Abstract
Reinforcement magnitude modulates the effects of the antidepressants fluvoxamine and desipramine in the pigeon. Increasing reinforcement magnitude diminishes the rate-dependent effects of these drugs. Whether this is also the case in other species is unknown. Rats were trained to respond under a multiple fixed-interval (FI 300-s) schedule of reinforcement. In one FI component, rats earned 2 food pellets, and in the other component they earned 10 food pellets when they completed the FI requirement. The effects of fluvoxamine (3, 5.6, 10, and 17.8 mg/kg) or desipramine (1, 3, 5.6, 10, 30 mg/kg) given 30-min, presession (i.p.) on overall response rate were examined. Local rates of responding (during each tenth of the component) increased throughout the FI as is typical, and were higher during the component reinforced with 10-pellets. Fluvoxamine and desipramine decreased overall response rates similarly in both components. Both drugs exerted limited rate dependent effects, demonstrated by a negative slope for the regression of LOG[drug rate/control rate] on LOG[control rate] using data from each tenth of the FI. However, the slope for the 2-pellet condition was significantly steeper than the slope for the 10-pellet condition following 3 and 10 mg/kg fluvoxamine and following 30 mg/kg desipramine. This result is consistent with those obtained in pigeons and demonstrates that reinforcement magnitude can modulate rate-dependent effects of fluvoxamine and perhaps desipramine in rats.
Keywords: behavioral momentum, rate-dependent, antidpressant
Introduction
Previous results from our laboratory showed that under certain conditions in pigeons, the antidepressant drugs fluvoxamine and desipramine exert rate-dependent effects which are modulated by reinforcement magnitude. Specifically, as reinforcement magnitude increases, the rate-dependent effects of fluvoxamine and desipramine decreased. Rate-dependent drug effects have been reported for a wide variety of drugs across several different species. Although some antidepressants (particularly those targeting the norepinephrine transporter) appear to exert rate-dependent effects in pigeons, such effects are generally not seen in other species, especially rats. The magnitude of reinforcement has been shown to influence disruption of behavior by extinction or prefeeding, however studies examining whether reinforcement magnitude can similarly influence disruption by drug treatments have shown mixed results. These observations suggest that reinforcement magnitude dependent modulation of rate-dependent effects of fluvoxamine and desipramine in pigeons might have limited generality. Alternatively, previous failures to observe rate-dependent effects of antidepressants, particularly in rats, could be due to conditions under which reinforcement magnitude was large enough to eliminate rate-dependent effects.
Work performed with pigeons demonstrated that reinforcement magnitude can modulate the rate-dependent effects of fluvoxamine and desipramine (Lamb and Ginsburg, 2008). In that study, pigeons responded under a multiple fixed-interval schedule reinforced by 2-, 4-, or 8-sec access to food. Generally, as fluvoxamine or desipramine dose increased, so did rate-dependent effects. However, rate-dependent effects were diminished by increasing the magnitude (access period) of grain presentation. While this result could influence the interpretation of many experiments involving disruption of behavior by drug treatments, the generality of reinforcement magnitude modulation of rate-dependent effects remains unclear across different drugs and species.
Rate-dependent effects appear to generalize across many (but not all) drug classes as well as across species (Dews, 1958; Kelleher and Morse, 1968; Dews and Wenger, 1977). Typically, rate-dependent effects are observed by comparing the rate of responding following drug treatment with the local control rate of responding across consecutive tenths of a fixed-interval. When analyzed in this way, differing drug effects are seen on low versus high local response rates (Kelleher and Morse, 1968). Specifically, low rates rates of responding are decreased less (or increased more) than high rates of responding.
While rate-dependent drug effects are most prominent for drugs such as amphetamine, many other classes of drugs have been shown to exert rate-dependent effects (Dews and Wenger, 1977). Rate-dependent effects of antidepressants are sometimes observed in pigeons (Zucarrelli and Barrett, 1980; Leander and Carter, 1984; Lamb and McMillan, 1989; Lamb and Ginsburg, 2008), but have not been reported in rats (Rastogi and McMillan, 1985; Lamb and McMillan, 1989). However, the results of the study by Lamb and Ginsburg (2008) might suggest that reinforcement magnitude conditions can influence whether rate-dependent effects are observable. If reinforcement magnitude is too great, rate-dependent effects of antidepressants might not be observable.
