Abstract
Klebsiella pneumoniae is the predominant pathogen isolated from liver abscess of diabetic patients in Asian countries. With the spread of multiple-drug-resistant K. pneumoniae, there is an increasing need for the development of alternative bactericides and approaches to block the production of bacterial virulence factors. Capsular polysaccharide (CPS), especially from the K1 and K2 serotypes, is considered the major determinant for K. pneumoniae virulence. We found that extracts of the traditional Chinese medicine Fructus mume inhibited the growth of K. pneumoniae strains of both serotypes. Furthermore, Fructus mume decreased the mucoviscosity, and the CPS produced in a dose-dependent manner, thus reducing bacterial resistance to serum killing. Quantitative reverse transcription polymerase chain reaction analyses showed that Fructus mume downregulated the mRNA levels of cps biosynthesis genes in both serotypes, possibly by increasing the intracellular iron concentration in K. pneumoniae. Moreover, citric acid, a major organic acid in Fructus mume extracts, was found to have an inhibitory effect on growth and CPS biosynthesis in K. pneumoniae. Taken together, our results indicate that Fructus mume not only possesses antibacterial activity against highly virulent K. pneumoniae strains but also inhibits bacterial CPS biosynthesis, thereby facilitating pathogen clearance by the host immune system.
1. Introduction
Klebsiella pneumoniae is an enteric gram-negative bacterium that causes community-acquired diseases, including pneumonia, bacteremia, septicemia, and urinary and respiratory tract infections, particularly in immunocompromised patients [1–4]. In Asian countries, especially in Taiwan and Korea, K. pneumoniae is the predominant pathogen responsible for pyogenic liver abscess in diabetic patients [2, 3, 5, 6]. In recent years, reports of Klebsiella liver abscess (KLA) in western countries have also been accumulating [6, 7]. Among the virulence factors identified in K. pneumoniae, capsular polysaccharide (CPS) is considered as the major determinant for K. pneumoniae virulence. Pyogenic liver abscess isolates often carry heavy CPS loads that could protect the bacteria from phagocytosis and killing by serum factors [6–8]. The capsular serotypes of K. pneumoniae have been classified into more than 77 known types [9, 10]. In Taiwan, a high prevalence of the K1 and K2 serotypes of K. pneumoniae has been documented in liver abscess in diabetes mellitus patients [11]. Extended spectrum β-lactamase- (ESBL-) producing K. pneumoniae, for which clinical treatment is difficult, has a wide distribution [4, 12]. As a result, there is an urgent need to develop a novel antimicrobial strategy to block the production of virulence factors.
In accordance with the significance of CPS in the physiology and pathogenesis of K. pneumoniae, the biosynthesis of CPS is controlled by a complex network of multiple regulators such as the Rcs system, RmpA, RmpA2, KvhR, KvgAS, and KvhAS [13–16]. Recently, we found that CPS production by K. pneumoniae was controlled by external iron and glucose concentrations via the regulation of ferric uptake regulator (Fur) and cAMP-dependent carbon catabolite repression (CCR), respectively [17, 18]. Iron availability has been demonstrated to affect multiple cellular functions such as oxidative stress, energy metabolism, acid tolerance, and virulence factor production in many bacteria [19–22]. Likewise, cAMP signaling has been demonstrated to regulate the expression of various genes encoding carbon metabolism enzymes and virulence factors, such as flagella, fimbriae, protease, exotoxin, and secretion systems [23–32]. These studies indicate that, in response to specific environmental signals, pathogenic bacteria express genes encoding virulence factors that help them to establish a successful infection.
Traditional Chinese medicine (TCM) has been a rich natural source of antimicrobial agents for treating various infectious diseases for more than 4,000 years. Mei, Prunus mume, has long played an important role in human diet and health. Fructus mume, the smoked fruit of Prunus mume, is a TCM that has been used to relieve cough, treat ulceration, and improve digestive function. Prunus mume extract has also been shown to inhibit Helicobacter pylori infection, which is associated with gastritis and gastric ulcers [33]. In addition, Fructus mume extract is a potential candidate for developing an oral antimicrobial agent to control or prevent dental diseases associated with several oral pathogenic bacteria [34–36]. A study involving high-pressure liquid chromatography (HPLC) analysis has also demonstrated that citric acid is the main organic acid in Fructus mume extract [35, 36].
In this study, we aimed to assess the antibacterial activity of Fructus mume against K. pneumoniae, and 2 highly virulent clinical strains, NTUH-K2044 and CG43S3, respectively, belonging to the K1 and K2 serotypes, were used in the following analyses. We found that Fructus mume not only possess antibacterial activity against the 2 K. pneumoniae strains but also reduced bacterial CPS production, thus decreasing the survival rate of bacteria in normal human serum. The regulatory effect of Fructus mume on cps gene expression in K. pneumoniae has also been clarified.
2. Materials and Methods
2.1. K. pneumoniae Strains and Primers
K. pneumoniae strains and primers used in this study are listed in Table 1. Bacteria were routinely cultured at 37°C in Luria-Bertani (LB) broth or agar plate.
Table 1.
Bacteria strains and primer used in this study.
