentration (BEC) was estimated from breath ethanol concentrations using a rat breathalyzer to confirm that rats were consuming ethanol earned in the session (Javors et al., 2005). Briefly, immediately after a single 30-min session, rats were placed into a modified commercially available restraint with their head inside a heated chamber. Expired air was collected in this chamber for 30-sec, then ethanol content was determined with gas chromatography. From the expired ethanol level we estimated the BEC.
3. Results
3.1 Behavior preceding the test session
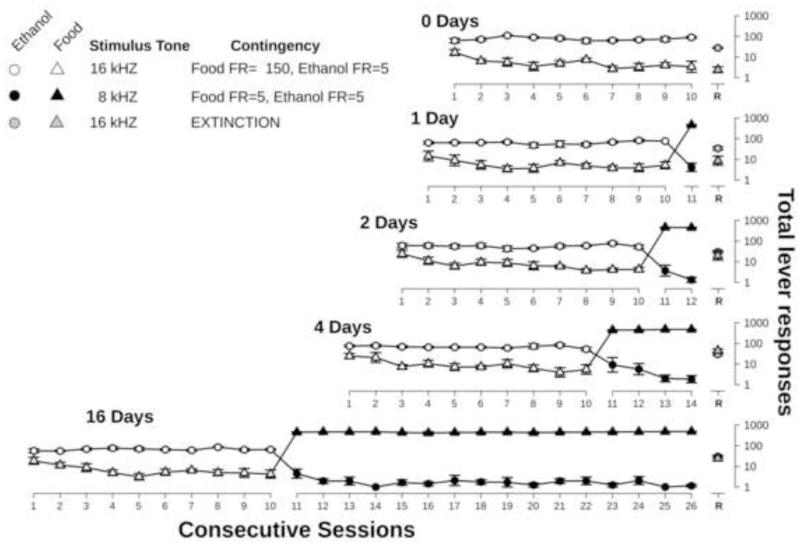
During ten consecutive sessions preceding the various intervention conditions in which food FR150 conditions were present, rats responded predominantly on the ethanol lever (88.7% ± 2.5; mean ± S.E.M, Table 1, and Figure 2 - open symbols). The mean number of ethanol deliveries per session was 15.8 ± 0.9, which was stable across sessions (F[9, 38]=1.7). This equates to an earned dose of 0.47 g/kg ethanol in 30-min. Over these sessions, there was a significant decrease in the total number of responses for food (F[9,236]=14.8, p<0.05). This decrease in food-maintained responding occurred over the first 3-4 sessions going from low levels to very low levels where it remained for the next 7 sessions. However, food responding was at such low levels initially that rats never completed a response requirement for food resulting in 100% ethanol choice across these sessions.
Table 1. Percentage of total responses on the ethanol-associated lever per session for each subject.
| 16 kHz sessions |
8 kHz sessions |
Test Sessions (by intervention condition) | |||||
|---|---|---|---|---|---|---|---|
| Subject | 0 | 1 | 2 | 4 | 16 | ||
| 1 | 77.9 | 0.4 | 92.6 | 69.4 | 37.9 | 39.7 | 28.7 |
| 2 | 91.3 | 1.0 | 85.0 | 98.0 | 65.8 | 75.8 | 46.7 |
| 3 | 88.8 | 0.3 | 96.2 | 56.2 | 67.6 | 69.4 | 46.3 |
| 4 | 91.7 | 0.4 | 100.0 | 86.2 | 49.5 | 89.3 | 29.8 |
| 5 | 93.8 | 1.3 | 96.2 | 73.5 | 54.3 | 19.7 | 49.0 |
| Group | 88.7 | 0.7 | 94.0 | 76.7 | 55.0 | 58.8 | 40.1 |
Figure 2.
Total responses for ethanol (circles) and food (triangles) during each phase of the experiment. Points represent mean ± S.E.M. for 5 rats plotted on a log scale. White symbols represent exposure to only the 16 kHz tone and FR150 response requirement for food and FR5 for ethanol over ten consecutive sessions. Black symbols represent various periods of exposure to the 8 kHz tone and matched FR5 response requirements for food and ethanol. Grey symbols represent responses during test sessions following each period of exposure to matched (FR5) response requirements for food and ethanol.