One other study designed to examine whether reinforcement magnitude can influence antidepressant effects on behavior yielded mixed results. Harper (1999) showed that the antidepressant fluoxetine increased a relatively high rate of responding maintained by a relatively low reinforcement magnitude, but had no effect on a lower rate of responding maintained by a higher reinforcement magnitude. The author considered this result to be consistent with the concept of behavioral momentum as proposed by Nevin and Grace (2000). Behavioral momentum posits that behavior maintained by higher reinforcement magnitude is more resistant to disruption. This effect has been demonstrated in cases where responding was disrupted by prefeeding or extinction (Grace and Nevin, 2000). Despite the result reported by Harper (1999), whether disruption of responding by antidepressant treatment is also affected by reinforcement magnitude remains unclear.
The goal of the present study was to assess whether the antidepressants fluvoxamine and desipramine exert rate-dependent effects that are modulated by reinforcement magnitude in rats responding under similar conditions to those used with pigeons by Lamb and Ginsburg (2008). Rats were trained to respond under a multiple fixed-interval schedule, with responding reinforced by either two or ten food pellets in the two components. Effects of different doses of each drug on response rate over consecutive tenths of the fixed-interval were compared with the control rate of responding, to examine potential rate-dependent effects during each component.
Methods
Subjects
Male Lewis rats (Harlan, Inc. Indianapolis, IN) weighing 275g upon arrival were fed and watered ad libitum and weighed daily until their weight was 325g. Rats were then placed on a food restricted diet to maintain body weights of 325–335g by feeding each approximately 10–12g of rat chow daily in addition to food earned in the experimental session. Subjects were maintained under a 14/10 hour light dark cycle, and experiments were conducted in the light phase. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. Eight rats were used in fluvoxamine studies. Due to the death of one of these eight rats during desipramine experiments, only seven rats were included in desipramine studies.
Apparatus
All studies were conducted in commercially available test chambers (MedAssociates, Georgia, VT; Standard Rat Test Chamber). The chambers were equipped with two levers on (the left and right side of) one wall, and had stimulus lights located above them. Chambers were also equipped with a house light above the chamber, and a speaker connected to a tone generator for white noise. Food was delivered into a hopper located between the two levers. Test chambers were enclosed in a light and sound attenuating chamber (MedAssociates, Georgia, VT; ENV-022MD) equipped with an exhaust fan. Experimental contingencies were controlled by and data were collected with a computer running MedPC software (MedAssociates, Georgia, VT; MedPC Version IV).
Procedure
Rats were initially trained to respond on each lever for food pellet deliveries, in the presence of the associated stimulus light during separate components under a multiple schedule. Once rats reliably responded for food on both levers, the response requirements for both levers were gradually increased to a fixed-ratio 10. After this behavior stabilized (standard deviation < 20% of the mean response rate for 5 consecutive days), rats were introduced to the multiple fixed-interval schedule.
Fixed-interval schedule
During one component, completion of the response requirement resulted in delivery of 2-pellets. In the other component (indicated by illumination of the light above the alternative lever), responding was reinforced by delivery of 10-pellets. The order of component presentation was randomized under a blocked design to assure equal numbers of each component (2- and 10-pellets) and no intentional clustering of the components. Each component was presented six times during each daily experimental session. The interval during each component was increased from 30-sec gradually until rats responded under a 300-sec fixed interval schedule on both levers. During the interval, the light above the active lever was illuminated, white noise was present in the chamber, but no other lights were on and responses on levers were recorded but had no programmed consequences. A 10-sec limited hold began as soon as the interval expired. The first response during the limited hold turned off the stimulus light above the lever and white noise, illuminated the house light, and resulted in delivery of either two or ten pellets, depending on which lever was active. Responses during the limited hold were not included in the analysis. One pellet was delivered each second. A 10-sec post-reinforcement timeout was imposed after the first response during the limited hold, regardless of the number of pellets earned. If the subject failed to emit a response during the limited hold, the next fixed-interval began immediately. Studies of fluvoxamine effects began after this behavior stabilized (standard deviation < 20% of the mean response rate for 5 consecutive days), and were followed by studies of desipramine.