(a)
| Strains | Descriptions | Reference or source |
|---|---|---|
| K. pneumoniae | ||
| NTUH-K2044 | K1 serotype | From Dr. Jin-Town Wang |
| CG43S3 | K2 serotype | From Dr. Hwei-Ling Peng |
(b)
| Primer | Sequence (5′→3′) | TaqMan probes | Target |
|---|---|---|---|
| RT03 | CGTCATCCAGACCAAAGAGC | 83 | orf1 in K1 cps gene cluster |
| RT04 | CCGGTTTTTCAATAAACTCGAC | orf1′ in K2 cps gene cluster | |
| RT134 | TACCGGGACAGAGAATGAGC | 78 | orf3 in K1 cps gene cluster |
| RT135 | TAACTGGCCAACCCAAGGT | ||
| RT136 | CGTTTTATGGTAATGTTCTCCTCA | 26 | orf7 in K1 cps gene cluster |
| RT137 | TCTGCCCATAACCTCGAAAG | ||
| RT05 | CGATGACCGGCTTTTTAATG | 83 | orf3′ in K2 cps gene cluster |
| RT06 | CTAGCGGAGATTTGGTACTGC | ||
| RT07 | CAGTCCACCTTTATTCCGATTG | 67 | orf16′ in K2 cps gene cluster |
| RT08 | AGGTACGACCCCGACTGG |
2.2. Preparation of Fructus mume Extract
The concentrated herbal medicine, Fructus mume, was purchased from Chuang Song Zong Pharmaceutical Co. Ltd. (Kaohsiung, Taiwan) under the good manufacturing practice (GMP) criteria. Fructus mume powder was dissolved in LB broth by end-over-end mixing at room temperature for 2 h. The extract of Fructus mume was collected by centrifugation (13,000 rpm for 10 min) to remove starch and then filtered by 0.45 μm filter.
2.3. Antibacterial Activity of Fructus mume Extract
LB broth or LB broth supplemented with 5, 10, or 20 mg/mL Fructus mume extract was initially inoculated with K. pneumoniae NTUH-K2044 or CG43S3 (approximate 108 CFU/mL). Cultures were incubated at 37°C, and samples were taken at 0, 2, 6, and 24 h to determine the viable counts (CFU/mL) for each strain. The assay was performed in triplicate, each with triplicate samples.
2.4. Assessment of K. pneumoniae Mucoviscosity and CPS Production
The mucoviscosity of K. pneumoniae was measured by a low speed centrifugation as previously described [37]. Briefly, equal numbers of overnight-cultured bacteria were centrifuged at 6000 g for 5 min. Then, formation of the bacterial pellet could be observed. To further evaluate the CPS amount of K. pneumoniae, the bacterial CPS was extracted and then quantified as previously described [38]. The glucuronic acid content, representing the amount of K. pneumoniae K1 and K2 CPS, was determined from a standard curve of glucuronic acid (Sigma-Aldrich) and expressed as micrograms per 109 CFU [39].
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNAs were isolated from early-exponential-phase grown bacteria cells by use of the RNeasy midi-column (QIAGEN) according to the manufacturer's instructions. RNA was DNase-treated with RNase-free DNase I (MoBioPlus) to eliminate DNA contamination. RNA of 100 ng was reverse-transcribed with the Transcriptor First Strand cDNA Synthesis Kit (Roche) using random primers. qRT-PCR was performed in a Roche LightCycler 1.5 Instrument using LightCycler TaqMan Master (Roche). Primers and probes were designed for selected target sequences using Universal ProbeLibrary Assay Design Center (Roche-applied science) and listed in Table 1. Data were analyzed using the real time PCR software of Roche LightCycler 1.5 Instrument. Relative gene expressions were quantified using the comparative threshold cycle 2−ΔΔCT method with 23S rRNA as the endogenous reference.
2.6. Bacterial Survival in Serum
Normal human serum, pooled from healthy volunteers, was divided into equal volumes and stored at −70°C before use. Bacterial survival in serum was determined with minor modifications [37]. In brief, LB broth or LB broth supplemented with 5, 10, or 20 mg/mL Fructus mume extract was initially inoculated with K. pneumoniae NTUH-K2044 or CG43S3 (approximate 108 CFU/mL). After overnight incubation at 37°C, the bacteria were collected, washed twice with phosphate-buffered saline (PBS), and then adjusted to approximately 1 × 106 CFU/mL. The reaction mixture containing 250 μL of the cell suspension and 750 μL of pooled human serum was incubated at 37°C for 15 min. The number of viable bacteria was then determined by plate counting. The survival rate was expressed as the number of viable bacteria treated with human serum compared to the number of pretreatment. The assay was performed in triplicate, each with triplicate samples.
2.7. Streptonigrin Sensitivity
To measure bacterial susceptibility to the iron-activated antibiotic streptonigrin, overnight grown K. pneumoniae were 1 : 10 diluted in LB or LB broth supplemented with different concentrations of Fructus mume extract. After 2 h incubation with or without streptonigrin (2 μg/mL) at 37°C with agitation, aliquots (5 μL) of cultures serially diluted tenfold in LB broth were spotted onto LB agar. The plates were incubated at 37°C overnight and photographed.
