Abstract
Cordyceps sinensis, an edible mushroom growing in Himalayan regions, is widely recognized in traditional system of medicine. In the present study, we report the efficacy of Cordyceps sinensis in facilitating tolerance to hypoxia using A549 cell line as a model system. Treatment with aqueous extract of Cordyceps sinensis appreciably attenuated hypoxia induced ROS generation, oxidation of lipids and proteins and maintained antioxidant status similar to that of controls via induction of antioxidant gene HO1 (heme oxygenase-1), MT (metallothionein) and Nrf2 (nuclear factor erythroid-derived 2-like 2). In contrast, lower level of NFκB (nuclear factor kappaB) and tumor necrosis factor-α observed which might be due to higher levels of HO1, MT and transforming growth factor-β. Further, increase in HIF1 (hypoxia inducible factor-1) and its regulated genes; erythropoietin, vascular endothelial growth factor, and glucose transporter-1 was observed. Interestingly, Cordyceps sinensis treatment under normoxia did not regulate the expression HIF1, NFκB and their regulated genes evidencing that Cordyceps sinensis per se did not have an effect on these transcription factors. Overall, Cordyceps sinensis treatment inhibited hypoxia induced oxidative stress by maintaining higher cellular Nrf2, HIF1 and lowering NFκB levels. These findings provide a basis for possible use of Cordyceps sinensis in tolerating hypoxia.
1. Introduction
Acclimatization is a major problem for people travelling to high altitudes for the first time. Adverse environmental conditions such as extreme cold, hypoxia, low humidity, high wind velocity, and high intensity of solar radiation [1–3] result in the risk of altitude sickness worldwide. The common problems are acute mountain sickness (AMS), insomnia, lack of appetite, tiredness, lethargy, upset stomach, disinclination to work, bone and muscle degradation, high-altitude pulmonary edema (HAPE), high-altitude cerebral edema (HACE), and all resulted in decrease in physical and mental performance in unacclimatized individuals [4–8]. These problems may escalate rapidly and the results may also be lethal sometimes.
For an individual or cells to adapt to hypoxic conditions, they must be able to sense changes in oxygen tension and respond accordingly. The initiation of these responses can be rapid involving biochemical homeostasis, reprogramming of transcription factors and gene expression, and these changes lead to the production of proteins that exert a protective effect on the cell. Major oxygen and redox-sensitive transcriptional factors (TFs) are nuclear factor- (erythroid-derived 2-) like 2 (Nrf2), hypoxia-inducible factor 1 (HIF1), and nuclear factor kappa-B (NFκB). Expression of antioxidant-responsive-element- (ARE-) driven genes and enzymes is directed by Nrf2 cap'n'collar bZIP transcription factors. These include glutathione peroxidase (GPx), glutathione-S-transferase (GST), heme oxygenase (HO), superoxide dismutase (SOD), and ferritin. HIF1 is selectively stabilized in hypoxia and this in turn leads to activation of several genes such as erythropoietin (EPO) that promotes erythropoiesis, nitric oxide synthase (NOS) that modulates vascular tone, vascular endothelial growth factor (VEGF) that promotes angiogenesis, and glucose transporter-1 (GLUT1) that regulates energy metabolism. Conversely, NFκB activates genes particularly involved in the inflammatory response, as well as in modulating the cellular response to oxidative injury. NFκB plays an important role in the innate and adaptive immunity and cellular survival through the induction of genetic networks [9, 10]. All these events are essential for the survival of cell to hypoxic environment.
Herbs have been used throughout history to enhance physical performance, but scientific scrutiny with controlled clinical trials has only recently been used to study such effects. Several herbs are currently used to enhance physical performance regardless of scientific evidence of effect: one potential supplement not yet well-characterized is Cordyceps sinensis (Berk.) Sacc, a fungus endemic to Himalayan alpine habitats (at an elevation of 3600–5000 m). Purported effects of the fungus suggested a wide range of biological functions such as use as an aphrodisiac [11], analgesic [12], and immune modulator [13] and free radical scavenger [14]. Human clinical trials have demonstrated the effectiveness of Cordyceps sinensis fermented mycelia in combating decreased libido and virility [15, 16]. In a clinical study of elderly patients with chronic fatigue, results indicated that most of the subjects treated with Cordyceps sinensis pure mycelium reported a significant clinical improvement in the areas of fatigue, cold intolerance, dizziness, frequent nocturia, tinnitus, hyposexuality, and amnesia, while no improvement was reported in the placebo group [17–19]. Inhabitants in the high altitude mountain regions of Tibet and Nepal consume Cordyceps sinensis claiming that it gives them energy and offsets the symptoms of high altitude sickness while in West it is consumed by both athletes and elderly people. In recent years, Cordyceps sinensis has been investigated in animal and in vitro studies for antiaging effects, activity on sexual function, and immune modulation, among other potential uses [20, 21].
Our study on the protective effect of Cordyceps sinensis supplementation on hypoxia-induced oxidative stress in lung epithelium cells (A549) is the first of its kind, wherein the effectiveness of Cordyceps sinensis in ameliorating the oxidative stress to hypoxia was studied at the molecular level. Further, evaluation of Cordyceps sinensis in acclimatization process in high altitude ailments could also enhance the possibility use Cordyceps sinensis as a potent naturaltherapeutic agent. In view of above, the present study was designed to evaluate the molecular mechanisms of action of Cordyceps sinensis in the process of hypoxia tolerance.
2. Material and Methods
2.1. Apparatus
HPLC Waters, ASE 350 Dionex Corporation (Sunnyvale, CA, USA), Spectrophotometer Bio-Rad. ELISA reader (Molecular Devices, USA), Spectrofluorimeter (Varian, USA).
2.2. Reagents
1,1′-Diphenyl-2-picrylhydrazly [DPPH], 3,4,5-trihydroxybenzoic acid [Gallic Acid], TPTZ [2,4,6-tripyridy-s-triazine], 6 Hydroxy-2,5,7,8-Tetramethylchroman-2-Carboxylic acid [Trolox], Rutin, Folin-Ciocalteu reagent and all other chemicals were from Sigma Aldrich Chemicals, USA. Specific antibodies against all HIF1, NFκB, Nrf2, TNFα, TGFβ, VEGF, EPO, GLUT1, HO1, MT1 and HRP conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc., California, USA.
2.3. Collection of Plant Material
Cordyceps sinensis mycelia (Voucher specimen DIP-CS/2011) were obtained from Defence Institute of Bio Energy Research, Haldwani. Cordyceps sinensis was collected during the rainy season (May-June) from wood logs and tree stumps from the hilly regions (at an altitude of over 4000 m) of the Northwest Himalayas at different locations in Pithoragarh, Uttarakhand, India, where the plant grows widely under natural conditions. Only the mature fruiting bodies (seen as reddish-brown open caps) were selected, removed, and washed with nanopure water, dried under shade in a clean, dust-free environment, and milled into powder using pestle and mortar.
2.4. Extraction Procedure
Water extract of Cordyceps sinensis was prepared using Accelerated Solvent Extraction system (ASE 350) equipped with a solvent controller unit (Dionex Corporation Sunnyvale, CA, USA). The extraction was carried out in triplicate using water as a solvent. The extraction procedure was as follows: (i) the powdered sample was loaded into cell, (ii) cell was filled with highly pure grade water up to a pressure of 1500 psi, (iii) static extraction was for 15 min, (iv) cell was rinsed with extraction solvent (60% of cell volume) and the solvent is purged from cell with N2 gas (viii), and finally depressurization takes place. Between extractions, a rinse of the complete system was made in order to overcome any extract carryover. The extract was lyophilized (Allied Frost, India) and stored at 4°C.
