Abstract
Breast cancer mortality is primarily due to the occurrence of metastatic disease. We have identified a novel potential therapeutic agent derived from an edible root of the plant Colocasia esculenta, commonly known as taro, that has demonstrable activity in a preclinical model of metastatic breast cancer and that should have minimal toxicity. We have shown for the first time that a water-soluble extract of taro (TE) potently inhibits lung colonizing ability as well as spontaneous metastasis from mammary gland-implanted tumors, in a murine model of highly metastatic ER, PR and Her-2/neu negative breast cancer. TE modestly inhibits proliferation of some, but not all, breast and prostate cancer cell lines. Morphologic changes including cell rounding were observed. Tumor cell migration was completely blocked by TE. TE treatment also inhibited prostaglandin E2 (PGE2) synthesis and downregulated cyclooxygenase (COX) 1 and 2 mRNA expression. We purified the active compound(s) to near homogeneity with antimetastatic activity comparable to stock TE. The active compound with a native size of approximately 25 kD contains two fragments of nearly equal size. The N-terminal amino acid sequencing of both fragments reveals that the active compound is highly related to three taro proteins; 12 kD storage protein, tarin and lectin. All are similar in terms of amino acid sequence, post-translational processing and all contain a carbohydrate-binding domain. This is the first report describing a compound(s) derived from taro, that potently and specifically inhibits tumor metastasis.
Keywords: Taro, Breast cancer, Antimetastatic activity, Tumor, Cancer therapy
Introduction
Breast cancer is the second leading cause of cancer deaths in women in the United States. Breast cancer mortality is primarily due to the occurrence of metastatic disease. Several agents derived from foods have demonstrable chemopreventive and chemotherapeutic activities by multiple mechanisms including enhanced detoxification of carcinogenic intermediates, inducing apoptosis, perturbing cell cycle progression and inhibiting angiogenesis and metastasis [1–7]. Research into food-derived bioactive components for cancer prevention as well as cancer therapy is growing due to the relatively low or no detectable toxicity and better bioavailability. We have identified and purified potentially novel therapeutic compound(s) derived from an edible root of the plant Colocasia esculenta, commonly known as taro. Using two highly metastatic, ER, PR and Her-2/neu negative murine mammary tumor cell lines (66.1, 410.4) transplanted to immune competent syngeneic mice, we have now shown that a water soluble extract of the raw taro corm (TE) can significantly inhibit the lung colonizing ability as well as spontaneous metastasis of these cells. Protective effects are also observed in a therapy model where treatment is initiated after tumors are established. It is well established that Cyclooxygenase-2 (COX-2) and its product PGE2 are associated with aggressive breast cancer [8,9]. In our previous work we have shown that pharmacologic inhibition of both COX-1 and 2 reduces tumor cell proliferation, PGE2 synthesis, tumor growth and more importantly metastasis in a murine model of breast cancer [10–12]. We now report that TE inhibits PGE2 synthesis and downregulates COX (1 and 2) mRNA expression. Migration in vitro is also inhibited by TE. Addition of TE to some human and murine cancer cell lines profoundly affects cellular morphology as well as cell proliferation in a dose-dependent manner. Others have shown that cooked mashed corm of the taro plant, known as poi, has antiproliferative activity against the rat YYT colon cancer cell line in vitro [13]. We have tentatively identified the active compound(s) as three closely related proteins. Other than our own preliminary data, little is known regarding potential anticancer or antimetastatic activity of taro in vivo.
Methods
Ethics statement
All animal experiments were approved by the IACUC committee of the University of Maryland School of Medicine (IACUC protocol # 0708005) and carried out in strict accordance with the recommendations in the guide for the Care and Use of Laboratory Animals of the National Institute of Health (U.S.).
Mice
Syngeneic Balb/cByJ female mice were purchased from Jackson Laboratories, Bar Harbor, ME. All mice were housed in microisolator cages, fed conventional, autoclaved chow, and provided drinking water ad libitum.
Taro extract (TE) preparation
Commercially obtained taro corm was peeled, combined with PBS in a weight: volume ratio of 1:3, blended at low speed, followed by high speed to liquefy. After centrifugation at 1200 r.p.m. for 15 min at 4°C, the supernatant was subjected to high speed centrifugation (15,000 r.p.m., 20 min at 4°C) and filter sterilized. Protein concentration of the stock TE was determined using Coomassie Plus Protein Assay Reagent (Pierce) and ranged from 1.69 mg/ml to 3.43 mg/ml. For the following experiments, stock TE of 2 µg/µl protein was used, unless otherwise indicated.
Isolation of active components
Stock TE was centrifuged through Amicon Ultra 10 K (10,000) Nominal Molecular Weight Limit (NMWL) devices (Millipore Corporation), at 4000g for 45 min at 25°C. After filter sterilization, the high m.w. fraction as well as the low m.w. fraction were used without further treatment.
Column chromatography and electrophoresis
Preparative size exclusion chromatography (SEC) was performed on a Biosuite 250, 13 µ, 21.5 × 300 mm column (Waters Corp., Milford. MA) using Dulbecco’s phosphate buffered saline with calcium and magnesium, at a flow rate of 2 ml/min. 0.5 min. fractions were collected and pooled based on UV absorbance at 220 nm. Preparative Anion Exchange Chromatography was performed on an HQ/20, 10 × 100 mm column (Applied Biosystems, Inc., Foster City, CA), using a 30 minute gradient of 0–30% B at a flow rate of 5 ml/min.: Buffer A = 50 mM Tris, pH 8.0, Buffer B = 50 mM Tris pH 8 + 1.0 M NaCl. 0.5 min. fractions were collected and pooled based on UV absorbance at 220 nm. The pooled samples were concentrated using Centricon Plus 70 10K Nominal Molecular Weight Limit (NMWL) devices (Millipore). Buffer exchange was done using Zeba Desalt Spin Columns, Pierce Protein Research Product (Thermo Scientific). Analytical reversed phase liquid chromatography (RPLC) was performed using a Jupiter C5 300Å column (Phenomenex, Torrance, CA), employing a 40 minute gradient of 1–100% B at 1 ml/min. Buffer A = 0.1% trifluoroacetic acid (TFA) in water, Buffer B = 0.1% TFA in water: acetonitrile (20:80), with UV detection at 215 nm. All chromatography was done on Beckman Coulter HPLC systems with System Gold V8 or 32 Karat software packages. Isolated proteins were further purified using reversed phase high performance liquid chromatography (rpHPLC) on a Waters 2695 HPLC system. Absorbance was monitored with an Applied Biosystems 785 UV detector at 214 nm. Proteins were separated on a Waters Symmetry 300 3µ C4 1mmx150mm column with a gradient of 0.1% trifluoroacetic acid (TFA) in water (solvent A) and 0.09% TFA in acetonitrile (solvent B). Automated Edman degradation was performed on an Applied Biosystems 494 HT. Proteins were identified by a similarity search of the nr database using the BLASTP 2.2.24+ algorithm via the NCBI web interface [14]. Sub fraction 1.1 was analyzed by SDS-PAGE under reducing condition.