2.8. Statistical Method
An unpaired t-test was used to determine the statistical significance, and values of P < 0.05 and P < 0.01 were considered significant. Each sample was assayed in triplicate, and the mean activity and standard deviation are presented.
2.9. Ethics Statement
For isolation of normal human serum from healthy volunteers, the procedure and the respective consent documents were approved by the Ethics Committee of the China Medical University Hospital, Taichung, Taiwan. All healthy volunteers provided written informed consent.
3. Results
3.1. Fructus mume Inhibits the Growth of K. pneumoniae
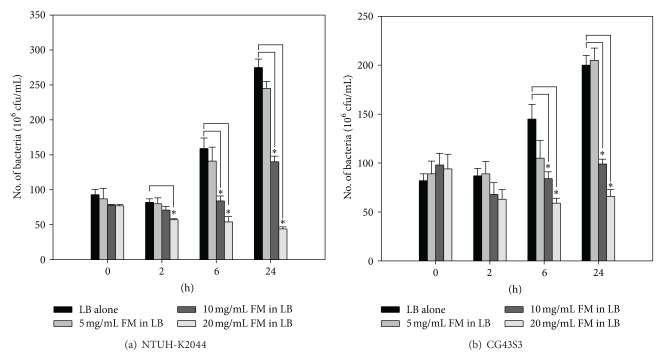
To examine the antibacterial activity of Fructus mume, K. pneumoniae NTUH-K2044 and CG43S3 were cocultured with increasing amounts of Fructus mume extract, and bacterial growth was monitored by plate counting. As shown in Figure 1, compared to the results for the control groups, addition of 5 mg/mL Fructus mume extract to LB broth did not obviously influence the growth of the 2 strains at any time interval, while the addition of 10 or 20 mg/mL Fructus mume extract caused significant growth reduction after 6 and 24 h incubations. This inhibitory effect of Fructus mume extract is dose dependent. Besides, Fructus mume extract appeared to exert a stronger growth inhibitory effect in the case of NTUH-K2044 than in the case of CG43S3. Bactericidal activity of the 20 mg/mL Fructus mume extract was observed for NTUH-K2044 but not CG43S3. Additionally, the pH values of LB broth supplemented with 5, 10, and 20 mg/mL FM extract were ∼5.0, 4.4, and 3.6, respectively.
Figure 1.
Antibacterial activity of the Fructus mume extract against K. pneumoniae. The addition of different concentrations of Fructus mume extract (FM), as indicated, to LB broth affects the growth of K. pneumoniae NTUH-K2044 (a) or CG43S3 (b). LB broth only inoculated with the bacteria serves as a negative control. *, P < 0.05 compared to the indicated group.
3.2. Fructus mume Reduces the Biosynthesis of CPS
In the case of K. pneumoniae strains, high mucoviscosity, resulting from a large amount of surface CPS, has been correlated with increased pathogenicity [8, 40]. To investigate whether Fructus mume affects this mucoviscosity, NTUH-K2044 and CG43S3 were, respectively, cocultured with increasing amounts of Fructus mume extract. After 24 h of incubation, the sedimentation test revealed that the addition of Fructus mume extract to LB broth obviously decreased mucoviscosity in the case of 2 K. pneumoniae strains; K. pneumoniae cocultured with Fructus mume extract formed a compact pellet after centrifugation, while the control group could not be pelleted down (Figure 2). Since the addition of 5 mg/mL Fructus mume extract did not influence bacterial growth (Figure 1), we suggest that Fructus mume affected the mucoviscosity by regulating the biosynthesis of CPS. As shown in Table 2, in comparison with the control group, NTUH-K2044 produced a larger amount of CPS, approximately 1.55 fold, than that of CG43S3. The addition of Fructus mume extract reduced CPS production in both strains in a dose-dependent manner. Moreover, Fructus mume extract appeared to exert a stronger inhibitory effect on NTUH-K2044 than on CG43S3 (Table 2).
Figure 2.
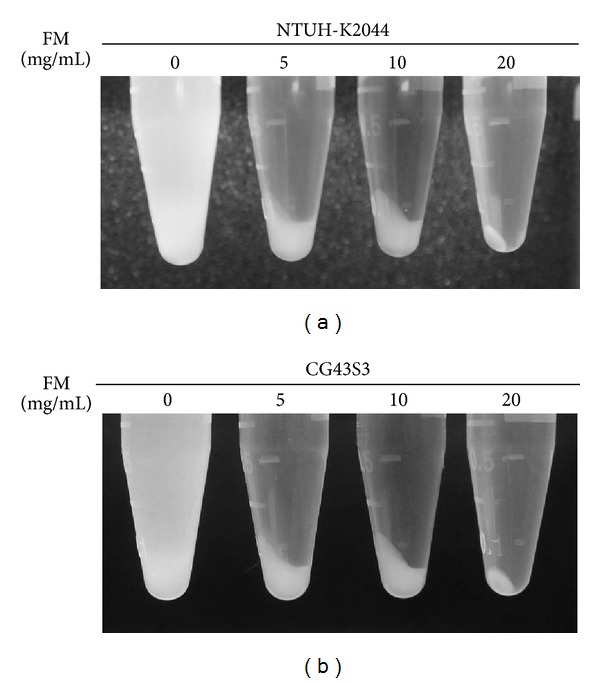
Fructus mume reduces K. pneumoniae mucoviscosity. Different concentrations of Fructus mume extract (FM), as indicated, were added to LB broth inoculated with K. pneumoniae NTUH-K2044 (a) or CG43S3 (b). After overnight incubation at 37°C, the bacterial mucoviscosity was assessed by a low speed centrifugation. LB broth only inoculated with the bacteria serves as a negative control.