2.5. HPLC Fingerprinting
HPLC analysis of the water extract of Cordyceps sinensis was performed using Waters HPLC system (Waters Corporation, USA) equipped with Waters 515 HPLC pump, Waters 717 plus autosampler, and Waters 2487 UV detector. Separation was performed in a symmetry C18 250 mm × 4.7 mm ID, 5 μm column (Waters, USA) by maintaining the isocratic flow rate (1 mL/min) of the mobile phase (0.01 M KH2PO4 pH 3.7 : methanol 90 : 10) and peaks were detected at 260 nm absorbance.
2.6. Determination of Total Phenol Content
The total phenolic content in extract was estimated using Folin-Ciocalteu reagent (FCR) based assay using 20 μL of stock solution (1 mg/mL) of the extract, 80 μl of water, and 500 μL of FCR [22]. After 5 min incubation in dark at room temperature, 400 μL of 7.5% sodium carbonate solution was added. The mixture was further incubated in dark for 30 min at room temperature and absorbance was read at 765 nm. Total phenols (mg/g) in the extract were expressed as gallic acid equivalent (GAE), using standard curve prepared from gallic acid (0.1 mg/mL) solution.
2.7. Determination of Total Flavonoid Content
Total flavonoid content was estimated by aluminum chloride (AlCl3) colorimetric assay as described elsewhere [23] using rutin as a standard. 1 mL of extract was added to 4 mL distilled water and subsequently 0.3 mL of 5% NaNO2 solution was added. After 5 min, 0.3 mL of 10% AlCl3 solution was added and allowed to stand for 5 min, and then 0.2 mL of 4% NaOH solution was added to the mixture and the volume was adjusted up to 10 mL with distilled water. Absorbance of the mixture was read at 510 nm. Total flavonoid content (mg/g) in the extract was expressed as rutin equivalent.
2.8. FRAP Assay
The FRAP assay was estimated using method described elsewhere [24]. The fresh FRAP working solution was prepared by mixing 25 mL of 300 mM acetate buffer (3.1 g C2H3NaO2 3H2O and 16 mL C2H4O2, pH 3.6), 2.5 mL of 10 mM TPTZ (2,4,6-tripyridyl-s-triazine in 40 mM HCl), and 2.5 mL of 20 mM FeCl3·6H2O solution which was warmed at 37°C prior to use. 150 μL of extract (1 mg/mL) was allowed to react with 2850 μL of the FRAP solution for 30 min in dark and the absorbance of the color developed was read at 593 nm. Results were expressed as mg Trolox equivalent/g of extract, using standard curve prepared from Trolox solution.
2.9. DPPH Assay
The DPPH assay was estimated using method described elsewhere [25]. DPPH assay stock solution was prepared by dissolving 24 mg DPPH with 100 mL methanol and then stored at −20°C until needed. The working solution was obtained by mixing 10 mL stock solution with 45 mL methanol to obtain an absorbance of 1.10 ± 0.02 units at 515 nm using the spectrophotometer. 150 μL of leaf extract solution was allowed to react with 2850 μL of the DPPH solution for 2 h in the dark. Then the absorbance was measured at 515 nm. The standard curve was linear between 25 and 200 ppm Trolox. Results are expressed in mg of TE/g of extract.
2.10. 2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) Diammonium Salt (ABTS) Assay
The ABTS assay was performed using method described elsewhere [26]. The stock ABTS solution (7 mM) and potassium persulphate (2.45 mM) was mixed in 1 : 1 ratio and incubated overnight in dark at room temperature. The assay was performed by incubation of 250 μL of chemically generated ABTS•+ radical in potassium persulfate with 10 μL of extracts or ascorbic acid standard. Absorbance was measured spectrophotometrically at 734 nm for intervals of 1, 2, 5, 10, and 15 min after addition of extract or standard. The extracts without ABTS•+ were used as assay control.
2.11. Cells and Culture Conditions
A549 cells (1 × 105 cells/mL) were plated in tissue culture flasks and incubated in normoxia (21% O2, 74% N2, and 5% CO2) at 37°C in DMEM medium (Sigma, St. Louis, USA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St. Louis, USA) and penicillin-streptomycin (50 μg/mL, Invitrogen). The hypoxic conditions were achieved by culturing cells in an incubator (Jouan, Saint-Nazaire, France) with a 0.5% O2, 5% CO2, and 94% N2 atmosphere. Cells were treated with Cordyceps sinensis extract (2.5 mg/mL stock) dissolved in culture medium and after an hour of treatment, cells were exposed to hypoxia.
2.12. MTT (Methylthiazole Tetrazolium) Cytotoxicity Assay
The MTT assay, which is based on conversion of yellow tetrazolium salt to purple-formazan crystals by metabolically active cells, provides a quantitative determination of viable cells. Cells seeded at the density of 10,000 per well in 96 well tissue culture plates were allowed to adhere for 24 h at 37°C. Cells were then treated with 2.5, 5, 10, 25, 50, 100, 250, 500, and 1000 μg/mL concentrations of Cordyceps sinensis dissolved in DMEM media. Cells were exposed to normoxia and hypoxia for 24 h or 48 h and cytotoxicity was assessed by MTT assay. Briefly, 50 μL of MTT (1 mg/mL) was added to each well and incubated for 4 h at 37°C. Formazan crystals were solubilized in 100 μL of DMSO by incubating in shaking condition at room temperature for 5 min. Absorbance was taken at 570 nm with 630 nm as reference filter. Absorbance given by untreated cells was taken as 100% cell survival.
2.13. Biochemical Analysis
After normoxic and hypoxic exposure, the cells were harvested by trypsinization and washed with sterile phosphate buffered saline (PBS; pH 7.4). The cells were then sonicated for 10 sec in PBS and centrifuged (Sigma Co., Munich, Germany) at 1500 g for 10 min at 4°C. The pellet containing tissue/cell debris was discarded and the supernatant was used to determine ROS, GSH, lipid peroxidation, the protein oxidation, GR, GPx, and SOD activities. The protein content in the homogenate was determined by the method of Lowry et al. [27].
2.14. Assay for Intracellular Redox State
Intracellular redox state levels were measured using the fluorescent dye 2,7-dichlorofluorescein diacetate (H2-DCFH-DA). Briefly, cells were washed once with HBSS and incubated in the same buffer containing 5–10 μg of DCFH-DA for 30 min at 37°C. Intracellular fluorescence was detected with excitation at 485 nm and emission at 530 nm using Spectra Max Gemini EM (Molecular Devices, Sunnyvale, CA).
2.15. Lipid Peroxidation
Lipid peroxidation was assessed by measuring malondialdehyde (MDA) formed by thiobarbiturate (TBA) reaction as thiobarbituric acid reactive substances (TBARS), using the method described elsewhere [28]. Thiobarbiturate was used as the standard, and the level of lipid peroxides was expressed as nmol MDA/mg protein.
2.16. Protein Oxidation
Protein oxidation was measured by determining the carbonyl groups after derivatization with 2,4-dinitrophenyl hydrazine (DNPH) [29]. Carbonyl content was calculated from its molar absorption coefficient as 22,000 m−1 cm−1 and results were expressed as nmol protein carbonyl per mg protein. Briefly, equal volumes of supernatant and 10 mM DNPH/2M HCl were incubated for 60 min at 50°C. Protein was then precipitated with 20% trichloroacetic acid (TCA), and unreacted DNPH was removed by centrifugation at 1400 g for 10 min. The precipitate was washed three times with a cold ethanol/ethyl acetate (1 : 1) mixture, and finally the precipitate was dissolved in 1 M NaOH. The absorbance was measured at 450 nm and the carbonyl content was obtained as nmol/mg protein.