Tumor cell lines
Two murine mammary tumor cell lines (66.1 and 410.4) [15] were maintained in DMEM supplemented with 10% FBS (Gemini Bio-Products, Inc., Calabasas, CA, USA), 2 mM glutamine, penicillin (100u/ml), streptomycin (100µg/ml) and 0.1 mM nonessential amino acids in a 10% and 5% CO2 humidified atmosphere, respectively. The nontumorigenic murine mammary epithelial cell line EpH4 derived from a Balb/c mouse was maintained in the growth medium described for murine tumor cell lines but without nonessential amino acids and in a 5% CO2 atmosphere. Human breast cancer cell lines MCF-7 [16], MDA-MB-231[17] and T47D [16] were cultured in the growth medium described for murine tumor cell lines but in a 5% CO2 atmosphere and MDA-MB-435 [17] cells were grown in MEM with Earle’s salt, L-glutamine (2mM), sodium pyruvate (1mM), non essential amino acids (0.1mM), vitamins (2X) and 5% fetal calf serum. MCF10A [18], an immortalized nontumorigenic epithelial cell line derived from tissue from a reduction mammoplasty, was grown in equal parts DMEM (with 4.5 g/L glucose and without L-glutamine) and HyQ Ham’s F-12 supplemented with 5% horse serum (Biosource, Camarillo, CA), 10 µg/ml insulin, 500 ng/ml hydrocortisone, 100 ng/ml cholera toxin, and 20 ng/ml epidermal growth factor in a 5% CO2 atmosphere. Human prostate cancer cell lines DU145 [19] and LNCaP [20] were grown in RPMI 1640, 10% fetal calf serum, penicillin (100 u/ml) and streptomycin (100 µg/ml). Human prostate cancer cell line PC3 [19] was grown in DMEM/F12 (Gibco), L-glutamine (2mM), 5% fetal calf serum, penicillin (100 u/ml) and streptomycin (100 µg/ml). For proliferation assays cells were seeded in 24-well plates and PBS or TE was added at time 0. Forty-eight hours later, cells were trypsinized and the viable cell number determined by trypan blue staining or MTT assay.
Tumorigenicity and metastasis assays
PBS (150µl or 200µl) or TE (150µl or 200µl) was injected i.p. into syngeneic Balb/cByJ female mice (Jackson Laboratories, Bar Harbor, ME) on days 1–4. On day 4, 1 × 10 line 66.1 or 410.4 tumor cells were injected into the lateral tail vein. Treatment with PBS or TE continued (200 µl) daily for an additional 6 days. Between days 16–21 post tumor cell injection, when control animals became moribund, mice were euthanized and surface lung tumor colonies were counted under a dissecting microscope [21]. To determine the effect of TE on established tumors, 5×105 line 410.4 tumor cells were injected subcutaneously proximal to the mammary gland of Balb/cByJ female mice. Two hundred micro liter of either PBS or TE was injected i.p. daily for 18 days, starting on day 5 when tumors became palpable. Tumor growth was monitored by caliper measurement as described in [12]. When tumors achieved an average diameter of 18 mm, or earlier if animals appeared moribund, mice were euthanized individually and soft tissues examined for spontaneous metastasis.
Prostaglandin assays
410.4 and 66.1 cells were seeded in 12 ml growth media. TE was added at a final concentration of 6.25–100 µg protein /ml. Control dishes contained PBS. Twenty four hours later media was collected and PGE2 level was determined by EIA (Cayman Chemicals, Ann Arbor, MI).
Real Time-PCR
Total RNA was extracted from cultured cells using TRIzol® (Invitrogen), and single-stranded cDNA was generated from 1 µg of total RNA via reverse transcription using qScript ™ cDNA SuperMix (Quanta Biosciences,Inc. Gaithersburg, MD). Quantitative PCR amplification was performed using iQ™ SYBR® Green Supermix iQ™ (Bio-Rad, Hercules, CA.) and the following gene-specific primers: 5’- CCGAGGTGTATG TAT GAGTGTG -3’ (sense) and 5’-TGAAGTGGGTAAGTATGTAGTGC -3’ (anti-sense) for mouse COX-2, 5’-GGTGACAACTGGAGGGAGGAG-3’ (sense) and 5’-TCTGGGAGTGG ATGGATGTGC-3’ (anti-sense) for COX-1, and 5’-GCCTTCC GT GTTCCTACC-3’ (sense) and 5’-GCCTGCTTCACCACCTTC-3’ (anti-sense) for mouse GAPDH. The obtained threshold cycle (Ct) values were processed for further calculations according to the comparative Ct method. Expression levels of target genes were normalized to the housekeeping gene GAPDH, giving the ΔCt value. Finally, the gene expression level was calculated as 2−ΔCt, giving the final value that is normalized to the housekeeping gene.
Migration assay
Calcein AM (Molecular Probes, Eugene, OR) labelled tumor cells were pretreated with TE at a final concentration of 6.25–50 µg/ml and were placed in the upper well of Millicell tissue culture plate well inserts (Millipore Ireland Ltd, Ireland). 2% fetal bovine serum (FBS) was placed in the bottom chamber. To some wells base medium or as a positive control 2% fetal bovine serum (FBS) were placed in the bottom chamber and untreated cells were placed in the upper well. Twenty-four hours later migration was assessed after removing non-migrating cells from the upper chamber using a DTX 880 multimode detector measuring fluorescence at 485nm. Results expressed as OD (mean±SE) of triplicate wells.
Statistical analysis
Data were summarized using descriptive statistics means and standard errors, medians and ranges. Depending on the data distribution, the Student’s t-test, or its non-parametric alternative, the Wilcoxon test, were used to compare distribution of metastases between treatment groups. All statistical tests were exact and done at the two-sided 0.05 level of significance.