Drugs
Fluvoxamine HCl (Solvay Pharmaceuticals, Weesp, Netherlands) and desipramine HCl (Sigma Inc, St. Louis, MO) were dissolved in 0.9% saline at concentrations of 3, 5.6, and 10 mg/ml. In addition, fluvoxamine was dissolved at a concentration of 17.8 mg/ml and desipramine was dissolved at concentrations of 1 and 30 mg/ml. Drugs were administered i.p. 30-min prior to the start of the experimental session on Tuesdays and Fridays. Saline was administered each Thursday to establish control performance. Dose effect curves were determined twice in each subject in mixed order.
Analysis
Control Response Rate
For each component, local response rates over consecutive bins were calculated.
Quarter Life
The bin in which ¼ of the total responses (quarterlife) for each component was determined (see Herrnstien and Morse, 1957).
Overall Response Rate
Overall rate across each component (2 versus 10 pellets) was averaged for each subject following saline and each dose of drug. Response rate for each dose was normalized to a percentage of the average saline control rate. Saline control rate was determined by averaging the response rates for each subject on the Thursdays of the weeks either fluvoxamine or desipramine was administered. Drug effects on responding for 2 or 10 pellets were compared using ED50.
ED50
For each drug, ED50 was calculated for responding maintained by 2- and 10-pellets. For each subject, a linear regression was performed on responding maintained by 2- or 10-pellets across log transformed doses. Only doses resulting in less than 80% or greater than 20% of control rates of responding were included in the regression, unless the only points that defined the regression line at 50% fell outside of this range. The ED50 was estimated for each subject based on his own regression. A two-tailed, paired Student's t-test was performed to compare ED50s for each drug under 2- and 10-pellet components.
Rate-dependency
Rate-dependent drug effects were assessed as described previously (Lamb and Ginsburg, 2008). Briefly, response rate during each tenth of the interval (bin) was calculated for both components (2- and 10-pellets) for each subject. Bins in which the control response rate was zero were excluded from analysis. Drug effects that resulted in a response rate of zero were also excluded from analysis. For each remaining bin, drug effects expressed as log10[Drug-rate/Control-rate × 100] were plotted against log10[Control-rate]. A linear mixed-effects regression was performed for each dose effect on responding for 2- and 10-pellets. The linear model used was: (log10(100*(Drug-Rate/Control-Rate))=log10(Control-Rate), with subject used as a within-subjects variable. The slopes for the 2- versus 10-pellet conditions were compared for each drug dose with a Student's t test using parameters (slope, standard error, degrees of freedom) derived from the linear mixed effects model. T-values that included 95% or more of the appropriate distribution were considered significant (p<0.05).
Software
ED50 calculations and t-tests were performed using OpenOffice Calc (Mandriva S.A., Paris, France; Mandriva version 2.2.1). Graphs and linear regressions were performed with R: A language and environment for statistical computing (Vienna, Austria; Version 2.5.1). Graphs were created using the gplots package, and linear mixed effects were determined with the nlme package.
Results
Control Responding
Response rates were higher for intervals resulting in delivery of 10-pellets versus 2-pellets for every subject (data not shown). Average response rates plotted by tenth of the fixed interval are shown in Fig. 1 for each component. For each rat, the quarterlife (point at which responses reach ¼ of the total) was determined to be between 50–60% of the total interval length indicative of an accelerating pattern of responding across the interval. Thus the pattern responding in each component was similar, and also similar to those expected under fixed-interval schedules (Herrnstein and Morse, 1957).
Figure 1.