2.17. Enzymatic and Nonenzymatic Antioxidants
Reduced glutathione (GSH) levels were measured fluorometrically by the method described elsewhere [30]. The activities of glutathione peroxidases (GPx), superoxide dismutase (SOD), and glutathione reductase (GR) were determined using commercial kits (Randox) as per the manufacturer's instructions.
2.18. Cellular Protein Preparation and Western Blotting
Following indicated treatments, cells were washed thrice with ice-cold phosphate buffered saline (PBS) and lysed in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5, with 120 mM NaCl, 10 mM sodium fluoride, 10 mM sodium pyrophosphate, 2 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1% NP-40, and protease inhibitor cocktail). Cellular lysates were clarified by centrifugation at 12,000 rpm for 30 minutes. 40 μg of protein was resolved on 10–12% SDS-polyacrylamide gel which was subsequently transferred onto a nitrocellulose membrane (0.45 μ). The membranes were probed with respective primary antibodies (Nrf2, HO1, MT, NFκB, TNFα, TGFβ EPO, GLUT1, VEGF) followed by HRP conjugated secondary antibodies. Immunoblots were detected by chemiluminescence reagent (Sigma, USA) and the bands were developed using X-ray films (Kodak, Rochester, NY, USA). Quantification was performed by densitometry using Labworks software (UVP BioImaging Systems).
2.19. Data Analysis
All the experiments were performed on three different occasions and data are presented as mean ± SE. The data was analyzed by one-way ANOVA followed by Dunnet's test for comparing control and the various groups, using GraphPad software. Statistical significance was estimated at the 5% level.
3. Results
3.1. Chemical Standardization of Water Extract of Cordyceps Sinensis by Its Total Phenolics, Flavonoid Content, Antioxidant Activity, and Fingerprinting
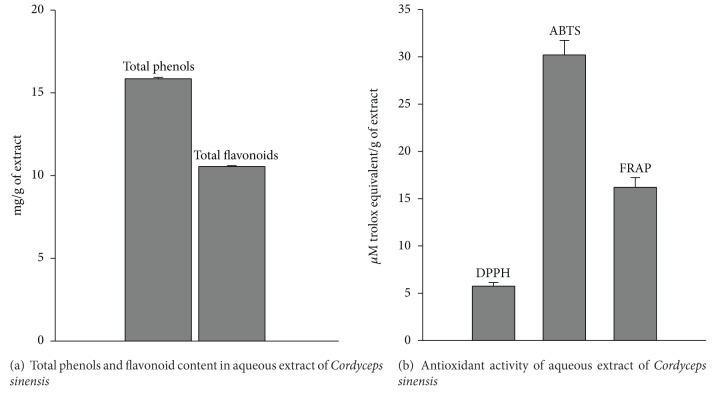
Figure 1 depicts % yield, total phenolics, flavonoid content, and antioxidant activity of water extract of Cordyceps sinensis. The extract showed 15.9 ± 0.08 mg/g of total phenols and 10.6 ± 0.05 mg/g of flavonoid content (Figure 1(a)). The antioxidant activity of extract was found to be 5.75 ± 0.38, 30.2 ± 1.54, 16.2 ± 1.02 μM TE/g of extract as evident by DPPH, ABTS, and FRAP assays, respectively (Figure 1(b)). Figure 2 shows HPLC chromatograms at 260 nm. The presence of adenine, the marker compound of Cordyceps sinensis, was evident in the extract with a retention time of 6.43. Adenine was found to be 0.71% w/w.
Figure 1.
Total phenols, flavonoid content and antioxidant activity of aqueous extract of Cordyceps sinensis.
Figure 2.
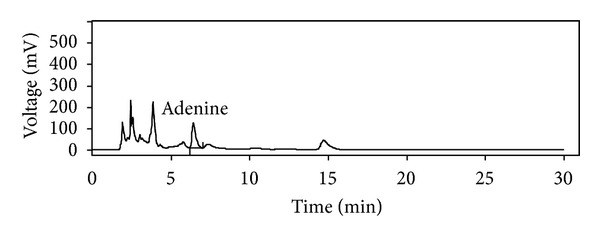
RP-HPLC chromatogram of aqueous extract of Cordyceps sinensis.
3.2. Effect of Cordyceps sinensis on Cell Viability and ROS Production in Hypoxia Exposed A549 Cells
We determined the effect of hypoxia alone using different percentages of oxygen (0.1%, 0.5%, 1%, and 5% O2) or in combination with various concentrations of Cordyceps sinensis extract (5–250 μg/mL) on cell viability and generation of reactive oxygen species. Cells exposed to varying percentage of oxygen showed increased reactive oxygen species and cell death. The cytotoxic effect of hypoxia was dependent on the concentration of oxygen (Figures 3(a)–3(d) and 4(a)–4(d), dark gray bars). The treatment of cells with various concentrations of extract prevented cell death and generation of reactive oxygen species following hypoxia exposure and the protective effects were dose dependent (Figures 3(a)–3(d) and 4(a)–4(d), light gray bars). Because 0.5% O2 showed approximately 51% cell death and 250 μg/mL of Cordyceps sinensis extract showed maximal effects on cell viability and ROS generation, all subsequent experiments were conducted using the above-mentioned percentage of oxygen and concentration of extract.
Figure 3.
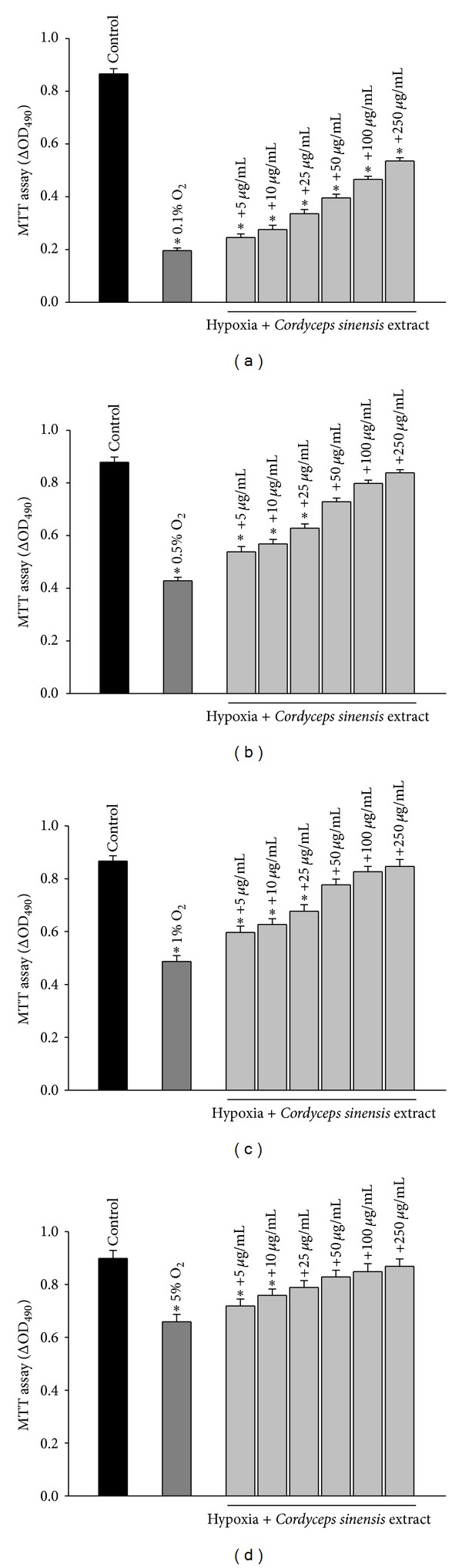
Cytoprotective effects of aqueous extract of Cordyceps sinensis in A549 cells exposed to different oxygen percentages (0.1, 0.5%, 1%, or 5% O2; (a)–(d) for 48 h. Cells exposed to hypoxia showed cell death (dark gray bar) in comparison to unexposed cells (black bar) which was dependent on oxygen percentage. Treatment of cells with aqueous extract of Cordyceps sinensis increased cell survival following hypoxia exposure and the efficacy of extract was dose dependent (light gray bars). *P < 0.05.