Results
TE inhibits lung colonization
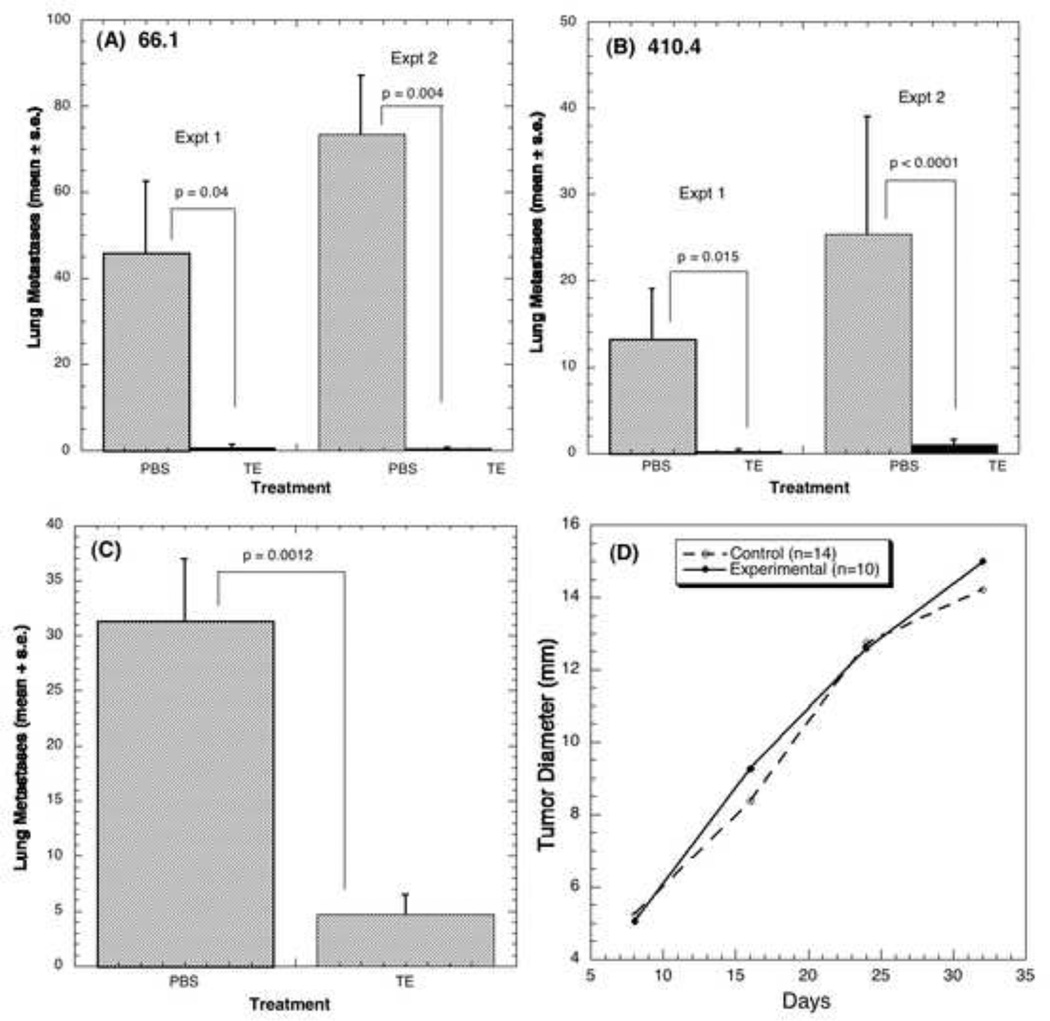
We first examined the effect of stock TE on lung colonizing ability of highly metastatic murine breast cancer cell lines 66.1 and 410.4; the latter forms colonies in both the lung and the heart after i.v. administration. PBS (control) or TE was injected i.p. daily into syngeneic Balb/cByJ female mice on days 1–10. On day 4, either 66.1 or 410.4 tumor cells were injected into the lateral tail vein. Between days 16–21 post tumor cell injection, when control animals became moribund, mice were euthanized and surface lung tumor colonies were counted. Figures 1A and 1B summarize the effect of TE on lung colonization by 66.1 and 410 4 cell lines, respectively, in two independent experiments, carried out with two independently prepared extracts. In all experiments, TE profoundly inhibited the ability of tumor cells to colonize the lungs. TE treatment resulted in a 98–99% reduction in lung tumor colony formation by either tumor cell line. In the case of 410.4, TE also significantly inhibited tumor colonies in the heart. In two experiments, tumor colonies were detected in 4/5 and 5/9 control mice, whereas none (0/5) and only 1/10 TE-treated mice showed heart involvement (data not shown).
Figure 1. TE inhibits lung colonization and spontaneous metastasis.
The effect of TE on lung colonizing ability of highly metastatic lines 66.1 (A) and line 410.4 (B) was determined. (A) 150µl PBS or TE or (B) 200 µl PBS or TE was injected i.p. daily for 10 days. On day 4, 1 × 105 line 66.1 or 410.4 tumor cells were injected into the lateral tail vein. On day 21 post tumor cell injection, mice were euthanized and surface lung tumor colonies were counted. (C) 5×105 line 410.4 tumor cells were injected subcutaneously proximal to the mammary gland of mice. When tumor became palpable (day +5) 200 µl of either PBS or TE was injected i.p. daily for the next 18 days. Tumor growth was monitored and when tumors measured 18 mm in average diameter or earlier if animals appeared moribund, mice were euthanized and spontaneous lung metastases enumerated. (D) Shows the null effect of TE on tumor growth.
TE inhibits established tumor metastasis
To determine the effect of TE on established tumors and spontaneous metastasis, mice were transplanted subcutaneously with 5 × 105 410.4 cells proximal to the right inguinal mammary gland. Beginning on day 5, when tumors became palpable, 200 µl of either PBS or TE was injected i.p. daily for 18 days. When tumors achieved an average diameter of 18 mm, or earlier if animals appeared moribund, mice were euthanized individually and soft tissues examined for spontaneous metastasis. Delaying initiation of TE therapy until tumors are well established still resulted in a significantly reduced number (85% inhibition) of spontaneous lung metastases (Fig. 1C) but had no effect on the size of the locally growing tumors (Fig. 1D).
Effect of TE on cell morphology and cell proliferation
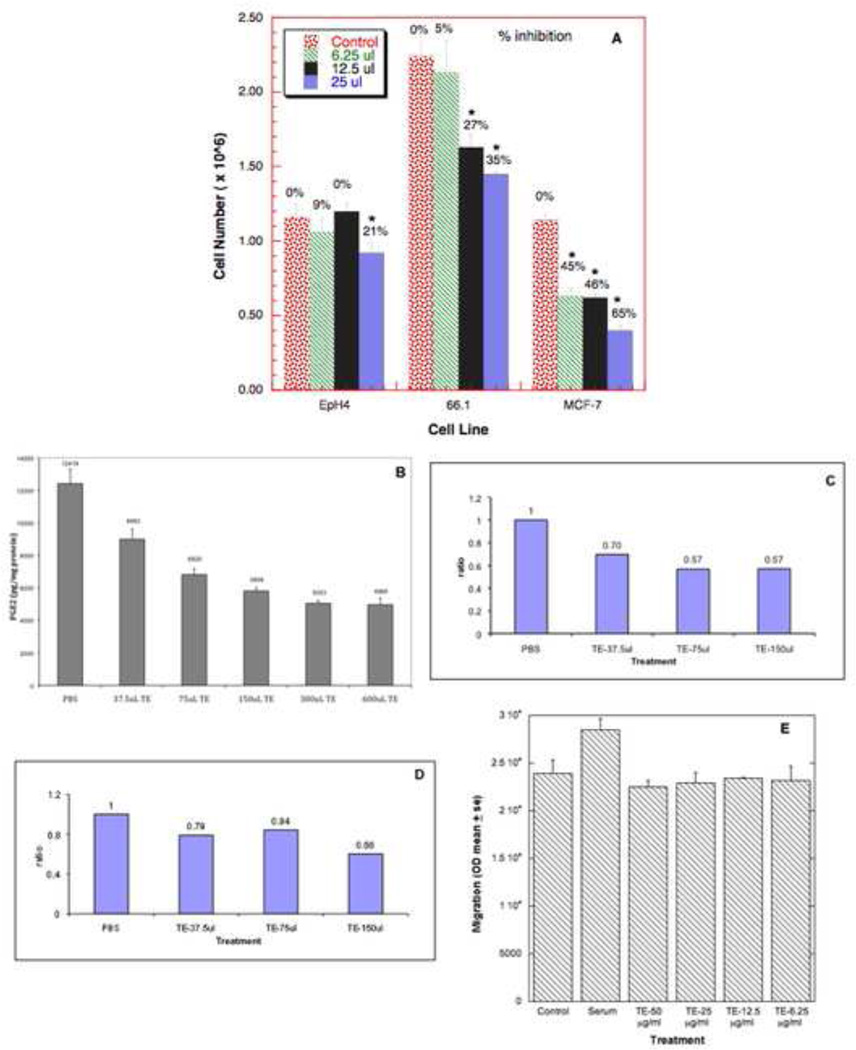
To investigate the mechanisms responsible for TE-mediated inhibition of metastasis, we examined the effect of TE on the morphology and proliferation of a panel of murine (66.1, 410.4) and human (MCF-7, MDA-MB-231, MDA-MB-435 and T47D) breast cancer cell lines, human prostate cancer cell lines (DU145, LNCaP, PC3) and immortalized murine mammary (EpH4) and human mammary (MCF10A) epithelial cell lines. TE profoundly affected the morphology of some (66.1, MCF-7, LNCaP) but not all tumor cells, resulting in the retraction of cellular foot processes and cell rounding whereas the appearance of immortalized normal mammary epithelial cell lines MCF10A (Human) and EpH4 (murine) remained unchanged even at the highest concentration of TE (data not shown). TE significantly inhibited proliferation of 66.1, MCF-7, 410.4, MDA-MB-231 and MCF10A cells in a dose dependent manner. At the highest TE concentrations (50 µg/ml) line 66.1 and MCF-7 cells were reduced by 35% and 65% (Fig. 2A). EpH4 cell number was reduced only at the highest TE concentration employed (Fig. 2A). Likewise, growth of lines 410.4, MDA-MB-231 and MCF10A were reduced by 24%, 26%, 31% (data not shown). The proliferation of the other breast cancer cell lines including MDA-MB-468 or T47D cells was not affected. The observation that some, but not all, cells were adversely affected by TE, suggests that the antiproliferative effects of TE are not likely to be due to nonspecific toxicity. In addition, the antiproliferative effect of TE is reversible with time. Polymyxin B treatment of TE did not reduce the antiproliferative activity ruling out endotoxin contamination as a likely explanation for these observations. We have also determined that the antiproliferative activity of TE is markedly reduced by boiling or by TCA precipitation of the extract prior to addition to cells (not shown).
