Abstract
Noni has been used in traditional medicine and as food for thousands of years. While the fruits serve as food and internal medicine, leaves were traditionally used only topically. In recent years, concern regarding the possible content of anthraquinones in noni has led to scrutiny by the European Food Safety Authority. Little research existed on the content of anthraquinones in different noni preparations, with no information about the potential effect of harvest and preparation methods. Our research focused on lucidin, alizarin, and rubiadin, the most important anthraquinones from a health perspective. We found that the production process (fermentation/juice production versus drying/lyophilization) has no effect on the anthraquinone content. The source product, however, does have implications: noni fruit puree from which seeds had been removed as well as consumer products produced from such puree had no detectable amounts of any anthraquinones. Products that did contain seed or leaf material in all cases did contain partly significant amounts of anthraquinones. To alleviate safety concerns, we suggest that noni products, whether fermented or unfermented juice or powder, should be derived only from fully ripe noni fruits, and that any seed material needs to be removed during the production process.
1. Introduction
Noni (Morinda citrifolia L., Rubiaceae) probably originated in the Indonesian archipelago and was widely distributed during the Polynesian migration as one of the important “canoe plants,” finally reaching French Polynesia and Hawai'i. Traditionally, the fruits were used as food a treatment for and intestinal problems, while the leaves served for the treatment of wound infections, arthritis, swellings, and similar conditions [1, 2]. Recent research indicated anti-inflammatory and antioxidant properties [3, 4]. During the last decade, noni, mostly marketed as a fermented juice, has become a widely traded food supplement worldwide, based on health claims related to some of its compounds, in particular flavonoids [5–7].
Starting in 2005, some reports on the hepatotoxicity of noni preparations raised health concerns [8, 9] and led the European Food Safety Authority to conduct further research. A conclusion of this work was that the regular intake of noni juice would most likely not cause any toxic effects [10]. Analyses sponsored by Tahitian Noni, the main global provider of noni juice, reported no toxicity from consumption of the product [11–13]. The main health concerns were based on the possible content of carcinogenic anthraquinones, in particular alizarin, rubiadin, and lucidin in noni. Anthraquinone and its derivatives are common aromatic compounds in plant pigments and are used to make dyes and paper, as well as bird repellants. The US National Toxicology Program investigations concluded that anthraquinones caused cancer of the kidney, urinary bladder, liver, and thyroid in rats and mice [14]. Comparative studies reported the presence of these compounds in madder roots (Rubia tinctorum, Rubiaceae) and animal models led to the conclusion that these compounds could possibly have genotoxic and carcinogenic effects [15, 16]. The same compounds were reported from the wood [17, 18], stems [19], and roots [7, 20–22] of M. citrifolia. Smaller amounts were reported from flowers [23], leaves [24], and to some extent from fruits [23, 25–27]. However, no studies had attempted to quantify anthraquinones in noni preparations until Deng et al. [6] developed a method to elucidate the anthraquinone content of noni based on noni pulp samples from Tahitian Noni and some noni leaf tea products. None of the tested materials contained anthraquinones in higher amounts. However, these studies did not provide any indication as to under which production conditions plant material in commerce might in fact contain higher anthraquinone amounts, and if the removal of certain plant compounds from preparations might lower the possible anthraquinone content.
The present study attempted to remedy this situation by examining the real content of anthraquinones in different noni preparations and to include information about the potential effect of harvest and preparation methods. Our research focused on alizarin, lucidin, and rubiadin, the most important anthraquinones from a health perspective, and used a variety of different preparations (fermented and unfermented; juice and powders; with and without seeds, leaves, and leaf fragments).
2. Materials and Methods
2.1. Materials for Sample Preparation
For high-performance liquid chromatography (HPLC) analysis and liquid chromatography-mass spectrometry (LC-MS), fresh plant material was wild collected in Honolulu (O'ahu) and Kalapana (Hawai'i) and identified by researchers at the William L. Brown Center at the Missouri Botanical Garden. Samples for commercially sold noni preparations were supplied by Arogya Inc., Honolulu. Lucidin and rubiadin (pro analysi—analysis grade) were obtained from Cfm Oskar Tropitzsch e.K., Marktredwitz, Germany. Reference chromatograms and ultraviolet (UV) spectra for lucidin, rubiadin, and anthraquinone were established.