Figure 4.
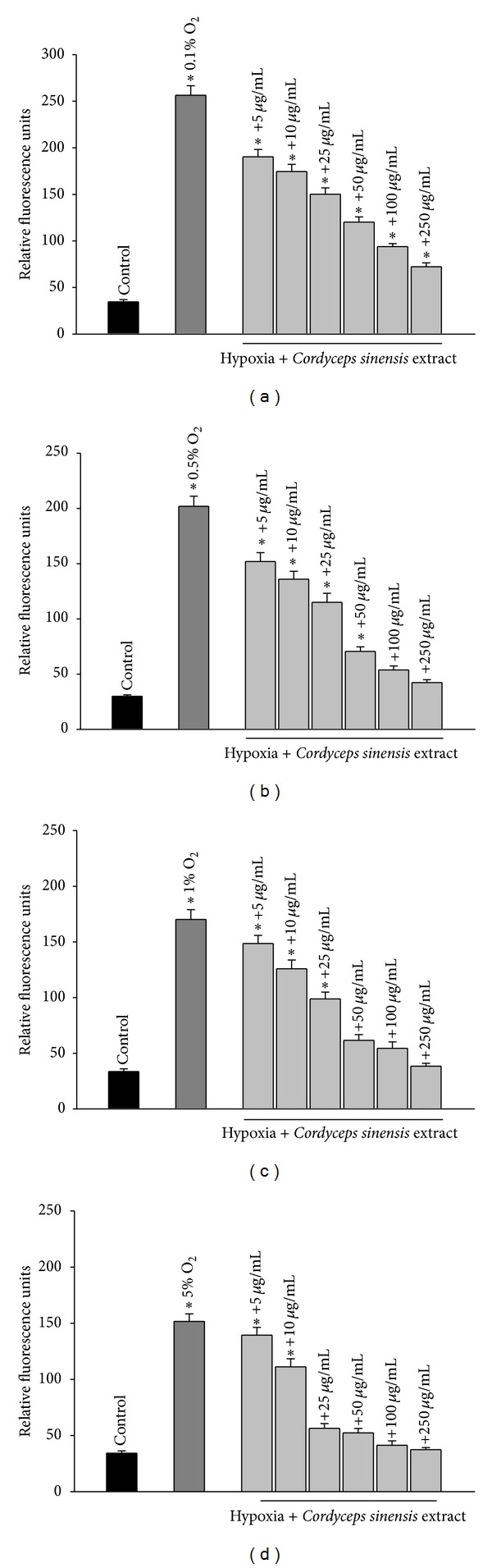
Antioxidative effects of aqueous extract of Cordyceps sinensis in A549 cells exposed to different oxygen percentages (0.1, 0.5%, 1%, or 5% O2; (a)–(d)) for 48 h. Cells exposed to hypoxia showed elevated levels of reactive oxygen species (dark gray bars) compared to unexposed cells (black bars) which was dependent on oxygen percentage. Cordyceps sinensis decreased reactive oxygen species following hypoxia exposure and the efficacy of extract was dose dependent (light gray bars). *P < 0.05.
3.3. Lipid Peroxidation
Since hypoxia exposure led to increased oxidative stress, we determined the levels of lipid peroxidation. Exposure to hypoxia caused a marked increase in lipid peroxidation as observed by an increase in MDA levels. However, Cordyceps sinensis treatment significantly inhibited the hypoxia-induced lipid peroxidation as compared with the respective hypoxia exposed cells (Figure 5(a); P < 0.05). No significant difference was observed in the normoxic cells and Cordyceps sinensis treated cells kept in normoxic conditions.
Figure 5.
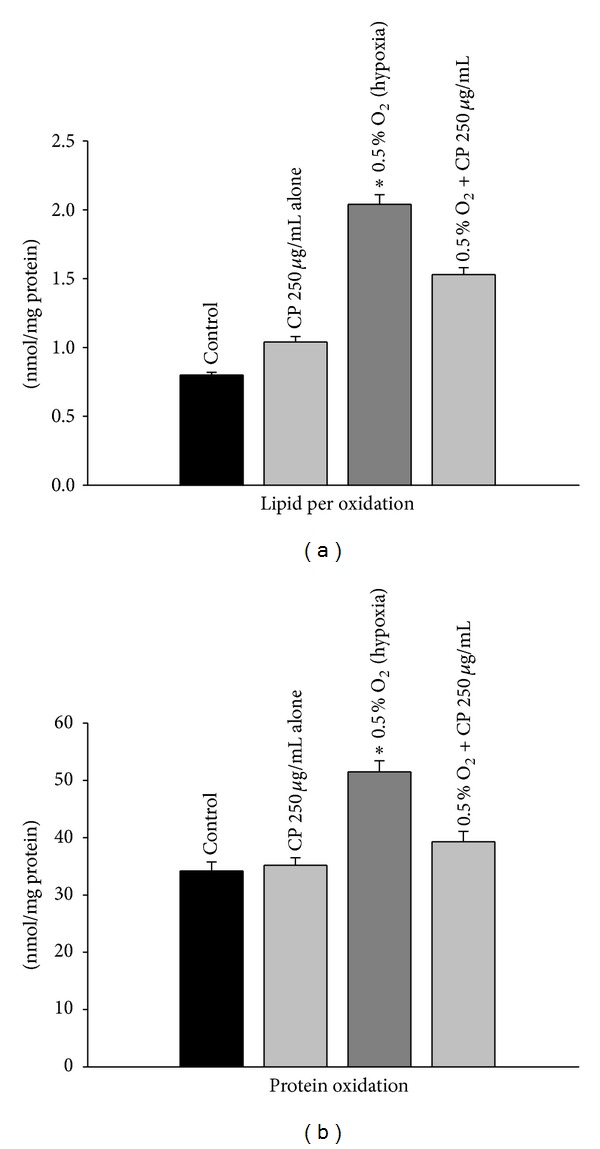
Lipid peroxidation (TBARS, thiobarbituric acid reactive substance) and protein oxidation (carbonyl groups after derivatization of proteins with dinitrophenylhydrazine) in A549 cells were determined after (0.5% O2) hypoxia exposure for 48 h by spectrophotometric measurement. A significant increase in both lipid peroxidation and protein oxidation was observed in cells exposed to hypoxia and such changes were attenuated in hypoxic cells treated with Cordyceps sinensis extract. Experiments were done in triplicate. Values are mean ± SE of three individual experiments. *P < 0.05.
3.4. Protein Oxidation
The effect of hypoxia on oxidation of proteins was measured by determining protein carbonyl contents in A549 cells after derivatization with DNPH. The results showed a considerable increase in protein oxidation in cells exposed to hypoxia as compared to the control. Cordyceps sinensis treatment appreciably inhibited the formation of protein carbonyls levels (Figure 5(b); P < 0.05) after exposure to hypoxia. No significant change was observed in protein oxidation in cells treated with Cordyceps sinensis kept in normoxic conditions relative to normoxic control cells.