Figure 2. TE inhibits cell proliferation, PGE2 synthesis, COX mRNA expression and migration.
(A) Line EpH4, 66.1 or MCF-7 cells were seeded at 2.5×105 cells/well/1.0 ml media in triplicate in 24 well plates. PBS or TE (6.25, 12.5, 25 µl) was added. Forty-eight hours later, cell number was determined. (* P <0.05).
(B) 6× 106 of line 410.4 cells were seeded in 12 ml media in triplicate. TE from a stock concentration of 2µg/µl was added to the media in the indicated amounts. Control dishes contained PBS at the highest amount used with TE. Twenty-four hours later, cell-conditioned media was collected and PGE2 level was determined by EIA. Data expressed as pg PGE2/ mg protein as mean±SE. (P <0.05). (C,D) 6× 106 line 410.4 cells were seeded in 12 ml media and 24 hours after plating, TE was added as in B. Six hours later RNA was isolated, reverse transcribed, and amplified using primer specific for COX-2 (C) and COX-1 (D). (E) Line 410.4 tumor cells, untreated or pretreated with TE (6.25–50 µg/ml) for 1 hour were placed in the upper chamber of a modified Boyden assay. Media or media containing 2% serum was added to the lower chamber. Cells traversing the membrane were quantified 24 hours later.
TE inhibits cyclooxygenase activity
Increased PGE2 production is a very common feature in human malignancies. PGE2 levels are positively correlated with increased tumorigenic and metastatic potential [10]. We examined the effect of TE on PGE2 production by 410.4 and 66.1 cells. TE inhibits PGE2 production significantly by 410.4 cells in a dose dependent manner (Fig. 2B) and in 66.1 cells (data not shown). At the highest concentration of TE, PGE2 release was reduced by 63% compared to PBS-treated control cells. PGE2 production is mediated by two COX isoforms. We treated 410.4 cells with TE for 6 hours, mRNA was harvested and levels of COX-2 and COX-1 mRNA were determined by quantitative PCR. TE treatment inhibited expression of COX-2 mRNA and, to a lesser extent, COX-1 (Figs. 2C, 2D).
TE inhibits cell migration
Tumor cell migration is an important aspect of metastasis. We asked if TE treatment modulates migratory ability. Tumor cells migrate in response to FBS. TE completely inhibited tumor cell migration in response to serum at all concentrations examined (Fig. 2E).
The effect of high and low molecular weight fraction of TE on cell proliferation
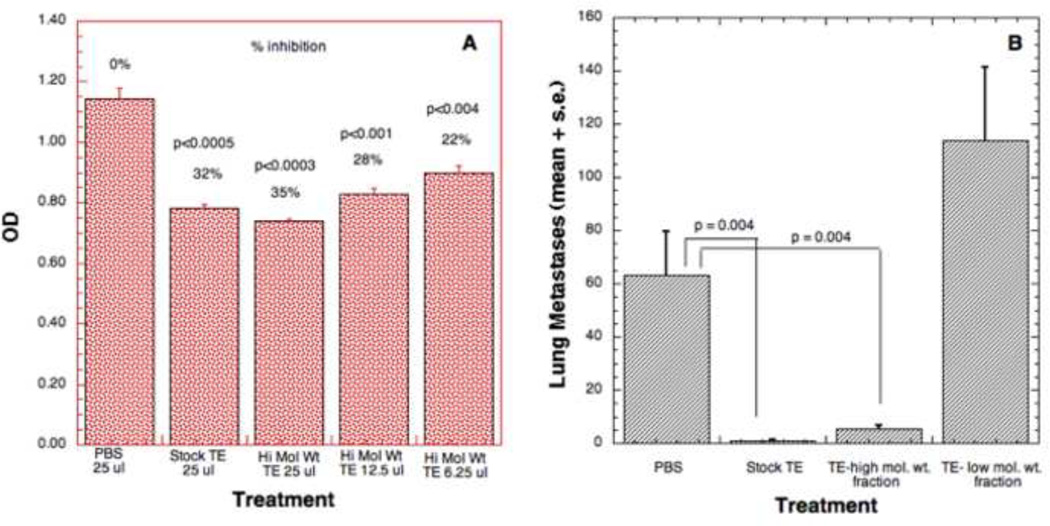
To begin to identify the active component of TE, we compared the antiproliferative activity of high and low m.w. subfractions to TE at 6.25, 12.5 and 25 µl doses. Significant reduction of cell number was observed in response to treatment with stock TE (32%) or the high m.w. fraction (22%, 28%, 35% respectively) (Fig. 3A). No significant effect on cell number was observed in the presence of the low m.w. fraction at any concentration (data not shown). Thus, the inhibitory effect of TE on cell growth resides chiefly in the high m.w. fraction.
Figure 3. Effect of high and low m.w. TE on cell proliferation and metastasis.
(A) Line 66.1 cells were seeded in 24-well plates and PBS or high molecular weight fraction of TE was added at 6.25, 12.5 and 25 µl doses at time 0. Forty-eight hours later, cell number was determined by MTT assay. (B) 100 µl high m.w. fraction, 200 µl of PBS, stock TE or low m.w. fraction of TE was injected i.p. for 10 days. On day 4 of treatment, 2 × 105 line 66.1 tumor cells were injected i.v. On day 19 post tumor cell injection, mice were euthanized, and surface lung and heart tumor colonies counted. P values by Wilcoxon exact two-sided test at the 0.05 level of significance.