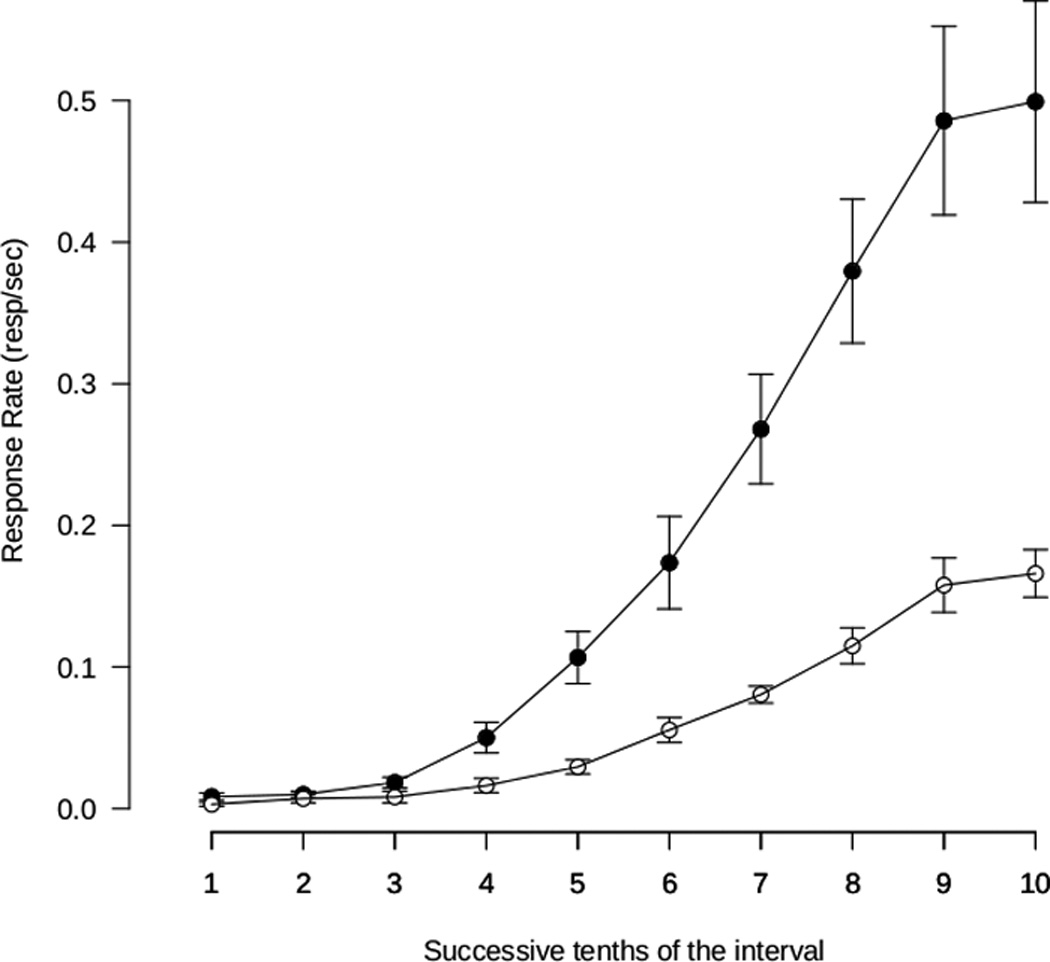
Control response rates during each tenth of the interval reinforced by 2- or 10-pellets (open and filled symbols, respectively). Points represent the mean ± S.E.M. response rate for the group during each tenth of the interval.
Overall Response Rate
The overall control rates of responding were: 0.06 ± 0.01 (mean ± SEM) responses/sec for the 2-pellet component and 0.20 ± 0.02 responses/sec for the 10-pellet component. As described for results shown in Figure 2, the ED50s for fluvoxamine effects on response rate for 2-pellets (9.9 ± 1.2 mg/kg) versus 10-pellets (8.2 ± 1.2 mg/kg) did not differ. Likewise, the ED50s for desipramine effects on response rate for 2-pellets (7.5 ± 1.3 mg/kg) versus 10-pellets (7.4 ± 1.2 mg/kg) did not differ. Thus, reinforcement magnitude did not alter the effects of fluvoxamine or desipramine on overall response rate.
Figure 2.
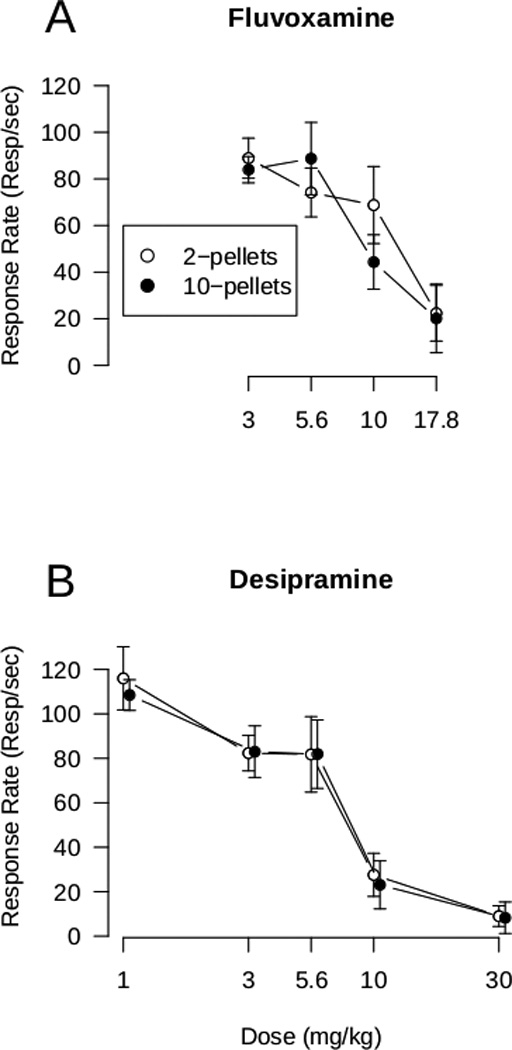
Effects of fluvoxamine (A) and desipramine (B) on overall response rate for the entire fixed-interval in which responding was reinforced by either 2- or 10-pellets. Points represent the mean ± S.E.M. for the group.