3.5. Glutathione Status and Antioxidant Enzyme Activity
To assess the level of endogenous nonenzymatic antioxidant, GSH was determined. Exposure of A549 cells to hypobaric hypoxia resulted in a significant decrease in GSH levels as compared with normoxic control cells (Table 1; P < 0.05). Cordyceps sinensis treatment significantly increased GSH levels after exposure to hypoxia though no difference was observed between Cordyceps sinensis treatment normoxic cells and control normoxic cells (Table 1).
Table 1.
Protective effects of aqueous extract of Cordyceps sinensis on antioxidant status of cells exposed to hypoxia.
| Treatment | GPx (U/L) | SOD (U/mL) | GR (U/L) | GSH (mM) |
|---|---|---|---|---|
| Control | 400.7 ± 38.8 | 24.4 ± 1.74 | 42.3 ± 2.23 | 9.01 ± 0.32 |
| Hypoxia | 328.0 ± 22.2* | 48.4 ± 3.46* | 14.9 ± 0.82* | 3.34 ± 0.26* |
| Control + extract | 400.9 ± 18.9 | 26.6 ± 2.02 | 43.8 ± 2.84 | 8.83 ± 0.33 |
| Hypoxia + extract | 386.9 ± 20.4 | 32.7 ± 2.46* | 44.7 ± 2.62 | 7.86 ± 0.38 |
A549 cells were exposed to hypoxia (0.5% O2) for 48 h in the absence or presence of aqueous extract of Cordyceps sinensis (250 μg/mL). A significant decrease in GSH, GPx, and GR levels observed after hypoxia was restored in Cordyceps sinensis treated cells. Further, an increase in SOD activity was observed in cells exposed to hypoxia and the effect was nearly neutralized by Cordyceps sinensis treatment. Results are means ± SE of three individual experiments. *P < 0.05.
As shown in Table 1, exposure to hypoxia resulted in a significant increase in SOD activity and a decrease in GPx and GR activities relative to normoxic cells (P < 0.05). Cordyceps sinensis treatment maintained these enzymes levels similar to the control values, indicating reduced oxidative stress.
3.6. Heme Oxygenase-1 and Metallothionein Levels
Considering that Cordyceps sinensis treatment has significantly inhibited ROS and maintained antioxidant status of hypoxia exposed cells, we sought whether this ability to prevent oxidative stress is mediated via Nrf2, HO1 and MT. Therefore, the relative levels of Nrf2, HO1 and MT were determined by immunoblotting. Exposure of cells to hypoxia resulted in a significant increase in Nrf2, HO1, and MT relative to normoxic control cells (Figure 6). Cordyceps sinensis treatment and hypoxia exposure further increased expression of Nrf2, HO1, and MT levels compared to normoxic cells.
Figure 6.
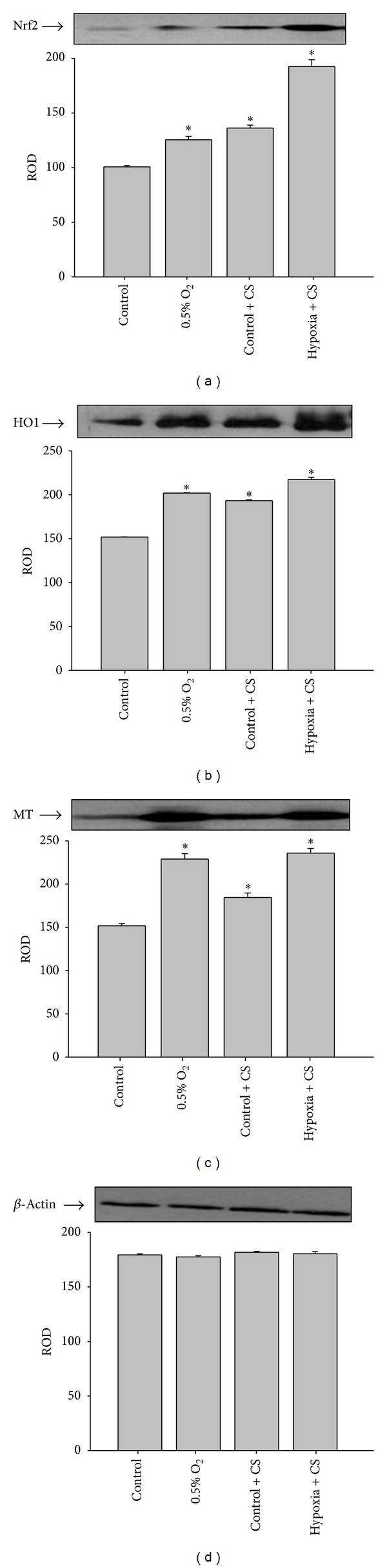
Effect of Cordyceps sinensis treatment on the expression of antioxidant transcription factor (Nrf2; (a)) and its regulated genes heme oxygenase-1 (HO1; (b)) and metallothionein 1 (MT; (c)) in A549 cells (0.5% O2) hypoxia exposure for 48 h. There was a significant increase in Nrf2, HO1, and MT levels following hypoxia exposure and also in the presence of Cordyceps sinensis extract. The figures are representative of three independent experiments. These were normalized with actin to observe any change in expression.
3.7. Role of NFκB in Cellular Protection
To investigate the role of NFκB-mediated mechanisms in the induction of protection, the effect of Cordyceps sinensis treatment on NFκB and its target genes were examined. It is a key transcriptional factor that regulates inflammatory mediators. We determined the relative levels of active NFκB by immunoblotting. Exposure of cells to hypoxia resulted in a marked increase in NFκB levels; however, Cordyceps sinensis treatment significantly attenuated NFκB expression (Figure 7).
Figure 7.
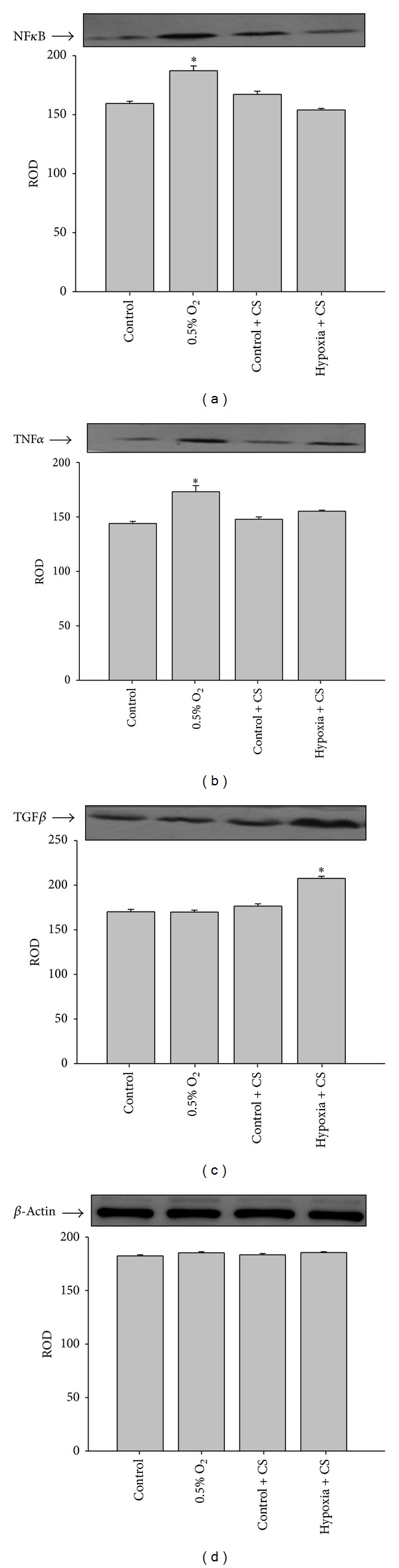
Western blot analyses showed significant increase in NFκB (a) and pro-inflammatory cytokines TNFα (b) while no change was observed in levels of anti-inflammatory cytokines TGFβ (c) in A549 cells following hypoxia (0.5% O2) exposure for 48 h. The treatment of cells with extract prevented increase in NFκB and TNFα levels following hypoxia exposure. Further, an increase in TGFβ levels was also evident in Cordyceps sinensis treated cells. The figures are representative of three independent experiments. These were normalized with β-actin (d) to observe any change in expression.