Table 2.
Quantification of CPS amount of K. pneumoniae strains cocultured with Fructus mume extract.
| Fructus mume (mg/mL) | CPS amounta (% relative to the control group) | |
|---|---|---|
| NTUH-K2044 | CG43S3 | |
| 0 | 27.33 ± 3.45 (100) | 17.61 ± 1.53 (100) |
| 5 | 15.46 ± 2.18 (56.6) | 13.15 ± 1.0 (74.7) |
| 10 | 11.42 ± 1.69 (41.8) | 10.86 ± 1.05 (61.7) |
| 20 | NDb | NDb |
aglucuronic acid content (μg/109 cfu).
bND: not determined.
3.3. Fructus mume Reduces the Serum Resistance Activity of K. pneumoniae
CPS plays a crucial role in the resistance of K. pneumoniae to serum killing. Since the Fructus mume extract decreased the CPS production of K. pneumoniae, the effect of the extract on bacterial serum resistance was further analyzed. NTUH-K2044 or CG43S3 was, respectively, cocultured with 5, 10, or 20 mg/mL Fructus mume extract, and the bacteria were then collected, washed, and subjected to incubation with pooled human sera. After 15 min of incubation, the survival rate of the bacteria was determined by plate counting. As shown in Figure 3, Fructus mume extract obviously decreased the serum resistance activity of NTUH-K2044 and CG43S3 in a dose-dependent manner, possibly due to reduced CPS production by the bacteria.
Figure 3.
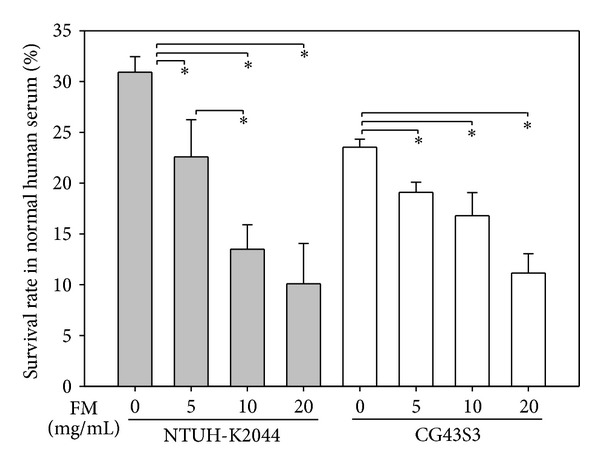
Effect of Fructus mume on K. pneumoniae susceptibility to normal human serum. Different concentrations of Fructus mume extract (FM) were added to LB broth inoculated with K. pneumoniae NTUH-K2044 (a) or CG43S3 (b), as indicated in the margin. After overnight incubation at 37°C, the bacterial serum resistance was determined. LB broth only inoculated with the bacteria serves as a negative control. *, P < 0.05 compared to the indicated group.
3.4. Effect of Fructus mume on cps Transcription
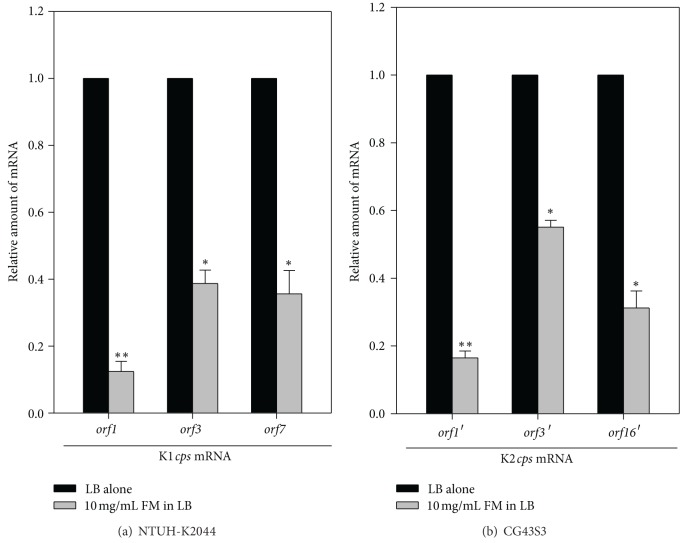
The biosynthesis of K. pneumoniae K1 and K2 CPS is controlled by 20 and 17 genes, respectively [41, 42]. Both the K1 and K2 cps gene clusters contain 3 transcriptional units: orf1-2, orf3-6, and orf7-20 in the K1 cps gene cluster and orf1′-2′, orf3′-15′, and orf16′-17′ in the K2 cps gene cluster [41, 42]. To investigate how Fructus mume extract affects the biosynthesis of K. pneumoniae CPS, NTUH-K2044 and CG43S3 were cocultured with 10 mg/mL Fructus mume extract, and the mRNA levels of the 3 transcripts belonging to the K1 or K2 cps gene cluster were measured by qRT-PCR. As shown in Figure 4, compared to the control group, the addition of 10 mg/mL Fructus mume extract obviously reduced the mRNA levels of all the cps transcripts, suggesting that Fructus mume regulated CPS biosynthesis at the transcriptional level.