Quarter Life
No effect on quarter life was observed for either drug at any reinforcement magnitude.
Rate-dependency
Individual data points included in the analysis are shown in Figures 3 and 4. Results of linear mixed effects regressions are shown in Table 1. Rate-dependent effects were observed at every dose of fluvoxamine tested. Following a dose of 3 mg/kg fluvoxamine, the slope relating drug rate with control rate was negative under both reinforcement magnitude conditions, and the slope under the smaller reinforcement magnitude condition (2 pellets) was significantly steeper than the slope under the larger reinforcement magnitude condition (10 pellets). Following a dose of 5.6 mg/kg fluvoxamine, the slope under the smaller reinforcement condition was negative, but not significantly different from zero. The slope for the larger reinforcement magnitude condition was significantly negative, but not significantly different from the slope under the smaller reinforcement magnitude condition. At 10 mg/kg fluvoxamine, significant rate-dependent effects were seen on response rates during both reinforcement magnitude conditions, and the slope for the small reinforcement magnitude condition was significantly steeper than the slope for the large reinforcement magnitude condition. At 17.8 mg/kg, significant rate-dependent effects were seen under both reinforcement conditions though the rate-dependent effects did not depend on the reinforcement magnitude as the slope did not differ.
Figure 3.
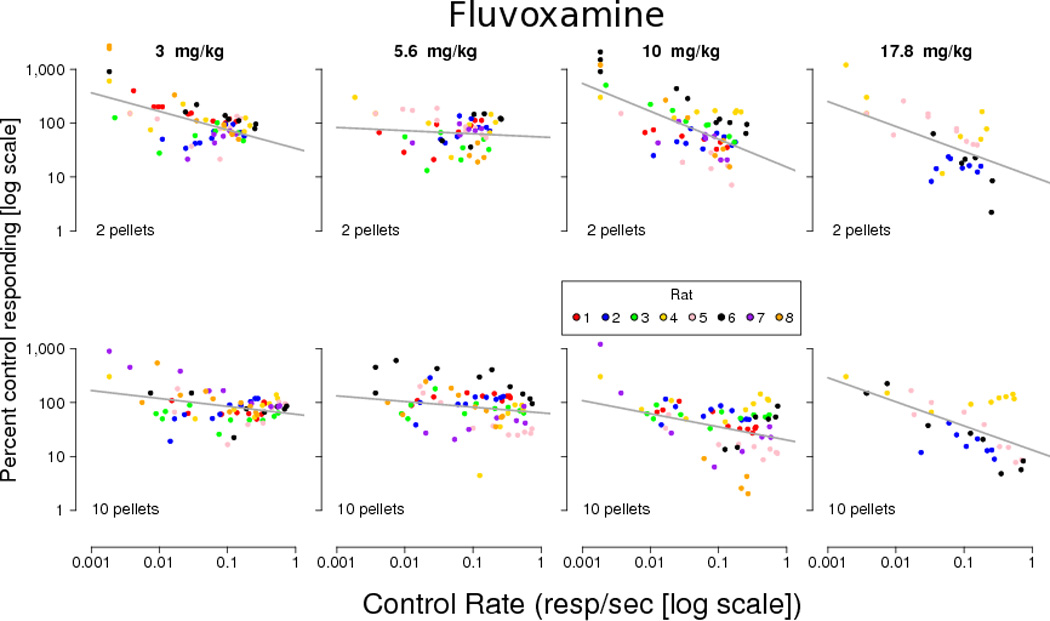
Effects of fluvoxamine on responding (as a percentage of the local control rate) for 2-pellets (upper panels) or 10-pellets (lower panels) expressed as a function of control rate. Control rate and drug rates (expressed as drug rate/control rate × 100) are log transformed. Each subject is represented by a different color. Control rates and drug effects equal to zero resp/sec were excluded from the plot and analysis. The line represents the linear mixed-effects regression for each panel.