Since NFκB regulates inflammatory mediators, we determined pro- and anti-inflammatory molecules like TNFα and TGFβ. There was a considerable increase in TNFα levels upon hypoxic exposure relative to control cells. However, no change in TGFβ levels was noticed in the cells exposed to hypoxia. Cordyceps sinensis treatment significantly attenuated hypoxia induced increase in TNFα level. Interestingly, the levels of TGFβ increased upon Cordyceps sinensis treatment (Figure 7).
3.8. Effect of Cordyceps sinensis on HIF1 Expression and Its Regulated Genes
HIF1 level was measured in A549 cells by immunoblotting. The results showed a marked increase in HIF1 expression during exposure to hypoxia. Interestingly, Cordyceps sinensis treated cells under hypoxic condition showed further increase in HIF1 protein (Figure 8). To know whether increased HIF1 levels in hypoxia and Cordyceps sinensis treated cells resulted in increased expression of its downregulated genes, western blotting was performed for EPO, GLUT1, and VEGF, which promote erythropoiesis, glucose transport, and angiogenesis, respectively. A significant increase in VEGF and GLUT1 protein levels was observed in hypoxia and Cordyceps sinensis treated cells. EPO protein increased slightly following hypoxia and Cordyceps sinensis treatment (Figure 8).
Figure 8.
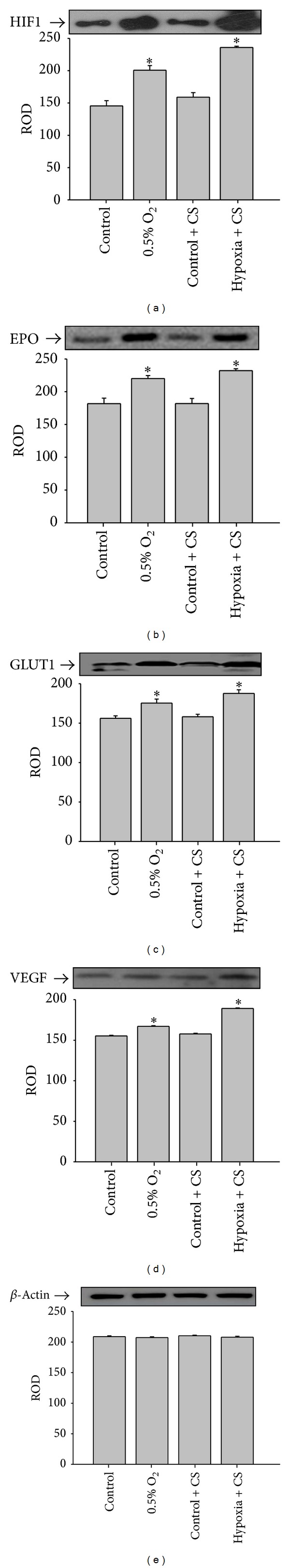
Immunoblot analysis of HIF1 (a) and HIF1 regulated genes, namely, EPO (b), GLUT1 (c), and VEGF (d) in A549 cells following hypoxia (0.5 % O2) exposure for 48 h. The treatment of cells with Cordyceps sinensis increased levels of HIF1, EPO, GLUT1, and VEGF following hypoxia exposure. The figures are representative of three independent experiments. These were normalized with β-actin (e) to observe any change in expression.
4. Discussion
In the preliminary experiments, A549 cells were exposed to different percentages of oxygen 0.1%, 0.5%, 1%, and 5% for 24–48 h duration and the results showed 70–77%, 42–51%, 32–44%, and 15–27% cell death with an increase of 6.6–7.4, 5.3–6.7, 4.3–5.1, and 3.5–4.3 fold in reactive oxygen species, respectively. The findings suggested that the cell death was due to increased oxidative stress governed by percentage of oxygen and duration of hypoxia exposure. Based on preliminary data optimal percentage of oxygen was found to be 0.5% O2 as 0.1% O2 percentage of oxygen reduced cell survival to 23–30% while 1% and 5% O2 resulted in minimal cell death. In a separate study, cells were exposed to various concentrations of Cordyceps sinensis extract (2.5–1000 μg/mL) for 24 h and 48 h, and A549 cells did not show any toxicity per se. The concentration of Cordyceps sinensis extract below 5 μg/mL was not effective and cells treated above 250 μg/mL could not provide additional improvement in efficacy of extract; hence, data of effective dose range 5–250 μg/mL is only presented in Figures 3(a)–3(d) and 4(a)–4(d). The effective dose range for aqueous extract of Cordyceps sinensis under hypoxia exposure of 48 h was found to be 5–250 μg/mL; however, the best results were obtained with 250 μg/mL against all the percentage of oxygen. Therefore, we have used 0.5% O2 and 250 μg/mL of Cordyceps sinensis extract in all our studies except MTT and ROS.
The present study shows that treatment of Cordyceps sinensis significantly improves tolerance to hypoxia as revealed by increased Nrf2 and HIF1 and decrease in NFκB transcription factors. This in turn led to increased antioxidant genes, antioxidant enzymes, EPO, VEGF, and GLUT1 levels, facilitating better oxygenation, oxygen delivery, and glucose transport. At the same time decrease in NFκB levels resulted in lower expression of proinflammatory cytokines like TNFα and increased expression of anti-inflammatory cytokine TGFβ. All these changes contributed to the increase in hypoxic tolerance of Cordyceps sinensis treated cells. To the best of our knowledge, we have demonstrated for the first time the potency of Cordyceps sinensis treatment in inducing tolerance to hypoxia.
During hypoxia, less oxygen is available to be reduced to H2O at cytochrome oxidase, causing accumulation of highly reactive oxygen intermediates (ROIs). Overproduction of ROIs can readily cause oxidative damage to various biological macromolecules resulting in DNA damage, oxidation of key amino acid side chains, formation of protein-protein cross-links, oxidation of the polypeptide backbone resulting in protein fragmentation, and lipid peroxidation. The present study also reports that exposure of A549 cells to hypoxia resulted in an appreciable increase in ROS levels which in turn could be responsible for the observed increase in oxidation of cellular protein and lipids. For hypoxia-associated free radicals, antioxidant can safely interact with free radicals and terminate the chain reaction before vital biological macromolecules are damaged. Cellular antioxidants defense network comprising the well-known endogenous enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as nonenzymatic antioxidants reduced glutathione (GSH). These participate in maintaining the proper balance of free radicals and antioxidants in the healthy cells. The cells exposed to hypoxia showed a decrease in GSH levels and to cope up with this hypoxia-induced stress, a marked increase in activities of cellular antioxidant enzymes such as SOD, GPx, and GR were observed. However, Cordyceps sinensis supplementation maintained the antioxidant enzymes levels similar to those of control values and restores the GSH level. The findings suggested that Cordyceps sinensis exerted its robust antioxidant defense abilities by activating endogenous antioxidant enzymes as well as inhibiting protein and lipid peroxidation due to presence of higher amount of phenols, flavonoids, nucleosides (adenosine), nucleobases (adenine and uracil), and polysaccharides; these compounds absorb and neutralize free radicals, quench singlet and triplet oxygen, or decompose peroxides [31]. This result is in agreement with other reports that aqueous extracts of natural and cultured Cordyceps species are more effective in free radical scavenging than in hydrophobic systems [32]. Yao's group [33] has reported that the water extracts of Cordyceps sinensis mycelia have direct and moderate-to-potent antioxidant activities involving the scavenging of superoxide anion radical, hydroxyl radical, and inhibition of lipid peroxidation. They also proposed that the antioxidant activities of the water extracts may be caused by a combined effect of proteins, polysaccharides, and mannitol or some other compounds in Cordyceps sinensis mycelia. However, Li et al. [34] have observed that the water extract of Cordyceps sinensis mycelia possessed a strong antioxidant activity and that partially purified polysaccharides from the water extract showed greater antioxidant activities (10-fold to 30-fold) than before purification. Therefore, polysaccharides may be regarded as the key components of the antioxidant activity of Cordyceps sinensis mycelia.