The high molecular weight fraction inhibits lung colonization
To determine if anti-metastatic activity is also present in the high m.w. fraction of TE, we compared the effect of stock TE, high m.w. fraction of TE and low m.w. fraction of TE on lung colonization. Since high m.w. compounds were concentrated in the upper fraction (protein concentration is twice the stock), 100 µl high m.w. fraction, 200 µl of PBS, stock TE or low m.w. fraction of TE was injected daily per mouse i.p. for 4 days. On day 4, 2 × 105 line 66.1 tumor cells were injected into the lateral tail vein. The PBS or TE treatments were continued i.p daily for an additional 6 days. On day 19 post tumor cell injection, mice were euthanized, surface lung tumor colonies were counted and the presence of heart metastases was noted (Fig. 3B). The results confirm our previous observation that stock TE significantly inhibits the ability of tumor cells to colonize the lungs as compared to the vehicle-treated control mice. Similarly, high m.w. fraction of TE also significantly inhibited lung colonization but the antimetastatic effect was completely absent from the low m.w. fraction. While 3/7 control animals and 4/8 mice treated with low m.w. fraction developed tumor colonies in the heart, only 2/10 and 0/10 mice treated with stock or high m.w. fraction, respectively, developed heart metastases.
Fraction profile by size exclusion chromatography (SEC)
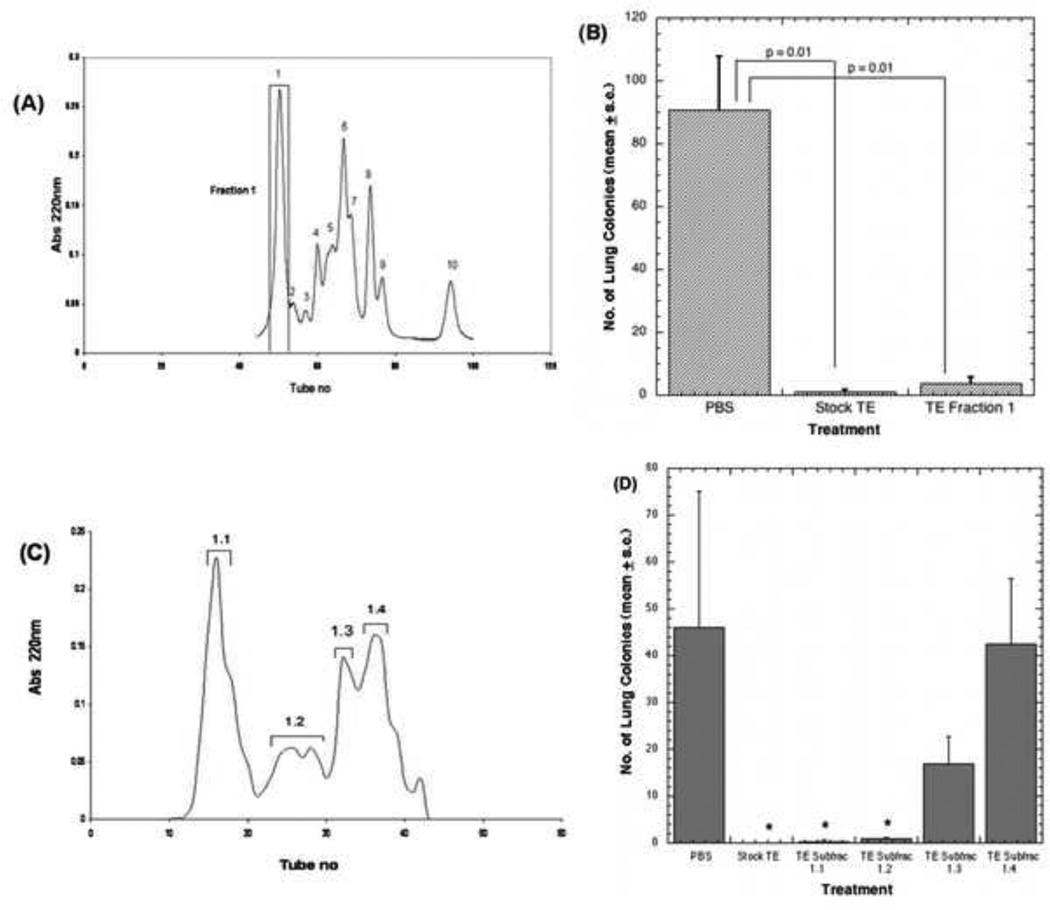
We established a protein purification scheme to identify the active component(s). Using size exclusion chromatography we obtained 10 fractions from Stock TE (Fig. 4A). All 10 fractions were tested for antiproliferative activity in vitro using 66.1 tumor cells but only fraction 1, approximately 30kD from SEC (calibrated using globular proteins), showed modest antiproliferative activity (data not shown). We evaluated the ability of fraction 1 to inhibit lung colonizing ability of 66.1 cells in comparison to stock TE or PBS. In spite of the modest effects on proliferation, fraction 1 contains potent antimetastatic activity comparable to stock TE (Fig. 4B). Fraction 1 was further purified by anion- exchange column chromatography. Four sub fractions obtained from this step (Fig. 4C) were assessed in the lung colonization assay and compared with the antimetastatic activity of stock TE (Fig. 4D). The majority of the antimetastatic activity was recovered from the first and the second sub fraction peaks. Activity gradually declines in the third peak and is almost lost in the fourth peak.
Figure 4. Fraction profile from size exclusion (SEC) and anion-exchange chromatography and effect on lung colonization.
(A) Stock TE was analyzed by preparative SEC and ten fractions were obtained. (B) PBS (control), 400µg TE stock or 20 µg fraction 1 from SEC injected i.p. for 10 days. On day 4, 1 × 105 line 66.1 tumor cells were injected i.v. On day 18 post tumor cell injection, mice were euthanized and surface lung tumor colonies enumerated. (C) Fraction 1 further purified by anion- exchange chromatography. (D) Four sub fractions from anion-exchange chromatography were assessed in the lung colonization assay using line 66.1. PBS, 400 µg TE stock or 20 µg sub fraction 1.1, 1.2, 1.3 or 1.4 injected i.p. and treatment continued as in B. P values by Wilcoxon exact two-sided test at the 0.05 level of significance (* p<0.0001).
Fraction profile from reversed phase liquid chromatography (RPLC)
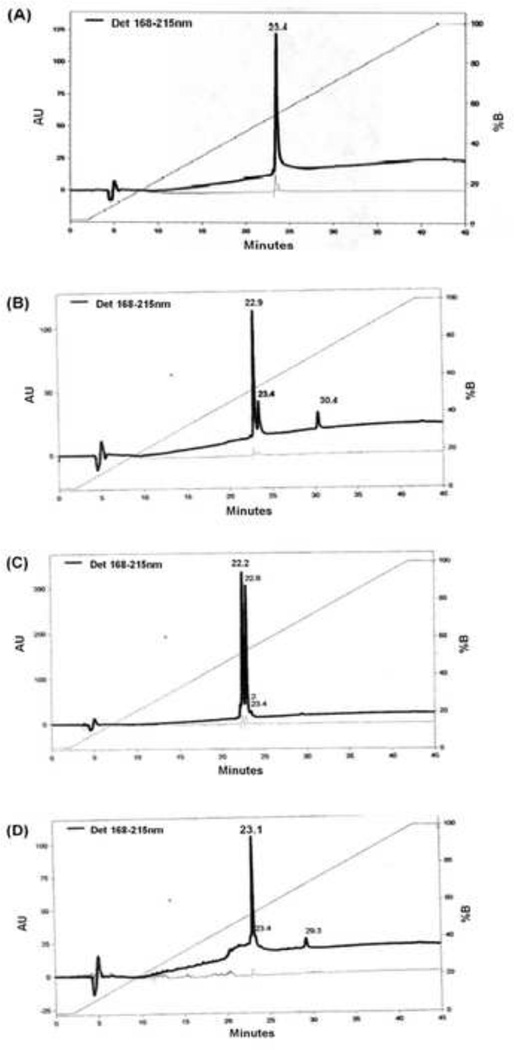
To determine the purity of each sub fraction, reversed phase chromatography was employed (Figs. 5A,B,C,D). Only one protein peak (at 23.4 minute) was eluted from sub fraction 1.1 (Fig. 5A), suggesting that the fraction containing antimetastatic activity is in almost pure form. When other sub fractions were analysed on RPLC, multiple peaks including sub fraction 1.1 (at 23.4 minute), were eluted as contaminants, in decreasing order (Figs. 5B,C,D). This probably explains why sub fraction 1.2 also shows significant antimetastatic activity but not sub fraction 1.4.