During these 10 sessions, in the presence of the 16 kHz tone, rats rarely responded more than five times on the food lever prior to completing five responses on the ethanol lever, including the first component of each session. Rats responded more than five times on the food lever in only 6.4% of the first components. When the entire session was considered, rats only completed more than five responses on the food lever prior to completing five responses on the ethanol lever in 2.3% of the components.
Rats were subsequently exposed to either 0, 1, 2, 4 or 16 sessions during which an 8 kHz tone was presented and only five responses were required to earn either food or ethanol. Under this condition ethanol-maintained responding was almost abolished (Table 1, Figure 2, black symbols). Across 1, 2, 4, or 16 sessions of this condition, responses on the ethanol lever accounted for only 0.7 ± 0.2% of total responses during a session. The length of exposure to these conditions did not affect this response proportion. Over all of these sessions, rats earned an average of 94.4 ± 2.7 food pellets per session. In contrast, rats did not complete a single fixed-ratio for ethanol in 86% of the sessions under this condition (97/115). In the 18 sessions where rats did earn ethanol, they earned between 1 and 3 ethanol deliveries in every case except one when a rat earned 7 deliveries. This resulted in ethanol choice of 0.0 – 0.5% across the subjects in these sessions.
3.2 Ethanol consumption
During this session only the 16 kHz tone, food FR150, ethanol FR5 conditions were presented and rats earned 18.4 ± 1.3 ethanol deliveries. This represents an earned dose of approximately 0.6 g/kg. BEC after this session was estimated at 0.07 ± 0.01 g/dL; a pharmacologically active concentration.
3.2 Order effects
There was no effect of order nor was there an interaction with intervention condition on the total number of ethanol responses during the test session (F[1, 11] = 0.07), nor was there an interaction with intervention condition (F[4,11] = 0.58). There was also no effect of order on the number of responses for food before the first five responses on the ethanol lever were completed (F[1,11]=0.01), nor was there an interaction between order and intervention condition (F[4,11] = 2.0). Finally, there was also no main effect of order on the number of food responses after the first five ethanol responses were completed (F[1, 11] = 0.00). There was, however an interaction between order and intervention condition on (F[4, 11] = 5.1). Further analysis revealed that this was largely driven by a singe animal who made a large number of food responses after the first five ethanol responses on the last test session (which happened to be a 2-session intervention). Removing this subject’s 2-session data from the analysis eliminates this interaction (F[4, 10]=0.52), but does not alter the reported results.
3.3 Responding during the test session
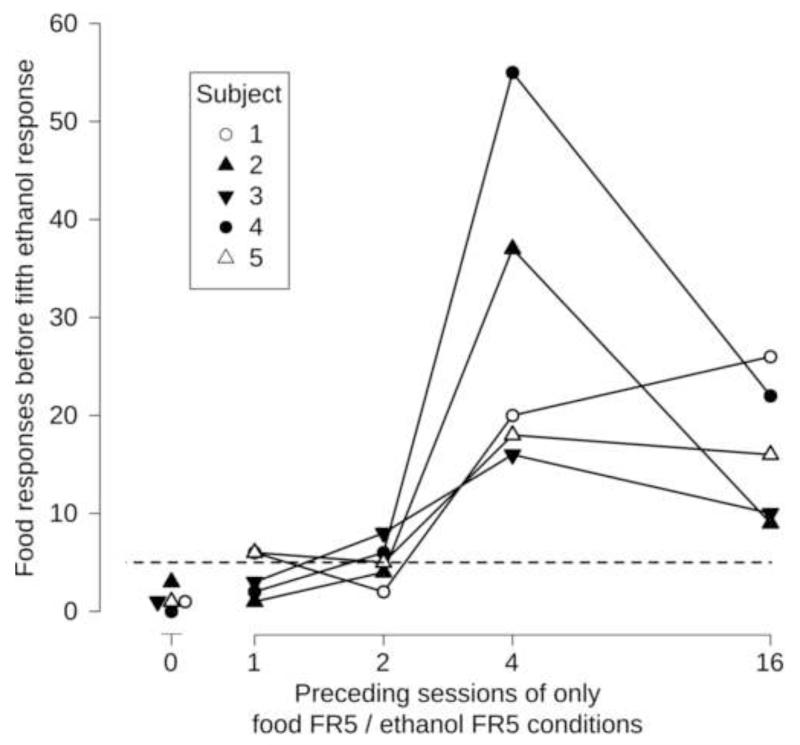