Figure 4.
Fructus mume downregulates cps transcription. qRT-PCR analyses of the expression of the K1 cps genes (orf1, orf3, and orf7) in NTUH-K2044 (a) or K2 cps genes (orf1′, orf3′, and orf16′) in CG43S3 (b) in LB or LB broth supplemented with 10 mg/mL Fructus mume extract (FM). *, P < 0.05 and **, P < 0.01 compared to LB alone.
3.5. Fructus mume Reduces CPS Biosynthesis by Regulating K. pneumoniae Intracellular Iron Concentration
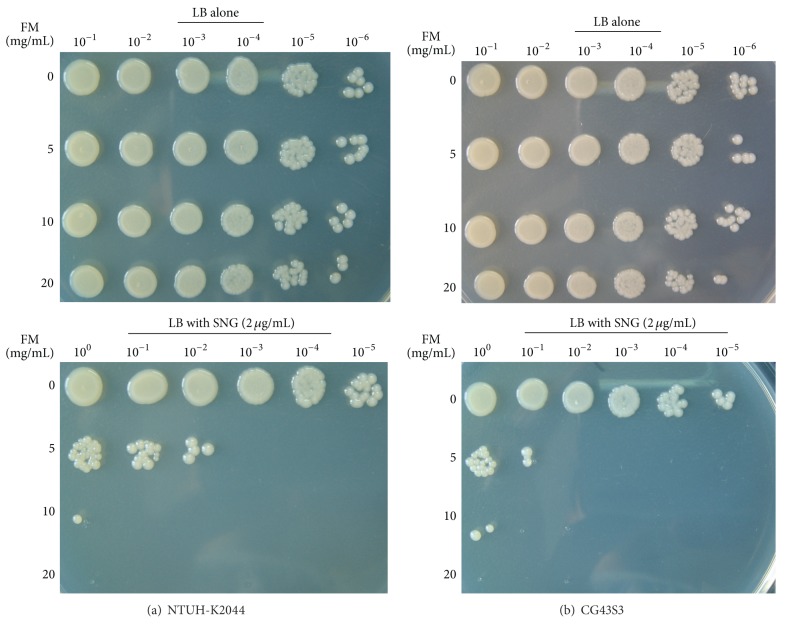
We have previously demonstrated that iron depletion activated CPS production in K. pneumoniae at the transcriptional level [18]. To analyze whether iron is involved in Fructus mume-regulated CPS production, we assessed intracellular iron levels in K. pneumoniae cocultured with increasing amounts of Fructus mume extract, using the iron-activated antibiotic streptonigrin, which requires iron for its bactericidal action that causes DNA degradation [43]. As shown in Figure 5, when NTUH-K2044 or CG43S3 was grown in LB broth only, the bacteria exhibited a streptonigrin-resistant phenotype. However, the streptonigrin susceptibility of the bacteria increased upon coculturing with increasing amounts of Fructus mume extract, suggesting that Fructus mume increased the intracellular level of free iron in a dose-dependent manner in K. pneumoniae.
Figure 5.
Fructus mume increases K. pneumoniae susceptibility to streptonigrin. K. pneumoniae NTUH-K2044 (a) or CG43S3 (b) cocultured with different concentrations of Fructus mume extract (FM) were grown in LB alone or LB that supplemented with 2 μg/mL streptonigrin (SNG) incubated for 2 h. Then, tenfold serial dilutions were spotted onto an LB agar to observe the colony formation.
3.6. Citric Acid Inhibits K. pneumoniae Growth and CPS Biosynthesis
Citric acid has been demonstrated to be the main organic acid in Fructus mume extract [35]. To examine if citric acid plays a role in the inhibitory effects of Fructus mume extract on K. pneumoniae growth and CPS production, NTUH-K2044 and CG43S3 were cocultured with various concentrations of citric acid, and then the growth curves of the bacteria were monitored. The result showed that citric acid obviously reduced the growth of NTUH-K2044 and CG43S3 in a dose-dependent manner (Figure 6(a)). Furthermore, CPS production in the 2 K. pneumoniae strains also decreased when citric acid was added to the growth medium (Figure 6(b)). These results indicated that citric acid is an active component of Fructus mume extract that has bactericidal activity and downregulates CPS biosynthesis in K. pneumoniae.
Figure 6.