Figure 4.
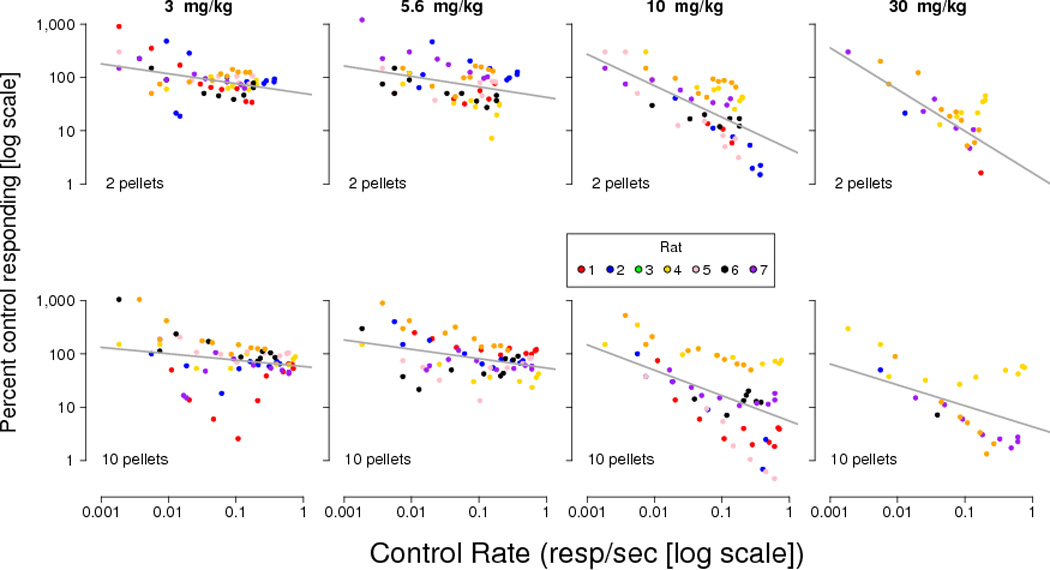
Effects of desipramine on responding (as a percentage of the local control rate) for 2-pellets (upper panels) or 10-pellets (lower panels) expressed as a function of control rate. Control rate and drug rates (expressed as drug rate/control rate × 100) are log transformed. Each subject is represented by a the same unique color used in Figure 3. Control rates and drug effects equal to zero resp/sec were excluded from the plot and analysis. The line represents the linear mixed-effects regression for each panel.
Table 1.
Regression results for fluvoxamine and desipramine dose effects on responding for 2 or 10 pellets
| Dose | Pellets | Slope | [95% confidence interval] |
Intercept | (SD) | R^2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Fluvoxamine | 3 | 2 | −0.34 | −0.45 – | −0.23 | 1.53 | (0.16) | 0.58 | |
| 3 | 10 | * | −0.15 | −0.23 – | −0.06 | 1.78 | (0.12) | 0.40 | |
| 5.6 | 2 | −0.06 | −0.19 – | 0.07 | 1.74 | (0.17) | 0.42 | ||
| 5.6 | 10 | −0.1 | −0.20 – | −0.01 | 1.81 | (0.22) | 0.52 | ||
| 10 | 2 | −0.52 | −0.61 – | −0.42 | 1.18 | (0.31) | 0.82 | ||
| 10 | 10 | * | −0.24 | −0.35 – | −0.14 | 1.31 | (0.37) | 0.67 | |
| 17.8 | 2 | −0.46 | −0.67 – | −0.26 | 1.01 | (0.31) | 0.71 | ||
| 17.8 | 10 | −0.45 | −0.57 – | −0.32 | 1.12 | (0.34) | 0.75 | ||
| Desipramine | 1 | 2 | −0.10 | −0.20 – | 0.01 | 1.92 | (0.18) | 0.47 | |
| 1 | 10 | −0.14 | −0.23 – | −0.05 | 1.91 | (0.11) | 0.33 | ||
| 3 | 2 | −0.18 | −0.29 – | −0.08 | 1.70 | (0.00) | 0.17 | ||
| 3 | 10 | −0.12 | −0.22 – | −0.02 | 1.76 | (0.26) | 0.54 | ||
| 5.6 | 2 | −0.20 | −0.29 – | −0.10 | 1.63 | (0.25) | 0.64 | ||
| 5.6 | 10 | −0.18 | −0.25 – | −0.10 | 1.73 | (0.23) | 0.66 | ||
| 10 | 2 | −0.59 | −0.71 – | −0.47 | 0.66 | (0.33) | 0.84 | ||
| 10 | 10 | −0.48 | −0.58 – | −0.37 | 0.74 | (0.54) | 0.86 | ||
| 30 | 2 | −0.78 | −1.02 – | −0.54 | 0.21 | (0.30) | 0.74 | ||
| 30 | 10 | * | −0.39 | −0.57 – | −0.22 | 0.63 | (0.39) | 0.80 | |
BOLD denotes slope<0, p<0.05
denotes 2-pellet slope significantly different from 10-pellet slope, p<0.05
For desipramine, rate dependent effects were seen following administration of every dose tested. Following a dose of 1 mg/kg, negative slopes were observed under both reinforcement magnitude conditions, though the slope under the smaller reinforcement magnitude condition was not different from zero. For all other doses and conditions, significantly negative slopes (less than zero) were observed. Following 30 mg/kg, the slope during the 2-pellet component differed from the slope during the 10-pellet component.
Discussion
Results of the present study provide limited evidence that reinforcement magnitude modulates rate-dependent effects of antidepressants in rats. Rate-dependent effects of fluvoxamine were apparent following every dose tested. Increasing the reinforcement magnitude diminished the rate-dependent effects of 3 and 10 mg/kg fluvoxamine. Rate-dependent effects of desipramine were also observed at every dose tested, however reinforcement magnitude only influenced rate-dependent effects of 30 mg/kg desipramine.