Figure 5. Fraction profile from reversed phase liquid chromatography (RPLC).
Each sub fraction from anion exchange column was analyzed by reversed phase chromatography of sub fraction 1.1 (A), 1.2 (B), 1.3 (C), 1.4 (D). The apparent peak of interest is at 23.4 minutes.
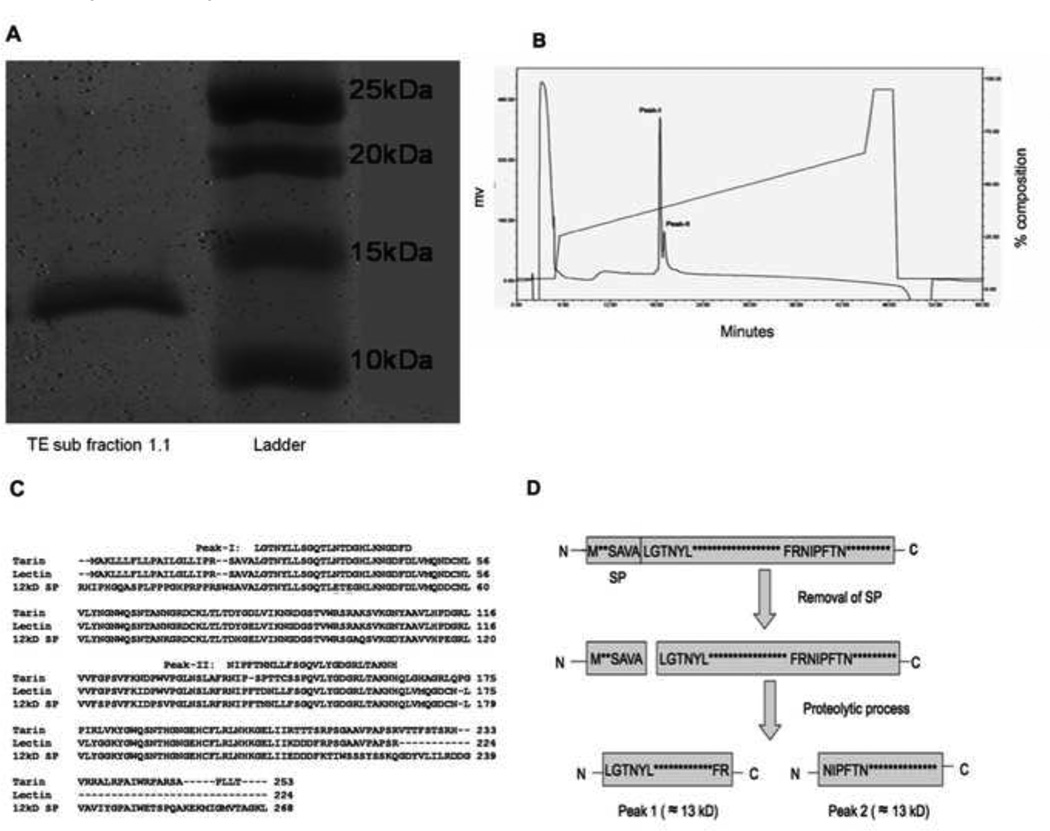
The active component(s) consists of two protein fragments of 12 and 13 kD
When the active component(s) was analyzed from sub fraction 1.1 by SDS-PAGE (Fig. 6A), two protein bands were seen with sizes of approximately 12 and 13 kD. Since the native size of the active component(s) determined by the sizing column is around 30 kD, the active component(s) likely consist of the two fragments seen in SDS-PAGE at 1:1 ratio. This hypothesis is further supported by the analyses of the N-terminal sequences of the active components (Table 1). When the pooled active components were subjected to automated Edman degradation, two major phenylthiohydantoin (PTH)-amino acid signals in each cycle were observed at nearly an equimolar ratio (Table 1). Taken together, the active component(s) appears to be a 25 kD protein that contains two subunits with sizes of 12 and 13 kD.
Figure 6. Protein identification.
(A) SDS PAGE of sub fraction 1.1 under reducing condition. (B) Additional purification of isolated proteins by rpHPLC. (C) The amino acid sequences of the three taro proteins (tarin, lectin and 12 kD storage protein) and the comparison of amino acid sequence location of peak 1 and peak 2 with these three proteins.
(D) A schematic representation of the proposed post-translational processing of the active compound. The three taro proteins: 12 kD storage protein, tarin and lectin appear to undergo an identical two-step maturation process to generate mature forms with two fragments: the signal peptides are removed by a cleavage at SAVA-LGTN followed by a second proteolytic cleavage at FR-NIP to generate two fragments.
Table 1. PTH-Amino acid yields from IEX fractions.
Automated Edman degradation was performed on an Applied Biosystems 494 HT. Mass spectrometric analysis was performed on a Shimadzu Axima CFR Plus using sinapinic acid as the matrix. Proteins were identified by a similarity search of the nr database using the BLASTP 2.2.24+ algorithm via the NCBI web interface.
| Cycle | Called Residues |
Yield (pmoles) |
|---|---|---|
| 1 | N L |
54.3 66.3 |
| 2 | G I |
53.1 58.7 |
| 3 | T P |
54.9 50.6 |
| 4 | N F |
41.5 49.3 |
The active component(s) is highly related to taro storage protein, tarin, and taro lectin
In order to obtain the N-terminal sequences of the two fragments, the active component(s) pooled from Poros HQ/20 column were purified further by rpHPLC (Fig. 6B). Two partially resolved peaks were obtained. The amino acid sequence for Peak-I (Fig. 6C) was determined to be LGTNYLLSGQTLNTDGHLKNGDFD and the sequence of the second peak in the fractions was deduced to be NIPFTNNLLFSGQVLYGDGRLTAKNH by subtracting the sequence obtained for Peak-I from the data obtained previously with both sequences (Table 1). A BLAST similarity search of the sequence data against the nr database reveals that the active component(s) is highly related to three taro proteins, including the taro 12 kD storage protein (accession number BAA03722) [22], tarin (accession number CAA53717) [23], and the taro lectin (accession number ABQ32294 ). The 24 N-terminal amino acid sequence of the protein in Peak-I is identical to both taro lectin and tarin which appear to be distinct gene products with identical N-terminal amino acid sequences that significantly diverge after amino acid 142. The N-terminal sequence of the Peak-I protein is also nearly identical to a third taro protein, 12 kD storage protein, with differences in only two amino acid residues. Interestingly, the 26 amino acid sequence identified in the Peak-II protein is identical to that of the 12 kD storage protein. The N-terminal sequence of the Peak-II protein is also highly related to tarin with 72% identity (18 out of 25 amino acid residues) and taro lectin with 96% identity (24 out 25 amino acid residues). Examination of the original sequence data from the ion-exchange fractions showed that the yield of PTH-glutamic acid in cycles thirteen and fifteen was near background level indicating that the N-terminal polypeptide chain (residues 28–143) of 12kD storage protein was not present or was below the level of detection. The three taro proteins appear to undergo an identical two-step maturation process to generate mature forms with two fragments: the signal peptides are removed by a cleavage at SAVA-LGTN followed by a second proteolytic cleavage at FR-NIP to generate two fragments (Fig. 6D). Since the active component(s) contains two fragments with N-terminal sequences matching to the two cleavage sites, respectively, the active component(s) resembles the taro proteins in terms of the maturation process as summarized (Fig. 6D). All three proteins; 12 kD storage protein, tarin and taro lectin, contain a carbohydrate binding domain.