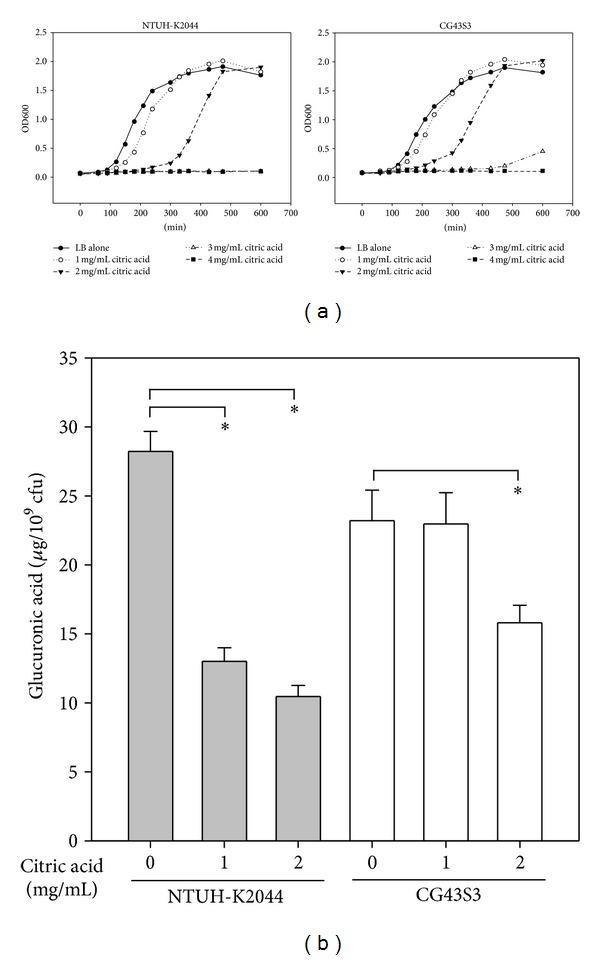
Citric acid inhibits K. pneumoniae growth and CPS levels. K. pneumoniae NTUH-K204 or CG43S3 cocultured with different concentrations of citric acid, as indicated, and the growth curve (a) as well as the CPS levels (b) were measured. Bacteria were grown in LB broth at 37°C with agitation. *, P < 0.05 compared to the indicated group.
4. Discussion
Since the 1980s, K. pneumoniae is emerging as an important pathogen in both community and hospital settings [44]. In the hospital environment, due to the extensive use of antibiotics, multiple drug resistance has been increasingly observed in K. pneumoniae, especially in ESBL-producing strains. Carbapenems are considered to be the preferred agents for the treatment of serious infections caused by ESBL-producing K. pneumoniae because of their high stability with respect to β-lactamase hydrolysis and the observed retained susceptibility of ESBL producers [45]. However, K. pneumoniae isolates resistant to carbapenems have been reported worldwide since the 2000s [46–49]. The emergence of carbapenem-resistant enterobacteria is worrisome because the option for antimicrobial treatment is further restricted. In this study, we screened a series of TCMs for the identification of new antibacterial agents (data not shown) and then focused on Fructus mume, the smoked fruit of Prunus mume, which has been demonstrated to efficiently inhibit H. pylori and other oral bacteria [33–36]. As shown in Figure 1, Fructus mume extract was found to have antibacterial activity against clinically isolated K. pneumoniae strains.
Clinically isolated K. pneumoniae strains usually produce a large amount of CPS, which confers not only a mucoid phenotype to the bacteria but also resistance to engulfment by phagocytes and to serum bactericidal factors [40, 50]. The degree of mucoviscosity has also been positively correlated with the successful establishment of infection [51, 52]. Among the 77 described capsular types of K. pneumoniae, K1 and K2 serotypes are highly virulent in experimental infection in mice and are often associated with severe infections in humans and animals [52–55], particularly with the tight association between the 2 serotypes and liver abscess [11, 56]. As shown in Figure 2 and Table 2, in K. pneumoniae strains of both the K1 and K2 serotypes, Fructus mume extract significantly reduced bacterial hypermucoviscosity and CPS levels and thus may result in the decreased resistance of K. pneumoniae to serum killing (Figure 3). On the other hand, we also found that heat inactivated serum did not have obvious bactericidal effects on K. pneumoniae (data not shown), suggesting an importance of the complement system.
Both prokaryotic and eukaryotic cells in natural environments are constantly challenged by various environmental stresses. K. pneumoniae, like many gastrointestinal pathogens, needs to penetrate the gastric acid barrier, face the challenge of the immune system, and cope with the limited supply of oxygen and nutrition to effect colonization and infection [57, 58]. Therefore, bacteria activate or repress the expression of virulence genes to adapt to environmental stimuli [59–62]. In K. pneumoniae, CPS biosynthesis is regulated by multiple environmental stimuli and protein regulators. Our previous studies have shown that ferric ions can repress K. pneumoniae CPS production through Fur regulation [18, 63]. Under iron-repletion conditions, Fur-Fe(II) can tightly repress cps transcription, resulting in lowered CPS production in K. pneumoniae [18]. To further investigate how Fructus mume reduces CPS levels in K. pneumoniae, we performed qRT-PCR analyses and found that CPS reduction was regulated at the transcriptional level (Figure 4). All 3 transcripts of K1 or K2 cps genes were obviously downregulated, especially orf1/orf1′ encoding GalF, a putative UDP-glucose pyrophosphatase (Figure 4). In addition, the streptonigrin sensitivity assay also indicated that Fructus mume could increase the intracellular iron levels of K. pneumoniae (Figure 5), implying that ferric iron and Fur participate in Fructus mume-mediated CPS reduction. Iron is essential to most bacteria for growth and reproduction, but iron overloading would lead to the formation of undesired reactive oxygen species (ROS) by the Fenton reaction to damage DNA or other biological macromolecules [64]. Although how Fructus mume affects bacterial iron-uptake remains unknown, the increase in intracellular iron leading to the formation of undesired ROS may account for part of its bactericidal activity.