2.2. Sample Preparation
2.2.1. HPLC Analysis
Plant Material. Seedless Arogya Noni (noni fruit pulp, freeze dried, with no seeds, and no skin parts), 2 samples of 0.5 g. Subsequently addition of 20 ml ethylacetate (HPLC grade), 30 min. agitation, and filtration of solids (filter paper soaked ethylacetate). Reduction to dryness in SPE-chamber and reconstitution of product in 500 ml methanol, filtration (45 min), folowed by induction in HPLC [6]. HPLC was conducted with a Macherey-Nagel, Nucleodur C18 Pyramid, 5 mcl column, with 0.1% trifluoroacetic acid (p.a. HPLC gradient grade) as solvent A and acetonitrile (p.a. HPLC gradient grade) as solvent B at 25°C. Flux velocity was set at 1.0 mL/min, detection set at 275 nm and 410 nm.
2.2.2. Liquid Chromatography-Mass Spectrometry (LC-MS)
Acetonitrile and formic acid (HPLC grade, Acros Organics), ammonium formate and tetrahydrofuran (HPLC grade, Sigma-Aldrich). Alizarin (dye content 97%) and purpurin (dye content 90%) were purchased from Sigma-Aldrich, and lucidin and rubiadin were obtained from the laboratory of natural product collection at the Donald Danforth Plant Science Center.
Dried and powdered samples (1.25 g) were stirred in 25 mL of ultrapure water for 1 h at 45°C. After cooling to room temperature, 50 mL of tetrahydrofuran-water-formic acid (1 : 1 : 0.005) was added and the mixture was stirred for an additional 30 min at room temperature. The supernatant was collected and filtered through a 0.2 mcm, 25 mm diameter PVDF membrane filter (PALL Life Sciences). Fresh serum samples (5 g) were freeze-dried with a lyophilizer, and then the same sample preparation procedure as for dried and powdered samples was applied. Fresh fruit samples (2.5 g) were freeze-dried with a lyophilizer, and ground into powder with a mortar and pestle. The same sample preparation procedure as for dried and powdered samples was then applied.
Samples were initially analyzed by HPLC at 254 nm and 280 nm. An LC-MS method (MRM-Multiple Reaction Monitoring) was developed to detect and quantify the anthraquinones in all samples. The liquid chromatography-mass spectrometry (LC-MS) system consisted of a CTC Pal autosampler (LEAP Technologies), a Shimadzu LC-20AD liquid chromatograph, and a 4000 QTRAP mass spectrometer (Applied Biosystem). Separation of (30 mcl) samples was achieved on a LiChroCART 250-4 HPLC column (Merck, 5 μm, LiChroaphor 60 RP select B) combined with a LiChroCART 4-4 HPLC cartridge (Merck, 5 μm, LiChroaphor 60 RP select B). The mobile phase total flow rate was set to 1.0 mL/min with binary gradient elution, using an ammonium formate formic acid buffer (0.2 M, pH 3) as solvent A and 90% acetonitrile as solvent B.
Compound-dependent parameters are described in Table 1. The Turbo V Ion Source (TIS) was used in negative mode and the following source parameters were used: CUR 30, CAD high, IS-4500, TEM 500, GS1 50, GS2 55, and EP-10 (Table 1).
Table 1.
Compound-dependent parameters for the LC-MS/MS method.
| Analyte | Collision energy (V) | Declustering potential (V) | Collision cell exit potential (V) | MRM transition | MRM transition |
|---|---|---|---|---|---|
| Alizarin | −40 | −80 | −10 | 239/211 | 239/167 |
| Rubiadin | −35 | −100 | −10 | 253/225 | 253/209 |
| Purpurin | −40 | −90 | −10 | 255/227 | / |
| Lucidin | −30 | −75 | −10 | 269/251 | / |
3. Results
3.1. HPLC
None of the samples contained detectable amounts of anthraquinone, lucidin, or rubiadin (Figure 1).
Figure 1.