After the completion of the first fixed-ratio, rats experienced extinction. Because of this, response allocation prior to completion of the first fixed ratio was analyzed. During these test sessions, the number of food responses prior to completion of the first 5 ethanol responses increased as the number of preceding food FR5 sessions increased. Before 5 responses occurred on the ethanol lever, the mean number of responses for food was less than 5 following 0, or 1 sessions of only food FR5 conditions (1.2 or 3.6 responses respectively; Figure 3), and equal to 5 following 2 sessions. However after 4 or 16 sessions, food-maintained responding before 5 responses occurred on the ethanol lever increased to approximately 20 responses (29.2 and 16.6, respectively). This was a significant effect (F[4,16]=10.0, p<0.05). It was also of interest to know how many subjects would have completed a fixed-ratio for food if the response requirement remained five, prior to completing a fixed-ratio for ethanol. The number of subjects completing five or more responses on the food lever before the first five responses on the ethanol lever increased as a function of intervention length (0-day: 0/5, 1-day: 2/5, 2-day: 3/5, 4-day: 5/5, 16-day: 5/5). This is shown in Figure 3 by the number of points on or above the dashed line at 5 responses; points on or above the dashed line completed 5 or more responses on the food lever before completing the first 5 responses on the ethanol lever. Thus, after 4 or 16 sessions, every subject would have completed a fixed-ratio for food had the response requirement from the preceding session remained in effect.
Figure 3.
Number of food responses that occurred before the first five ethanol responses were completed expressed as a function of preceding period of food-predominant responding. Points represent the number of responses on the food-associated lever prior to completion of the first five responses on the ethanol-associated lever during a test session following the indicated number of sessions of responding under only 8 kHz, food FR5, ethanol FR5 conditions. More than five food responses prior to completion of five responses on the ethanol lever (points above the horizontal dashed line) would have been reinforced under contingencies in place the previous session (except for the zero-day condition). Some points have been offset for clarity.
Once food-maintained responding during the test session extinguished, ethanol-maintained responding resumed. The persistence of ethanol-maintained responding under extinction within the test session was not affected by the duration of the preceding intervention (F[4, 20]=0.4), nor was the persistence of food-maintained responding after the completion of the first fixed-ratio (F[4,20]=1.1).
4. Discussion
Here, we present an innovative animal model of recovery and relapse: reducing ethanol intake by reinforcing an alternative behavior in the same context but in the presence of a distinct discriminative stimulus. More preceding sessions where food-maintained responding was reinforced increased the perseverance of food-maintained responding during extinction before ethanol-responding resurged. This increase in food-maintained responding occurred in the presence of a stimulus that had been associated with low rates of food-delivery and responding. This pattern of behavior reduces the likelihood that ethanol responding would be reinforced before the alternative behavior, and thus should reduce the likelihood of relapse. However, once ethanol-responding resurges, its persistence was not affected by the length of the preceding period of reduced ethanol-maintained responding. These findings using tones as discriminative stimuli are consistent with previous results using lights as stimuli in rats responding for ethanol or saccharin (Ginsburg and Lamb 2012a). This procedure may be useful because it parallels recovery in humans and may provide insight into the processes critical to successful recovery.