Effect of stock TE or sub fraction 1.1 treatment on weight
To examine the potential toxicity of TE, normal mice were treated i.p. with PBS, 400 µg stock TE or 20µg sub fraction 1.1 for 4 days. On day 5, mice were euthanized and organ wet weight was determined. Body weight was assessed on day 1 and day 5 and the difference recorded as body weight gain (Table 2). The gross appearance of the animals was not different among the control, stock TE or sub fraction 1.1-treated mice. Spleens and livers were significantly heavier in TE-treated mice, whereas, sub fraction 1.1-treated mice showed no differences in any organ weight when compared to PBS-treated control mice. To evaluate the long term effects on organ weight, three mice from PBS and TE-treated groups were euthanized on day 36 (32 days after treatment ended). Spleens from control mice weighed 124±2 mg versus 127±1 mg in TE-treated mice. Thus, the TE-induced splenomegaly is reversible when the treatment is stopped; sub fraction 1.1 does not induce spleen enlargement.
Table 2. Effect of TE on body and organ weight.
Normal mice were treated i.p. with PBS, 400µg TE or 20µg sub fraction 1.1 for 4 days. On day 5, mice were euthanized and organ wet weight was determined. Body weight was assessed on day 1 and day 5 and the difference recorded as body weight gain. Organ weights compared to PBS-treated mice and P values determined by Student’s t-test.
| Body weight gain (mg) |
Spleen (mg) |
Liver (mg) |
Kidney (mg) |
Heart (mg) |
Lung (mg) |
|
|---|---|---|---|---|---|---|
| PBS (n=3) |
73±10 | 94±6 | 1000+45 | 293±7 | 137±9 | 158±4 |
| Sub fraction 1.1 (n=3) |
89±17 | 121±9 | 1159±67 | 293±10 | 134±5 | 163±12 |
| Stock TE (n=3) |
78±17 | 183±12 p=0.006 |
1553±116 p=0.03 |
345±22 | 144±10 | 206±16 |
Values are: Mean±SE
Discussion
Taro (Colocasia esculenta) is a tropical plant that is a major dietary staple in many regions of Asia and Africa. Taro corms are very high in starch, and are a good source of dietary fiber. Raw taro contains sodium, carbohydrate, dietary fiber, sugars, protein, vitamins and minerals [24]. Some early studies suggested that poi, a pasty starch made from cooked, mashed corm of the taro plant might be useful for the treatment of allergies, failure-to-thrive in infants and certain gastrointestinal conditions [25]. Fiber derived from taro could adsorb the mutagens 1,8-dinitropyrene [26]. A soluble extract of cooked taro (poi) has previously been shown by Brown et al [13] to inhibit proliferation of the rat YYT colon cancer cell line in vitro but potential anti-cancer and antimetastatic activities of taro have not been investigated in vivo. Our study demonstrates for the first time that a water soluble extract of uncooked taro corm has potent antimetastatic activity in a murine model of metastatic breast cancer. Using two highly metastatic, ER, PR and Her-2/neu negative murine mammary tumor cell lines, we showed that TE treatment leads to nearly complete ablation of metastasis in a lung colonization model. In a more clinically relevant model, TE treatment was initiated after mammary tumors were established in mice. Significant inhibition of spontaneous metastases to the lung was observed in TE-treated mice. Thus TE is efficacious in both prevention and therapeutic models.
We sought the relevant mechanisms underlying the therapeutic effect of TE. TE modestly inhibited the proliferation of some murine and human breast and prostate cancer cell lines. These findings are somewhat similar to those of Brown et al [13]. We observed that the antiproliferative effect is reversible and is accompanied by morphologic changes; TE-treated cells were rounded and displayed fewer cellular extensions. Observations that some but not all cell lines are affected by TE argue against a non-selective toxicity. The modest effects on proliferation in vitro were not reflected in any demonstrable inhibition of the growth of subcutaneously implanted tumors. Furthermore, fraction 1 showed very little antiproliferative activity but significant antimetastatic activity comparable to stock TE suggesting that antiproliferative and antimetastatic activities are not by the same mechanism. Given the potent antimetastatic activity of TE, we were somewhat surprised that the growth of the mammary-gland implanted tumor was not reduced. It may be that higher doses are required to observe an effect on local growth. It is difficult to obtain sufficient SF-1.1 to determine if this fraction affects local tumor growth and this question must wait until recombinant proteins can be produced and evaluated. The current data suggests, however, that TE may specifically target mechanisms underlying tumor dissemination. The complete inhibition of tumor cell migration by TE is consistent with this hypothesis.
It is well established that there is a direct positive correlation between elevated level of prostaglandin synthesis and COX-2 expression with increased tumorigenicity as well as metastasis [8–10]. In this study we have shown that TE treatment inhibits PGE2 synthesis as well as COX-1 and COX-2 enzyme expression in vitro. The majority of PGE2 released by these cells is generated by the COX-2 isoform and is inhibitable with selective COX-2 inhibitors [10]. Future studies will explore the mechanisms by which TE downregulates COX mRNA.
Further purification of fraction 1 by anion exchange chromatography and RPLC indicate that potent anti-metastatic activity resides in sub fraction 1.1. Sub fraction 1.1 from IEX also showed one major peak but SDS PAGE, rpHPLC and Edman degradation analysis of the sub fraction 1.1 showed the presence of two peptides. It is possible that the native active protein(s) of similar size and sequence dissociated into two polypeptide chains. Two polypeptides were identified in sub fraction 1.1 in nearly equimolar amounts. Since Edman degradation requires a free amino-terminus for coupling of phenylisothiocyanate, proteins with a chemically blocked N-terminus will be refractory to this sequence analysis. Therefore, the presence of additional proteins with blocked N-termini cannot be ruled out based on this analysis. However, upon further analysis of sub fraction 1.1 by rpHPLC, only two peaks were observed. Peak-I yielded a single amino acid sequence that upon a BLAST search of the nr database showed an N-terminal sequence identical to two entries: tarin (accession CAA53717) and lectin (accession ABQ32294). Both of these database entries have identical amino acid sequences until position 142 after which their amino acid sequences begin to diverge significantly toward the C-termini. Since additional characterization was not performed, it is not clear whether one or both proteins are present in Peak-I.