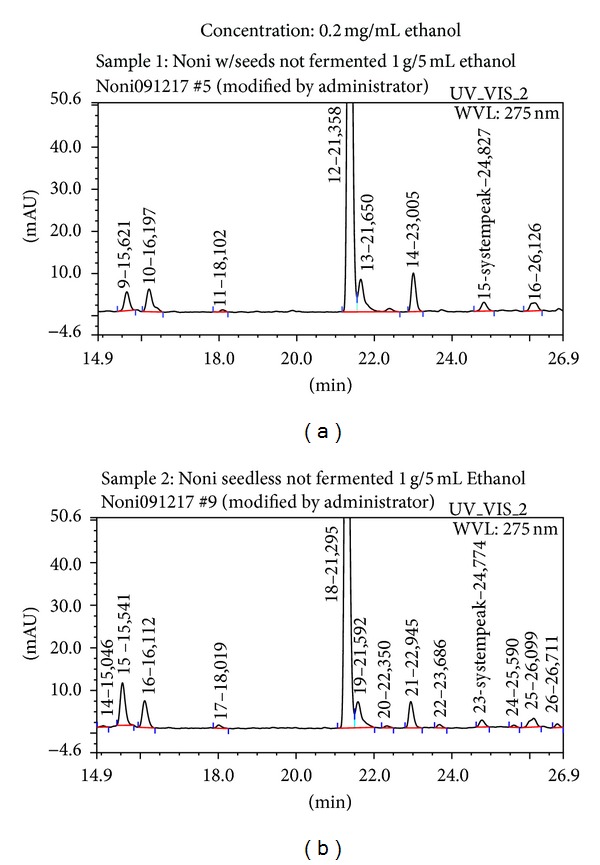
HPLC of representative noni samples. None of the samples contained detectable amounts of rubiacin or lucidin.
Additionally, HLPC studies confirmed that lucidin is not a very stable compound. It was already observed, in 1984, that the formation of lucidin ω (ω = Greek Omega) ethers could be artifacts derived from lucidin as methanol and chloroform were used as solvents for extraction, although there was no direct proof for this possibility [28]. Otherwise, authors [29] have shown in 2010 that lucidin and its derivatives can be activated in the metabolic pathway to react with the DNA [28].
Storing HPLC solution of lucidin in acetonitrile which was treated with a small amount of ethanol for better solubility, the formation of a new product was observed within several days. A retention time very similar to rubiadin was established for this compound in the chromatogram. Obviously this product is identical to the compound described as unknown anthraquinone [13]. Careful workup, isolation, and structural investigation by 1H-NMR and high resolution MS (negative mode) showed that this compound is ibericin (lucidin ethyl ether).
3.2. LC-MS/MS
The results presented in Table 2 indicate that all noni samples containing fragments of leaves or fruit skin did show traces of various anthraquinones. In clear contrast, samples that were produced under the removal of fruit skin and free of leaf material did not contain detectable amounts of anthraquinones. The inclusion of seed material did not influence the anthraquinone content.
Table 2.
Anthraquinone content in Noni samples.
| Sample ID | Lucidin 269/251 | Total alizarin mg/kg | Purpurin 255/227 | Rubiadin 253/225 | |
|---|---|---|---|---|---|
| Sample_#1 | / | / | / | / | Fresh noni seedless pulp, no skin |
| Sample_#2 | / | / | / | / | Fresh noni seeded pulp, no skin |
| Sample_#3 | / | 0.152 | / | Detectable | Fermented noni fruits |
| Sample_#4 | / | / | / | / | Noni powder, seedless, and no skin |
| Sample_#5 | / | 0.279 | / | Detectable | Ripe noni dried |
| Sample_#6 | Detectable | 0.337 | / | / | Overripe noni dried |
| Sample_#7 | / | 0.781 | / | / | Noni powder |
| Sample_#8 | Detectable | 0.334 | / | / | Noni powder (unfermented) |
| Sample_#9 | Detectable | 4.655 | / | Detectable | Noni powder (fermented) |
| Sample_#10 | Detectable | 0.365 | / | Detectable | Noni powder |
| Sample_#11 | Detectable | 7.797 | / | Detectable | Noni powder (fermented) |
| Sample_#12 | Detectable | 0.774 | / | / | Noni powder |
| Sample_#13 | Detectable | / | / | / | Noni powder |
| Sample_#14 | / | 8.612 | / | Detectable | Noni powder (Peru) |
| Sample_#15 | Detectable | 0.725 | / | Detectable | Noni powder |
| Sample_#16 | Detectable | 0.677 | / | Detectable | Noni powder |
| Sample_#17 | / | / | / | / | Juice, seedless, and no skin |
| Sample_#18 | / | / | / | / | Juice, seedless, and no skin |
| Sample_#19 | / | 0.053 | / | Detectable | Noni juice |
| Sample_#20 | / | / | / | / | Noni juice, seedless, and no skin |
| Sample_#21 | / | 0.281 | / | Detectable | Noni leaf tincture |
| Sample_#22 | / | / | / | / | Noni tonic |
| Sample_#23 | / | / | / | / | Maca/Cordia powder (comparison) |
| Sample_#24 | / | / | / | / | Chilchos coffee (for comparison) |
4. Discussion
The concern regarding the possible content of anthraquinones in noni products has led to scrutiny by the European Food Safety Authority. The present study indicates that the production process (fermentation and juice production versus drying or lyophilization) has no effect on the anthraquinone content.