Growing evidence has indicated that cellular redox plays an essential role not only in cell survival but also in cellular signaling pathways such as NFκB. Several laboratories have demonstrated that treatment of cells with H2O2 (ROIs) can activate the NFκB pathway as reviewed by Bauerle and Henkel [35]. Shih et al. [36] demonstrated that the hydroxyl radical was primarily responsible for activation of NFκB in Jurkat cells and macrophages. During hypoxia elevated levels of ROIs prompted speculation that ROIs may function as common mediators of NFκB activation also. Since NFκB is involved in inflammation, we therefore analyzed the expression of NFκB and its proinflammatory mediator TNFα in cells exposed to hypoxia. A marked increase in NFκB and TNFα levels was seen during hypoxia; however, Cordyceps sinensis treatment + hypoxia markedly inhibited NFκB and TNFα expression possibly due to reduction in hypoxia-induced oxidative stress indicating towards cells defense against the hypoxic stress. This hypothesis is in agreement with an earlier report in which evidence showing that nearly all pathways leading to NFκB activation could be blocked by a variety of antioxidants, including pyrrolidine dithiocarbamate (PDTC), N-acetyl-L-cysteine (NAC), glutathione (GSH), or thioredoxin (Txn), or by overexpression of antioxidant enzymes, including superoxide dismutase, glutathione peroxidase, or thioredoxin peroxidase. As aforementioned, Cordyceps sinensis possesses robust antioxidant properties.
Based on the results mentioned above and reported facts, we hypothesized that Cordyceps sinensis treatment inhibited oxidative stress and inflammation in the hypoxia exposed cells. Thus, we explored the probability of alterations in the expression of Nrf2 transcription factor, a master regulator of endogenous antioxidative systems [36–38] being involved in cell survival. We found that Cordyceps sinensis + hypoxia treatment resulted in a significant increase in Nrf2 levels. Having confirmed the expression of Nrf2, we investigated the levels of HO1 which is known to possess antioxidant and anti-inflammatory activity and regulated by Nrf2 [39]. Our results demonstrated that Cordyceps sinensis + hypoxia resulted in higher levels of HO1. We also measured other anti-inflammatory mediators such as TGFβ and MT levels in A549 cells. The study revealed significant upregulation of TGFβ and MT after Cordyceps sinensis + hypoxia treatment, and all these genes might be responsible for the observed fall in proinflammatory cytokines after exposure to hypoxia. Our results are in accordance with previous report [40] stating that activation of Nrf2 effectively reduces oxidative stress and inflammation in rats. Similarly overexpression of TGFβ, HO1, and MT facilitates acclimatization and maintains the redox balance in the cells [41, 42]. The capacity of Cordyceps sinensis in limiting the oxidative stress is partly mediated by Nrf2 pathway.
Hypoxia has been shown to activate HIF1, thus an attempt was made to investigate changes in the expression and to define a functional involvement of HIF1, in response to Cordyceps sinensis treatment. We found that Cordyceps sinensis treatment 1 h before hypoxia exposure resulted in a significant increase in HIF1 levels; this suggests that HIF1-mediated gene expression may be involved in hypoxia-induced tolerance. Therefore, we determined the relative levels of HIF1 regulated genes, EPO, GLUT1, and VEGF by immunoblotting in control and Cordyceps sinensis treated cells. Cordyceps sinensis treatment under hypoxia resulted in higher EPO levels, increased EPO levels, and increased O2 carrying capacity. Further the protein levels of GLUT1 mediating the transport of glucose were increased in hypoxia and Cordyceps sinensis + hypoxia cells compared to the control cells, indicating enhanced glucose uptake for continued energy generation in hypoxic environments. Our results are in accordance with previous studies suggest that CordyMax CS-4 supplementation effectively lowers blood glucose and plasma insulin and improves glucose metabolism by enhancing insulin sensitivity [43]. It is presumed that when cells in the tissue are deprived of oxygen, they release angiogenic factor that induce new capillary growth and hence better oxygen transport [6]. Of the known angiogenic factors FGF and VEGF are most commonly expressed [8]. In our study too, we found a significant increase in VEGF protein levels in hypoxic cells and Cordyceps sinensis + hypoxia exposed cells relative to control cells. These results indicate that the induction of specific HIF1 and its regulated genes in Cordyceps sinensis treated cells following hypoxia is an attempt by cells to initiate the process of acclimatization to escape death. Putative corelation among various transcription factors and mechanism of tolerance offered by aqueous extract of Cordyceps sinensis to hypoxia is schematically represented in Figure 9.
Figure 9.
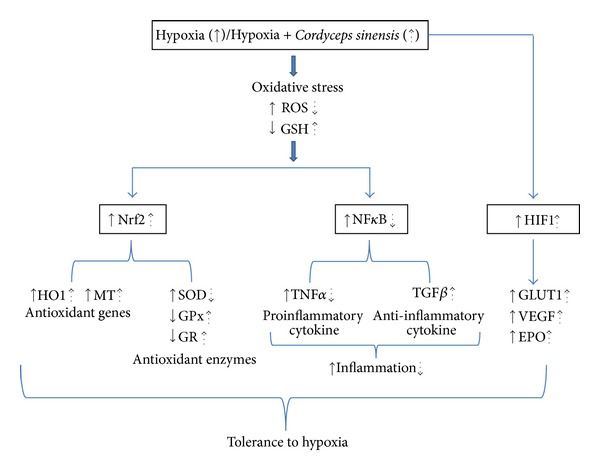
Schematic representations of effects of hypoxia and aqueous extract of Cordyceps sinensis in A549 cells. Putative corelation among various transcription factors and mechanism of tolerance to hypoxia, upregulation/downregulation after hypoxia (solid arrow), and upregulation/downregulation after Cordyceps sinensis treatment (dotted arrow).
To conclude exposure of cells to hypoxia resulted in a significant increase in oxidative stress; further, there was a marked increase in NFκB activity and inflammatory mediators TNFα. Treatment of cells by Cordyceps sinensis (250 μg/mL) significantly attenuated the oxidative stress in hypoxia. This was attributed to activation of Nrf2-ARE pathway, reduced NFκB activity, and increased oxygen availability via HIF1 signaling mechanisms. Further, higher levels of antioxidant, anti-inflammatory mediators, namely, TGFβ, HO1, and MT levels in Cordyceps sinensis treated cells might also be responsible for tolerance of cells to hypoxia. The findings of the study will help in the development of novel therapeutic strategies to use Cordyceps sinensis as a nutraceutical for promoting tolerance to high altitude.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgments
The authors are thankful to the Director of DIPAS, Delhi, for her constant support and constructive suggestions and the Director of DIBER, Haldwani, India, for providing study material. We acknowledge Dr. Sayeed Ahmed, Jamia Hamdard University, Delhi, for carrying out HPLC analysis of extract.