Reinforcement magnitude modulation of the rate-dependent effects of fluvoxamine is consistent with results in pigeons (Lamb and Ginsburg, 2008) and rats (Harper, 1999). In pigeons, rate-dependent effects of fluvoxamine diminished with increasing lengths of access to grain under a similar multiple fixed-interval schedule to the one used in the present study (Lamb and Ginsburg, 2008). In rats, Harper (1999) reported that fluoxetine (which selectively inhibits serotonin reuptake like fluvoxamine) increased the response rate for the low frequency reinforcement at a dose that had no effect on responding for the higher frequency reinforcement. The authors conclude that this effect is consistent with the notion that reinforcement magnitude confers increased resistance to disruption of responding by the drug.
In the present study, rate-dependent effects of desipramine were affected by reinforcement magnitude following 30 mg/kg. However, this dose virtually eliminated responding in all but three rats. Therefore, this result is based on only the rats that are less sensitive to the rate-decreasing effects of desipramine, and may represent an effect that is only present in this subset of rats. Following a dose of desipramine (10 mg/kg) that decreases, but does not eliminate responding for food, reinforcement magnitude does not alter rate-dependent effects of desipramine, even in this subset of rats (p<0.17).
Others have failed to clearly demonstrate rate-dependent effects of antidepressants that preferentially target norepinephrine reuptake (such as desipramine) in rats (Rastogi and McMillan, 1985; Lamb and McMillan, 1989). In the present study, rate-dependent effects were modest, as evidenced by the demonstration of rate-dependent effects with the more statistically powerful regression technique, but not with drug-dependent changes in quarterlife values. In one study, although desipramine did not change the quarterlife of FI responding in rats at any dose, greater decreases in responding occurred during the second half of the FI as compared with the first half, especially at a dose of 30 µM/kg, or approximately 8 mg/kg (Lamb and McMillan, 1989). No rate-increasing effects of desipramine were reported by Lamb and McMillan (1989), consistent with other studies utilizing differential reinforcement of low rates of responding schedules (Britton and Koob, 1989; Richards and Seiden, 1991). The results of the present study are generally in agreement with previous reports indicating that desipramine decreases high rates of responding to a greater extent than low rates, but does not increase low rates of responding.
Considering the results of the present study, the lack of robust reinforcement magnitude modulation of rate-dependent effects of desipramine, and the limited modulation of rate-dependent effects of fluvoxamine could be due to limited rate-dependent effects of antidepressants in rats. Thus, studies utilizing drugs such as amphetamine which have robust rate-dependent effects across a range of doses in both pigeons and rats will help further characterize the generality and range of reinforcement magnitude modulation of rate-dependent effects.