Fruit ripeness as such also did not have any influence on anthraquinone content. However, it is to be noted that the removal of seeds and fruit skin from fully ripe fruits is much easier than from unripe noni. This has serious implications on the production process.
The source product, however, does have implications: noni fruit puree from which seeds and skin fragments had been removed as well as products (juice as well as capsules) produced from such puree had no detectable amounts of any anthraquinones. In contrast, products that did contain fruit skin or leaf material did contain partly significant amounts of all anthraquinones in all cases.
To alleviate potential safety concerns, we suggest that commercial noni products in the market, whether fermented or unfermented juice or powder, should be derived only from fully ripe noni fruits, and that any seed material needs to be removed during the production process.
References
- 1.McClatchey W. From Polynesian healers to health food stores: changing perspectives of Morinda citrifolia (Rubiaceae) Integrative Cancer Therapies. 2003;1(2):110–120. doi: 10.1177/1534735402001002002. [DOI] [PubMed] [Google Scholar]
- 2.Wang MY, West BJ, Jensen CJ, et al. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacologica Sinica. 2002;23(12):1127–1141. [PubMed] [Google Scholar]
- 3.Yang J, Paulino R, Janke-Stedronsky S, Abawi F. Free-radical-scavenging activity and total phenols of noni (Morinda citrifolia L.) juice and powder in processing and storage. Food Chemistry. 2007;102(1):302–308. [Google Scholar]
- 4.Palu AK, Kim AH, West BJ, Deng S, Jensen J, White L. The effects of Morinda citrifolia L. (noni) on the immune system: its molecular mechanisms of action. Journal of Ethnopharmacology. 2007;115(3):502–506. doi: 10.1016/j.jep.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Deng S, Palu AK, West BJ, Su CX, Zhou B, Jensen JC. Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. Journal of Natural Products. 2007;70(5):859–862. doi: 10.1021/np0605539. [DOI] [PubMed] [Google Scholar]
- 6.Deng S, West BJ, Jensen CJ, Basar S, Westendorf J. Development and validation of an RP-HPLC method for the analysis of anthraquinones in noni fruits and leaves. Food Chemistry. 2009;116(2):505–508. [Google Scholar]
- 7.Deng S, West BJ, Jensen CJ. Simultaneous characterisation and quantitation of flavonol glycosides and aglycones in noni leaves using a validated HPLC-UV/MS method. Food Chemistry. 2008;111(2):526–529. doi: 10.1016/j.foodchem.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Millonig G, Stadlmann S, Vogel W. Herbal hepatotoxicity: acute hepatitis caused by a Noni preparation (Morinda citrifolia) European Journal of Gastroenterology and Hepatology. 2005;17(4):445–447. doi: 10.1097/00042737-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Stadlbauer V, Fickert P, Lackner C, et al. Hepatotoxicity of NONI juice: report of two cases. World Journal of Gastroenterology. 2005;11(30):4758–4760. doi: 10.3748/wjg.v11.i30.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Food Safety Authority. Opinion on a request from the commission related to the safety of noni juice (juice of the fruits of Morinda citrifolia) The EFSA Journal. 2005;376:1–12. [Google Scholar]
- 11.West BJ, Jensen CJ, Westendorf J, White LD. A safety review of noni fruit juice. Journal of Food Science. 2006;71(8):R100–R106. [Google Scholar]
- 12.West BJ, Tani H, Palu AK, Tolson CB, Jensen CJ. Safety tests and antinutrient analyses of noni (Morinda citrifolia L.) leaf. Journal of the Science of Food and Agriculture. 2007;87(14):2583–2588. doi: 10.1002/jsfa.3007. [DOI] [PubMed] [Google Scholar]