References
- 1.Ward M. Mountain Medicine: A Clinical Study of Cold and High Altitude. London, UK: Crosby Lockwood staples; 1975. [Google Scholar]
- 2.Moehrle M, Dennenmoser B, Garbe C. Continuous long-term monitoring of UV radiation in professional mountain guides reveals extremely high exposure. International Journal of Cancer. 2003;103(6):775–778. doi: 10.1002/ijc.10884. [DOI] [PubMed] [Google Scholar]
- 3.Pugh LG. Tolerance to extreme cold at altitude in a Nepalese Pilgrim. Journal of Applied Physiology. 1963;18:1234–1238. doi: 10.1152/jappl.1963.18.6.1234. [DOI] [PubMed] [Google Scholar]
- 4.Shukitt-Hale B, Banderet LE, Lieberman HR. Elevation-dependent symptom, mood, and performance changes produced by exposure to hypobaric hypoxia. International Journal of Aviation Psychology. 1998;8(4):319–334. doi: 10.1207/s15327108ijap0804_1. [DOI] [PubMed] [Google Scholar]
- 5.Frisancho AR. Functional adaptation to high altitude hypoxia. Science. 1975;187(4174):313–319. doi: 10.1126/science.1089311. [DOI] [PubMed] [Google Scholar]
- 6.Peacock AJ. Medical problems of high altitude. Journal of the Royal College of Physicians of Edinburgh. 2008;38(2):126–128. [Google Scholar]
- 7.Singh I, Khanna PK, Srivastava MC, Lal M, Roy SB, Subramanyam CS. Acute mountain sickness. The New England Journal of Medicine. 1969;280(4):175–184. doi: 10.1056/NEJM196901232800402. [DOI] [PubMed] [Google Scholar]
- 8.Sutton JR, Coates G, Houston H, editors. Hypoxia and Mountain Medicine: Proceedings of the 7th International Hypoxia Symposium Held at Lake Louise, Canada, February 1991. Vol. 84. Oxford, UK: Pergamon; 1992. The Lake Louise Consensus on the definition and quantification of altitude illness; pp. 327–330. (Advances in the Biosciences). [Google Scholar]
- 9.Barnes PJ, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. The New England Journal of Medicine. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 10.Karin M. The beginning of the end: IκB kinase (IKK) and NF-κB activation. Journal of Biological Chemistry. 1999;274(39):27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 11.Bhattarai NK. Folk herbal medicines of Dolakha district, Nepal. Fitoterapia. 1993;64(5):387–395. [Google Scholar]
- 12.Koyama K, Imaizumi T, Akiba M, et al. Antinociceptive components of Ganoderma lucidum. Planta Medica. 1997;63(3):224–227. doi: 10.1055/s-2006-957658. [DOI] [PubMed] [Google Scholar]
- 13.Gong XJ, Ji H, Lu SG, et al. Effects of polysaccharides from cultured Cordyceps sinensis on murine immunologic function. Zhongguo Yaoke Daxue Xuebao. 2000;31:53–55. [Google Scholar]
- 14.Yamaguchi Y, Kagota S, Nakamura K, Shinozuka K, Kunitomo M. Antioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensis . Phytotherapy Research. 2000;14(8):647–649. doi: 10.1002/1099-1573(200012)14:8<647::aid-ptr670>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Guo YZ. Medicinal chemistry, pharmacology and clinical applications of fermented mycelia of Cordyceps sinensis and JinShuBao capsule. Journal of Modern Diagnostics and Therapeutics. 1986;1:60–65. [Google Scholar]
- 16.Zhu JS, Rippe JM. CordyMax enhances aerobic capability, endurance performance, and exercise metabolism in healthy, mid-age to elderly sedentary humans. The FASEB Journal. 2004;18(4):p. A931. [Google Scholar]
- 17.Mizuno T. Medicinal effects and utilization of Cordyceps (Fr.) Link (Ascomycetes) and Isaria Fr. (Mitosporic fungi) Chinese caterpillar fungi, ‘Tochukaso’. International Journal of Medicinal Mushrooms. 1999;1(3):251–262. [Google Scholar]
- 18.Bao ZD, Wu ZG, Zheng F. Amelioration of aminoglycoside nephrotoxicity by Cordyceps sinensis in old patients. Chinese Journal of Integrated Traditional and Western Medicine. 1994;14(5):259–271. [PubMed] [Google Scholar]
- 19.Chen DG. Effects of JinShuiBao capsule on the quality of life of patients with heart failure. Journal of Administration of Traditional Chinese Medicine. 1995;5:40–43. [Google Scholar]
- 20.Ji DB, Ye J, Li CL. Antiaging effect of Cordyceps sinensis extract. Phytotherapy Research. 2009;23(1):116–122. doi: 10.1002/ptr.2576. [DOI] [PubMed] [Google Scholar]
- 21.Park DK, Choi WS, Park P-J, et al. Immunoglobulin and cytokine production from mesenteric lymph node lymphocytes is regulated by extracts of Cordyceps sinensis in C57Bl/6N mice. Journal of Medicinal Food. 2008;11(4):784–788. doi: 10.1089/jmf.2007.0550. [DOI] [PubMed] [Google Scholar]
- 22.Singleton VL, Rossi JA. Calorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- 23.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 1999;64(4):555–559. [Google Scholar]
- 24.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytotherapy Research. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Faro AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 28.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 29.Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 30.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Analytical Biochemistry. 1976;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 31.Costantino L, Albasini A, Rastelli G, Benvenuti S. Activity of polyphenolic crude extracts as scavengers of superoxide radicals and inhibitors of xanthine oxidase. Planta Medica. 1992;58(4):342–344. doi: 10.1055/s-2006-961481. [DOI] [PubMed] [Google Scholar]
- 32.Hui MY, Wang B-S, Huang SC, Duh P-D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. Journal of Agricultural and Food Chemistry. 2006;54(8):3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- 33.Dong C-H, Yao Y-J. In vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensis . Food Science and Technology. 2008;41(4):669–677. doi: 10.1016/j.lwt.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li SP, Li P, Dong TTX, Tsim KWK. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine. 2001;8(3):207–212. doi: 10.1078/0944-7113-00030. [DOI] [PubMed] [Google Scholar]
- 35.Bauerle PA, Henkel T. Function and activation of NK-κB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 36.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. Journal of Neuroscience. 2005;25(44):10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochimica et Biophysica Acta. 2000;1517(1):19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 38.Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. Journal of Biological Chemistry. 2000;275(21):16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 39.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends in Immunology. 2003;24(8):449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 40.Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. American Journal of Physiology—Renal Physiology. 2007;293(3):F914–F919. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- 41.Hollander J, Fiebig R, Gore M, et al. Superoxide dismutase gene expression in skeletal muscle: fiber-specific adaptation to endurance training. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 1999;277(3):R856–R862. doi: 10.1152/ajpregu.1999.277.3.R856. [DOI] [PubMed] [Google Scholar]
- 42.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological Reviews. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C-S, Yin W-T, Wang J-Y, et al. CordyMax™ Cs-4 improves glucose metabolism and increases insulin sensitivity in normal rats. Journal of Alternative and Complementary Medicine. 2002;8(3):309–314. doi: 10.1089/10755530260127998. [DOI] [PubMed] [Google Scholar]