The ingredients of Prunus mume extract are organic acids, including citric acid (the main ingredient), tartaric acid, oxalic acid, and other unknown components [35, 36]. Although the acidic components of Prunus mume showed some antibacterial activity, it is less compared to that of the original extract, implying that other active components possess considerable antibacterial activity [36]. As seen in Figure 6, we also showed that citric acid could inhibit growth and CPS production in K. pneumoniae. The acidic property may be one of the antibacterial mechanisms of Fructus mume; however, there should be other active components, since LB broth supplemented with 20 mg/mL Fructus mume, pH ∼3.6, has stronger bactericidal activity than LB broth adjusted to pH 3.5 using HCl (data not shown). For future clinical application, more studies are required to determine the active components of Fructus mume and its mechanism of action.
5. Conclusion
To our knowledge, this study is the first to report the antibacterial activity of Fructus mume against highly virulent K. pneumoniae isolates. Moreover, we found that Fructus mume reduced K. pneumoniae CPS biosynthesis at the transcriptional level, possibly by regulating the intracellular iron concentration of the bacteria, thereby helping the host immune system eliminate the pathogen.
Conflict of Interests
The authors declare no conflict of interests in this work.
Authors' Contribution
T.-H. Lin, S.-H. Huang, and C.-C. Wu contributed equally to this work.
Acknowledgments
The authors thank Professor Hwei-Ling Peng from National Chiao Tung University, Taiwan, for providing the K. pneumoniae CG43S3 and Dr. Jin-Town Wang from National Taiwan University Hospital for providing K. pneumoniae NTUH-K2044. The authors thank Yi-Ming Hong and Jing-Ciao Lin for their technical assistance during the study. The work is supported by the Grants from National Science Council (NSC 99-2320-B-039-002-MY3), China Medical University (CMU98-ASIA-01), and Buddhist Tzu Chi general hospital (TTCRD101-17).
References
- 1.Chou F, Kou H. Endogenous endophthalmitis associated with pyogenic hepatic abscess. Journal of the American College of Surgeons. 1996;182(1):33–36. [PubMed] [Google Scholar]
- 2.Han S-HB. Review of hepatic abscess from Klebsiella pneumoniae—an association with diabetes mellitus and septic endophthalmitis. Western Journal of Medicine. 1995;162(3):220–224. [PMC free article] [PubMed] [Google Scholar]
- 3.Lau Y, Hu B, Wu W, Lin Y, Chang H, Shi Z. Identification of a major cluster of Klebsiella pneumoniae isolates from patients with liver abscess in Taiwan. Journal of Clinical Microbiology. 2000;38(1):412–414. doi: 10.1128/jcm.38.1.412-414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng HL, Wang PY, Wu JL, Chiu CT, Chang HY. Molecular epidemiology of Klebsiella pneumoniae . Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1991;24(3):264–271. [PubMed] [Google Scholar]
- 5.Yang Y, Siu LK, Yeh K, et al. Recurrent Klebsiella pneumoniae liver abscess: clinical and microbiological characteristics. Journal of Clinical Microbiology. 2009;47(10):3336–3339. doi: 10.1128/JCM.00918-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. The Lancet Infectious Diseases. 2012;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 7.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. American Journal of Gastroenterology. 2005;100(2):322–331. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 8.Sahly H, Podschun R, Oelschlaeger TA, et al. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae . Infection and Immunity. 2000;68(12):6744–6749. doi: 10.1128/iai.68.12.6744-6749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung C, Hu B, Chang F, et al. A 5-year study of the seroepidemiology of Klebsiella pneumoniae: high prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. Journal of Infectious Diseases. 2000;181(6):2075–2079. doi: 10.1086/315488. [DOI] [PubMed] [Google Scholar]
- 10.Pan Y, Fang H, Yang H, et al. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. Journal of Clinical Microbiology. 2008;46(7):2231–2240. doi: 10.1128/JCM.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung C-P, Chang F-Y, Lee S-C, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut. 2002;50(3):420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. American Journal of Medicine. 2006;119(6) supplement 1:S20–S28, S60–S70. doi: 10.1016/j.amjmed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Huang T, Liang W, Peng H. Homologous response regulators KvgA, KvhA and KvhR regulate the synthesis of capsular polysaccharide in Klebsiella pneumoniae CG43 in a coordinated manner. Journal of Biochemistry. 2006;140(3):429–438. doi: 10.1093/jb/mvj168. [DOI] [PubMed] [Google Scholar]
- 14.Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annual Review of Microbiology. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman S, Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Molecular Microbiology. 1991;5(7):1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 16.Stout V. Regulation of capsule synthesis includes interactions of the RcsC/RcsB regulatory pair. Research in Microbiology. 1994;145(5-6):389–392. doi: 10.1016/0923-2508(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 17.Lin CT, Chen YC, Jinn TR, Wu CC, Hong YM, Wu WH. Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae . PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0054430.e54430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C, Wu C, Chen Y, et al. Fur regulation of the capsular polysaccharide biosynthesis and iron-acquisition systems in Klebsiella pneumoniae CG43. Microbiology. 2010;157(part 2):419–429. doi: 10.1099/mic.0.044065-0. [DOI] [PubMed] [Google Scholar]