In this procedure, the availability of ethanol did not change. However, the amount of behavior maintained by ethanol changed as a function of the response requirement for food. Similar results have been shown using sucrose-maintained responding instead of food pitted against ethanol in rats, and using food pitted against cocaine instead of ethanol in monkeys (Nader and Woolverton, 1992; Negus, 2003; Samson et al., 1982). In humans, reinforcing alternative behavior has also been shown to reduce problematic behavior (Athens and Vollmer, 2010), including opioid (Iguchi et al., 1997) and ethanol use (Hunt and Azrin, 1973). Others have suggested that increasing the occurrence of alternative behavior may be an effective approach to the initiation and maintenance of recovery (Bickel and Kelley, 1997; Schuster, 1986). Indeed, a recent report shows that providing access to a running wheel (which presumably functioned as alternative reinforcement) can reduce reinstatement of cocaine responding in rats (Smith et al., 2012).
The most important finding of the present work was that longer preceding periods where the response requirement for food is reduced result in greater perseverance of food-maintained responding when rats are re-exposed to a stimulus indicating conditions where ethanol-maintained responding had previously predominated over food-maintained responding. Thus, longer periods where alternative behavior is frequent increased its strength. This may reflect decreased control by the discriminative stimulus as the context (rather than the tone presented) begins to provide information about the prevailing contingencies and assumes greater control over behavior. Consistent with this interpretation, longer periods of low ethanol use due to alternative reinforcement is associated with a reduced ability of a range of stimuli to occasion ethanol-maintained responding (Ginsburg and Lamb, 2012b). This may also parallel clinical results and provide a mechanism for the clinical observation that longer periods of recovery are associated with reduced attentional bias to alcohol- or drug-related stimuli (Field and Cox, 2008).
In the present study, food-maintained responding was not reinforced during the test session, and ethanol-responding eventually resurged. This result is consistent with those from other resurgence and renewal studies. Reinforcing a competing response can decrease the likelihood of the original response and this decrease is similar in form (though steeper) to an extinction curve (Boe, 1964). However, if these responses are suddenly not reinforced, the original response returns and undergoes extinction (Leitenberg et al., 1970). These early results with food reinforcement have been extended to studies with drugs. For example, when ethanol- or cocaine-maintained responding is extinguished and replaced by food-maintained responding in the same context, subsequent extinction of food-maintained responding results in a resurgence of drug-maintained responding (Podlesnik et al., 2006; Quick et al., 2011). Together, these results demonstrate that unexpected changes in contingencies that maintain alternative behavior can lead to a resurgence of drug-seeking. Moreover, the relapse precipitated by such disruption of alternative behavior may occur with the same vehemence regardless of the length of recovery.
It is apparent that alcohol or drug-related stimuli may precipitate a relapse even after years or decades of recovery (O’Brien, 2008). This may result from disruption of alternative behavior cultivated during recovery. Yet if such a lapse occurs, the length of recovery may not affect the strength or persistence of the resumed alcohol- or drug-seeking. Therefore, treatment strategies must focus both on ways to strengthen alternative behavior to drug use and on ways to reduce the strength of drug-seeking when it resurges. This may be why treatment strategies that combine behavioral therapy which strengthens alternative behavior with pharmacotherapy which devalues drug use (such as methadone) are the most successful approaches currently available (Kleber, 2007; O’Brien, 2008).
While the persistence of ethanol-maintained responding during extinction once it resurged was not diminished by longer preceding periods of alternative reinforcement, neither did it increase. This lack of “incubation” contrasts with results from other relapse models (Agabio et al., 2000; Bienkowski et al., 2004; Grimm et al., 2003). Typically, greater persistence of responding is interpreted as greater motivation to seek the drug. Indeed, there is evidence that increasing deprivation level (and thus motivation) increases persistence of responding during extinction (Perin, 1942). In the present study, increasing the period of deprivation from ethanol (by reducing the amount of ethanol earned over longer intervention periods) did not affect the persistence of responding during extinction. This result contrasts with other results where longer periods of deprivation increase the persistence of responding during extinction and weakens the argument that such reinstated responding simply reflects increased motivation due to extended periods of no self-administration.