Zucarrelli and Barrett (1980) showed results in pigeons that could be interpreted as diminishing rate-dependent effects of amphetamine, cocaine, and perhaps imipramine as the FI decreased (effectively increasing the reinforcement density). However, this pattern was not apparent for rate-dependent effects of pentobarbital which suggests that different drug classes may be more susceptible to reinforcement magnitude modulation of rate-dependent effects. Whether similar effects are seen in rats has not been reported.
In conclusion, rate-dependent effects of 10 mg/kg fluvoxamine and 30 mg/kg desipramine were diminished by increasing the reinforcement magnitude in rats responding under a multiple FI schedule. This result is consistent with a similar study conducted in pigeons (Lamb and Ginsburg, 2008), though the effect was more limited. In contrast, rate-dependent effects of desipramine did not depend on reinforcement magnitude, perhaps a result of reinforcement magnitude of even the smallest condition (2-pellets) being too large. However, neither drug produced pronounced rate-dependent effects. These results suggest that reinforcement magnitude can modulate rate-dependent effects in rats, however additional studies are necessary to establish the generality of this effect.
References
- Britton KT, Koob GF. Effects of corticotropin releasing factor, desipramine and haloperidol on a DRL schedule of reinforcement. Pharmacology Biochemistry and Behavior. 1989;4:967–970. doi: 10.1016/0091-3057(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Dews PB. Studies on behavior IV: Stimulant actions of methamphetamine. Journal of Pharmacology and Experimental Therapeutics. 1958;122:137–147. [PubMed] [Google Scholar]
- Dews PB, Wenger GR. Rate-dependency of the behavioral effects of amphetamine. In: Thompson T, Dews PD, editors. Advances in behavioral pharmacology. New York: Academic; 1977. pp. 167–227. [Google Scholar]
- Grace RC, Nevin JA. Response strength and temporal control in fixed-interval schedules. Animal Learning and Behavior. 2000;28:313–331. [Google Scholar]
- Harper DN. Drug-induced changes in responding are dependent on basline stimulus-reinforcer contingencies. Psychobiology. 1999;27:95–104. [Google Scholar]
- Herrnstein RJ, Morse WH. Effects of pentobarbital on intermittently reinforced behavior. Science. 1957;127:929–931. doi: 10.1126/science.125.3254.929-a. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, McMillan DE. Some effects of desmethylimipramine and amitriptyline on the schedule-controlled behavior of pigeons and rats. Arch Int Pharmacodyn Ther. 1989;302:49–67. [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC. Fluvoxamine and desipramine on fixed-ratio responding: effects of reinforcement magnitude. Behav Pharmacol. 2005;16:573–578. doi: 10.1097/01.fbp.0000181594.01244.a2. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC. Reinforcement magnitude modulates the rate-dependent effects of fluvoxamine and desipramine on fixed-interval responding in the pigeon. Behav Pharmacol. 2008;19:51–60. doi: 10.1097/FBP.0b013e3282f3d093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander JD, Carter RB. Effects of norepinephrine and serotonin uptake inhibitors on the schedule-controlled behavior of the pigeon. Pharmacol Biochem Behav. 1984;20:391–395. doi: 10.1016/0091-3057(84)90277-6. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Grace RC. Behavioral momentum and the law of effect. Behav Brain Res. 2000;23:73–90. doi: 10.1017/s0140525x00002405. discussion 90–130. [DOI] [PubMed] [Google Scholar]
- Rastogi SK, McMillan DE. Effects of some typical and atypical antidepressants on schedule-controlled responding in rats. Drug Dev Res. 1985;5:243–250. [Google Scholar]
- Richards JB, Seiden LS. A quantitative interresponse-time analysis of DRL performance differentiates similar effects of the antidepressant desipramine and the novel anxiolytic gepirone. J Exp Anal Behav. 1991;56:173–192. doi: 10.1901/jeab.1991.56-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccarelli RR, Barrett JE. A comparison of the effects of d-amphetamine, cocaine, imipramine and pentobarbital on local and overall rates of responding maintained under a four-component multiple fixed-interval schedule. Pharmacol Biochem Behav. 1980;12:899–907. doi: 10.1016/0091-3057(80)90451-7. [DOI] [PubMed] [Google Scholar]