- 13.Westendorf J, Effenberger K, Iznaguen H, Basar S. Toxicological and analytical investigations of noni (Morinda citrifolia) fruit juice. Journal of Agricultural and Food Chemistry. 2007;55(2):529–537. doi: 10.1021/jf062130i. [DOI] [PubMed] [Google Scholar]
- 14.National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of anthraquinone (CAS No. 84-65-1) in F344/N rats and B6C3F1 mice (feed studies) National Toxicology Program Technical Report Series. 2005;494:1–358. [PubMed] [Google Scholar]
- 15.Westendorf J, Poginsky B, Marquardt H, Groth G, Marquardt H. The genotoxicity of lucidin, a natural component of Rubia tinctorum L., and lucidinethylether, a component of ethanolic Rubia extracts. Cell Biology and Toxicology. 1988;4(2):225–239. doi: 10.1007/BF00119248. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K, Yoshida M, Takahashi M, et al. Carcinogenic potential of alizarin and rubiadin, components of madder color, in a rat medium-term multi-organ bioassay. Cancer Science. 2009;100(12):2261–2267. doi: 10.1111/j.1349-7006.2009.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balakrishna S, Seshadri TR, Venkataramani B. Special chemical components of commercial woods and related plant materials. IX. Morindonin, a new glycoside of morindone. Journal of Scientific and Industrial Research B. 1960;19:433–436. [Google Scholar]
- 18.Srivastava M, Singh J. A new anthraquinone glycoside from Morinda citrifolia . International Journal of Pharmacognosy. 1993;31(3):182–184. [Google Scholar]
- 19.Do QV, Pham GD, Mai NT, Phan TPP, Nguyen HN, Jea YY. Cytotoxicity of some anthraquinones from the stem of Morinda citrifolia growing in Vietnam. Tap Chi Hoa Hoc. 1999;37:94–97. [Google Scholar]
- 20.Rusia K, Srivastava SK. A new anthraquinone from the roots of Morinda citrifolia Linn. Current Science. 1989;58 [Google Scholar]
- 21.Ohsawa Y, Ohba S. Damnacanthal. Acta Crystallographica C. 1993;49:2149–2151. [Google Scholar]
- 22.Deng Y, Chin YW, Chai H, Keller WJ, Kinghorn AD. Anthraquinones with quinone reductase-inducing activity and benzophenones from Morinda citrifolia (Noni) roots. Journal of Natural Products. 2007;70(12):2049–2052. doi: 10.1021/np070501z. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari RD, Singh J. Structural study of the anthraquinone glycoside from the flowers of Morinda citrifoli . Journal of the Indian Chemical Society. 1977;54:429–430. [Google Scholar]
- 24.Takashima J, Ikeda Y, Komiyama K, Hayashi M, Kishida A, Ohsaki A. New constituents from the leaves of Morinda citrifolia . Chemical and Pharmaceutical Bulletin. 2007;55(2):343–345. doi: 10.1248/cpb.55.343. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya K, Tanaka Y, Endang H, Umar M, Satake T. New anthraquinone and iridoid from the fruits of Morinda citrifolia . Chemical and Pharmaceutical Bulletin. 2005;53(12):1597–1599. doi: 10.1248/cpb.53.1597. [DOI] [PubMed] [Google Scholar]
- 26.Pawlus AD, Su B, Keller WJ, Kinghorn AD. An anthraquinone with potent quinone reductase-inducing activity and other constituents of the fruits of Morinda citrifolia (Noni) Journal of Natural Products. 2005;68(12):1720–1722. doi: 10.1021/np050383k. [DOI] [PubMed] [Google Scholar]
- 27.Akihisa T, Matsumoto K, Tokuda H, et al. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni) Journal of Natural Products. 2007;70(5):754–757. doi: 10.1021/np068065o. [DOI] [PubMed] [Google Scholar]
- 28.Chang P, Lee KH. Cytotoxic antileukemic anthraquinones from Morinda parvifolia . Phytochemistry. 1984;23(8):1733–1736. [Google Scholar]
- 29.Ishii Y, Okamura T, Inoue T, Fukuhara K, Umemura T, Nishikawa A. Chemical structure determination of DNA bases modified by active metabolites of lucidin-3-O-primeveroside. Chemical Research in Toxicology. 2010;23(1):134–141. doi: 10.1021/tx900314c. [DOI] [PubMed] [Google Scholar]