The lack of incubation observed in the present study may also reflect a limitation of the present study. It is unclear whether even longer periods of reinforcing alternative behavior would result in incubation or diminution of the persistence of ethanol-maintained responding in extinction. For example, rats deprived of ethanol access for 3 – 30 days show an increase in ethanol consumption in an alcohol deprivation procedure, while rats deprived for 90 or 180 days show no change in consumption, compared with non-deprived controls (Agabio et al., 2000). Similarly, Bienkowski et al.(2004) showed that the persistence of ethanol-maintained responding in extinction increases after 28 days of suspended training compared to rats with 1 day of suspended training. However, responding during extinction decreases after 56 days of suspended training compared with the 28 day condition (but remains higher than the 1 day group). Thus, although our time periods were reasonable, incubation may be a biphasic effect which we still failed to capture.
Despite this limitation, the ability to quantify an alternative behavior represents a clear strength of our procedure. The occurrence and reinforcement of alternative behavior may be critical to successful recovery. While others have developed procedures in which drug-maintained responding is reduced by means other than extinction, including punishment (Panlilio et al., 2003) or reinforcing abstinence (LeSage, 2009), these and the reinstatement procedures all share the limitation that no alternative behavior is measured. This contrasts with our procedure in which alternative operant behavior is quantified concurrently with drug-maintained responding.
When drug-maintained responding is diminished by extinction, punishment, or by reinforcing not responding for drug, alternative behaviors undoubtedly occur, but are typically not measured. With our procedure, the occurrence of an alternative behavior that has previously been reinforced in the same context is assessed. Indeed, with longer periods of reduced ethanol-maintained responding (and increased food-maintained responding), more food-maintained responding occurs before ethanol-maintained responding resurges. This finding demonstrates a critical advantage of the present procedure over traditional reinstatement procedures: assessment of an alternative behavior in the same subject during relapse.
The ability to assess an alternative behavior also provides a means to assess specificity of potential therapies (Griffiths et al., 1981). A major limitation of the reinstatement procedure is its inability to determine the effect of treatments that reduce reinstatement on other reinforced behavior. Often, the specificity of a treatment that reduces reinstatement is demonstrated by showing that the treatment does not affect responding on an inactive lever (a weak test, given the relatively low levels of responding that occur on the inactive lever). With our procedure, a treatment that reduces the likelihood of resumed ethanol-maintained responding should do so without substantially decreasing food-maintained responding. In fact, we would argue that an effective treatment should increase food-maintained behavior in this model as behavior is re-allocated from drug-maintained responding to the alternative reinforced behavior. This is a potentially important area of future investigation.
In conclusion, reinforcement of alternative behavior reduces the likelihood of ethanol-maintained responding versus food upon re-exposure to stimuli where ethanol-responding had previously predominated over food-maintained responding in a time-dependent manner. This reduced likelihood is due to increased persistence of the alternative behavior, and may reflect reduced control by stimuli that prompt ethanol-seeking. Reducing control by alcohol- or drug-related stimuli may represent an important mechanism to target for relapse prevention strategies that also include pharmacotherapy.
We reduce ethanol-seeking by reinforcing alternative behavior in the same context.
Food responding is more persistent upon re-exposure to a drug-associated stimulus.
This effect increases with longer periods of alternative reinforcement.
Once food seeking extinguishes, ethanol-seeking resurges.
Alternative reinforcement can decrease attention to drug-associated stimuli.