The sequence of the second major protein present in the IEX fractions was deduced by subtracting the sequence obtained from Peak-I from the previous sequence data containing the sequences of both proteins. The remaining sequence, when searched against the same nr database, showed sequence identity with 12kD storage protein (accession BAA03722). The 12kD storage protein is a mixture of two polypeptide chains (12.5 kD and 13.9 kD) and the N-terminal sequence of the sample protein aligned to the 13.9 kD polypeptide of the 12kD storage protein sequence beginning at position 144, which is the N-terminus of the 13.9 kD popypeptide chain. The N-terminal chain of the 12kD storage protein shares a high degree of sequence identity with that of the other mannose binding lectins identified in this study. The presence of the N-terminal chain can be distinguished from the other two mannose binding lectins by the PTH-amino acids observed in cycles thirteen and fifteen. A glutamic acid residue occupies these positions in the 12kD storage protein whereas asparagine and aspartic acid residues, respectively, occupy these positions in both tarin and lectin. Since there were only background levels of PTH-glutamic acid in these cycles it was concluded that the N-terminal chain of 12kD storage protein (which belongs to the 12.5 kD polypeptide chain) was either absent or below the level of detection. This sequence was also identical to that of the above database entry, lectin (accession ABQ32294) beginning at position 140. However, lectin is not known to be processed into two chains as is the 12kD storage protein. Considering the results from SEC, IEX, rpHPLC, SDS PAGE and amino acid sequencing, we conclude that the active compound may be tarin, lectin or 12 kD storage protein, however, a novel protein cannot be excluded.
Mice treated with TE appear healthy and have normal weight gain with the exception that TE caused splenomegaly which effect was fully reversible once treatment was stopped. Stock TE also increased liver weight. Histologic examination by a Clinical Pathologist (F. Z.) revealed no tissue abnormalities with the exception of B cell proliferation in the spleens of TE-treated mice. In contrast, sub fraction 1.1 induced no significant increase in whole body or individual organ weight indicating that the cause of this potential toxicity is absent from sub fraction 1.1.
We continue to explore the mechanisms underlying these promising results. Storage proteins have multiple functions in addition to their storage roles [23]. It will be important to determine if recombinant proteins, based on the predicted taro-related proteins, can replicate these observations. Few treatment options are available for metastatic breast cancer. This study identifies a new class of plant-derived compound(s) with potential antimetastatic activity which may prove useful for the therapy of metastatic disease.
Acknowledgements
This work was supported by Public Health Service grant RO1 CA120278 to A.M.F. from the National Institutes of Health and Human Services. We thank the Biostatistics, Confocal microscopy and Genomics shared services of the University of Maryland Greenebaum Cancer Center and the Protein Analysis Laboratory of the Center for Vascular and Inflammatory Diseases, University of Maryland School of Medicine.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelloff GJ, Crowell JA, Steel VE, Lubet RA, Malone WA, Boone CW, et al. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 3.Liu RH. Health benefits of fruit and vegetables and from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517s–520s. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 4.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 5.Milner JA. Molecular targets for bioactive food components. J Nutr. 2004;134:2492s–2498s. doi: 10.1093/jn/134.9.2492S. [DOI] [PubMed] [Google Scholar]
- 6.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104(1):339–356. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 7.Stoner GD, Chen T, Kresty LA, Aziz RM, Reinemann T. Protection against esophageal cancer in rodents with lyophilized berries: Potential mechanism. Nutr Cancer. 2006;54:33–46. doi: 10.1207/s15327914nc5401_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenasis (part I) J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 9.Ristimaki A, Sivula A, Lundin M, Salminen T, Haglund C, Joensuu H, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 10.Kundu N, Yang Q, Dorsey R, Fulton AM. Increased cyclooxygenase-2 (COX-2) expression and activity in a murine model of metastatic breast cancer. Int J Cancer. 2001;93:681–686. doi: 10.1002/ijc.1397. [DOI] [PubMed] [Google Scholar]
- 11.Kundu N, Smyth MJ, Samsel L, Fulton AM. Cyclooxygenase inhibitors block cell growth, increase ceramide and inhibit cell cycle. Breast Cancer Research and Treatment. 2002;76:57–64. doi: 10.1023/a:1020224503335. [DOI] [PubMed] [Google Scholar]
- 12.Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002;62:2343–2346. [PubMed] [Google Scholar]
- 13.Brown AC, Reitzenstein JE, Liu J, Jadus MR. The anti cancer effects of poi (Colocasia esculenta) on Colonic adenocarcinoma cells In Vitro . Phytother Res. 2005;19:767–771. doi: 10.1002/ptr.1712. [DOI] [PubMed] [Google Scholar]
- 14.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller FR, Miller BE, Heppner GH. Characterization of metastatic heterogeneity among subpopulations of a single mouse mammary tumor: heterogeneity in phenotypic stability. Invasion Metastasis. 1983;3:22–31. [PubMed] [Google Scholar]
- 16.Lu Y, Zhou H, Chen W, Zhang Y, Hamburger AW. The ErbB3 binding protein EBP1 regulates ErbB2 protein levels and tamoxifen sensitivity in breast cancer cells. Breast Cancer Res Treat. 2011;126:27–36. doi: 10.1007/s10549-010-0873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 18.Balzer EM, Whipple RA, Cho EH, Matrone MA, Martin SS. Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells. Breast Cancer Res Treat. 2010;121:65–78. doi: 10.1007/s10549-009-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DI, Sumbilla C, Lee M, Natesavelalar C, Klein MG, et al. Mechanisms of resistance and adaptation to thapsigargin in androgen-independent prostate cancer PC3 and DU145 cells. Arch Biochem Biophys. 2007;464(1):19–27. doi: 10.1016/j.abb.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Tang Y, Hamburger AW, Wang L, Khan MA, Hussain A. Int J Clin Exp Pathol. 2010;3(2):128–138. [PMC free article] [PubMed] [Google Scholar]
- 21.Kundu N, Zhang S, Fulton AM. Sublethal oxidative stress inhibits tumor cell adhesion and enhances experimental metastasis of murine mammary carcinoma. Clin Exp Metastasis. 1995;13:16–22. doi: 10.1007/BF00144014. [DOI] [PubMed] [Google Scholar]
- 22.Hirai M, Nakamura K, Imai T, Sato T. cDNAs encoding for storage proteins in the tubers of taro (Colocasia esculenta Schott) Jpn J Genet. 1993;68:229–236. doi: 10.1266/jjg.68.229. [DOI] [PubMed] [Google Scholar]
- 23.Bezerra IC, Csatro LAB, Neshich G, de Almeida ERP, Grossi de Sa’ MF, et al. A corm-specific gene encodes tarin, a major globulin of taro (Colocasia esculenta L. Schott) Plant Mol Biol. 1995;28:137–144. doi: 10.1007/BF00042045. [DOI] [PubMed] [Google Scholar]
- 24.Huang AS, Titchenal CA, Meilleur BA. Nutrient composition of taro corms and breadfruit. Journal of Food Composition and Analysis. 2001;13:859–64. [Google Scholar]
- 25.Brown AC, Valiere A. The medicinal uses of poi. Nutr Clin Care. 2004;7:69–74. [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson LR, Roberton AM, Mckenzie RJ, Watson ME, Harris PJ. Adsorption of a hydrophobic mutagen to dietary fiber from taro (Colocasia esculenta), an important food plant of the South Pacific. Nutr Cancer. 1992;17:85–95. doi: 10.1080/01635589209514175. [DOI] [PubMed] [Google Scholar]