Acknowledgments
The authors with to thank Olivia Dominguez and Gerardo Martinez for expert technical assistance. This work was funded by PHS grant AA016987 (NIH, BCG) and AA012337 (NIH, RJL) as well as by the Alcoholism Breakthrough Endowed Research Fund provided to the institution by a private donor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, et al. Development of short-lasting alcohol deprivation effect in sardinian alcohol-preferring rats. Alcohol. 2000;21:59–62. doi: 10.1016/s0741-8329(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Athens ES, Vollmer TR. An investigation of differential reinforcement of alternative behavior without extinction. J Appl Behav Anal. 2010;43:569–589. doi: 10.1901/jaba.2010.43-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57:289–300. Series B (Methodological) [Google Scholar]
- Bickel WK, Kelley T. Stimulus Control Processes in Drug Taking: Implications for Treatment. In: Baer DM, Pinkston EM, editors. Environment and behavior. WestviewPress; Boulder, Colo.: 1997. pp. 185–193. [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, et al. Time-dependent changes in alcohol-seeking behaviour during abstinence. European Neuropsychopharmacology. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Boe EE. Extinction As A Function Of Intensity Of Punishment, Amount Of Training, And Reinforcement Of A Competing Response. Can J Psychol. 1964;18:328–342. doi: 10.1037/h0083311. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. Am Psychol. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13:397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav. Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Discrete-Trial Choice Procedure: Effects of Naloxone and Methadone on Choice Between Food and Heroin. Pharmacol Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Choice between food and heroin: effects of morphine, naloxone, and secobarbital. J Exp Anal Behav. 1981;35:335–351. doi: 10.1901/jeab.1981.35-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su T-P, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GM, Azrin NH. A community-reinforcement approach to alcoholism. Behav Res Ther. 1973;11:91–104. doi: 10.1016/0005-7967(73)90072-7. [DOI] [PubMed] [Google Scholar]
- Iguchi MY, Belding MA, Morral AR, Lamb RJ, Husband SD. Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. J Consult Clin Psychol. 1997;65:421–428. doi: 10.1037//0022-006x.65.3.421. [DOI] [PubMed] [Google Scholar]
- Javors MA, Ginsburg BC, Friesenhahn G, Delallo L, Lamb RJ. Rat breathalyzer. Alcohol. Clin. Exp. Res. 2005;29:1853–1857. doi: 10.1097/01.alc.0000183228.07510.a2. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl.) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Masterton B. Auditory sensitivity of the albino rat. J Comp Physiol Psychol. 1977;91:930–936. doi: 10.1037/h0077356. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitenberg H, Rawson RA, Bath K. Reinforcement of competing behavior during extinction. Science. 1970;169:301–303. doi: 10.1126/science.169.3942.301. [DOI] [PubMed] [Google Scholar]
- LeSage MG. Toward a Nonhuman Model of Contingency Management: Effects of Reinforcing Abstinence from Nicotine Self-Administration in Rats with an Alternative Nondrug Reinforcer. Psychopharmacology (Berl) 2009;203:13–22. doi: 10.1007/s00213-008-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Choice between cocaine and food by rhesus monkeys: effects of conditions of food availability. Behav Pharmacol. 1992;3:635–638. [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academies Press; 1996. [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Evidence-based treatments of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3277–3286. doi: 10.1098/rstb.2008.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology (Berl.) 2003;168:229–235. doi: 10.1007/s00213-002-1193-0. [DOI] [PubMed] [Google Scholar]
- Perin CT. Behavior potentiality as a joint function of the amount of training and the degree of hunger at the time of extinction. Journal of Experimental Psychology. 1942;30:93–113. [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, Shahan TA. Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behav Pharmacol. 2006;17:369–374. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- Quick SL, Pyszczynski AD, Colston KA, Shahan TA. Loss of alternative non-drug reinforcement induces relapse of cocaine-seeking in rats: role of dopamine D(1) receptors. Neuropsychopharmacology. 2011;36:1015–1020. doi: 10.1038/npp.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol. Clin. Exp. Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Roehrs TA, Tolliver GA. Ethanol reinforced responding in the rat: a concurrent analysis using sucrose as the alternate choice. Pharmacol. Biochem. Behav. 1982;17:333–339. doi: 10.1016/0091-3057(82)90088-0. [DOI] [PubMed] [Google Scholar]
- Schuster CR. Implications of laboratory research for the treatment of drug dependence. In: Stolerman IP, Goldberg SR, editors. Behavioral Analysis of Drug Dependence. Academic Press Inc; London: 1986. pp. 357–386. [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121:54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretch R, Gerber GJ. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can J Psychol. 1973;27:168–177. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. The promise and pitfalls of animal and human models of relapse: comment on Leri and Stewart. Exp Clin Psychopharmacol. 2002;10:361–363. doi: 10.1037//1064-1297.10.4.361. 2002. discussion 364-366. [DOI] [PubMed] [Google Scholar]
- De Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl.) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]