- 19.Hassett DJ, Sokol PA, Howell ML, et al. Ferric uptake regulator (Fur) mutants of Pseudomonas aeruginosa demonstrate defective siderophore-mediated iron uptake, altered aerobic growth, and decreased superoxide dismutase and catalase activities. Journal of Bacteriology. 1996;178(14):3996–4003. doi: 10.1128/jb.178.14.3996-4003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochsner UA, Vasil ML. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(9):4409–4414. doi: 10.1073/pnas.93.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijlsma JJE, Waidner B, Van Vliet AHM, et al. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infection and Immunity. 2002;70(2):606–611. doi: 10.1128/iai.70.2.606-611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Vliet AHM, Stoof J, Poppelaars SW, et al. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori fur repressor. The Journal of Biological Chemistry. 2003;278(11):9052–9057. doi: 10.1074/jbc.M207542200. [DOI] [PubMed] [Google Scholar]
- 23.Båga M, Göransson M, Normark S, Uhlin BE. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli . The EMBO Journal. 1985;4(13):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lory S, Wolfgang M, Lee V, Smith R. The multi-talented bacterial adenylate cyclases. International Journal of Medical Microbiology. 2004;293(7-8):479–482. doi: 10.1078/1438-4221-00297. [DOI] [PubMed] [Google Scholar]
- 25.Skorupski K, Taylor RK. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West SEH, Sample AK, Runyen-Janecky LJ. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. Journal of Bacteriology. 1994;176(24):7532–7542. doi: 10.1128/jb.176.24.7532-7542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez M, Huang I-H, Ohtani K, Grau R, Shimizu T, Sarker MR. Carbon catabolite repression of type IV pilus-dependent gliding motility in the anaerobic pathogen Clostridium perfringens . Journal of Bacteriology. 2008;190(1):48–60. doi: 10.1128/JB.01407-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalivoda EJ, Stella NA, O’Dee DM, Nau GJ, Shanks RMQ. The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Applied and Environmental Microbiology. 2008;74(11):3461–3470. doi: 10.1128/AEM.02733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller CM, Åberg A, Straseviçiene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathogens. 2009;5(2) doi: 10.1371/journal.ppat.1000303.e1000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs EL, Brutinel ED, Klem ER, Fehr AR, Yahr TL, Wolfgang MC. In vitro and in vivo characterization of the Pseudomonas aeruginosa cyclic AMP (cAMP) phosphodiesterase CpdA, required for cAMP homeostasis and virulence factor regulation. Journal of Bacteriology. 2010;192(11):2779–2790. doi: 10.1128/JB.00168-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endoh T, Engel JN. CbpA: a polarly localized novel cyclic AMP-binding protein in Pseudomonas aeruginosa . Journal of Bacteriology. 2009;191(23):7193–7205. doi: 10.1128/JB.00970-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stella NA, Kalivoda EJ, O’Dee DM, Nau GJ, Shanks RMQ. Catabolite repression control of flagellum production by Serratia marcescens . Research in Microbiology. 2008;159(7-8):562–568. doi: 10.1016/j.resmic.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enomoto S, Yanaoka K, Utsunomiya H, et al. Inhibitory effects of Japanese apricot (Prunus mume Siebold et Zucc.; Ume) on Helicobacter pylori-related chronic gastritis. European Journal of Clinical Nutrition. 2010;64(7):714–719. doi: 10.1038/ejcn.2010.70. [DOI] [PubMed] [Google Scholar]
- 34.Wong RWK, Hägg U, Samaranayake L, Yuen MKZ, Seneviratne CJ, Kao R. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A pilot study. International Journal of Oral and Maxillofacial Surgery. 2010;39(6):599–605. doi: 10.1016/j.ijom.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seneviratne CJ, Wong RWK, Hägg U, et al. Prunus mume extract exhibits antimicrobial activity against pathogenic oral bacteria. International Journal of Paediatric Dentistry. 2011;21(4):299–305. doi: 10.1111/j.1365-263X.2011.01123.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Wong RWK, Seneviratne CJ, et al. The antimicrobial efficacy of Fructus mume extract on orthodontic bracket: a monospecies-biofilm model study in vitro. Archives of Oral Biology. 2011;56(1):16–21. doi: 10.1016/j.archoralbio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Lai Y, Peng H, Chang H. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. Journal of Bacteriology. 2003;185(3):788–800. doi: 10.1128/JB.185.3.788-800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domenico P, Schwartz S, Cunha BA. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infection and Immunity. 1989;57(12):3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blumenkrantz N, Hansen GA. New method for quantitative determination of uronic acids. Analytical Biochemistry. 1973;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 40.Lin J, Chang F, Fung C, et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes and Infection. 2004;6(13):1191–1198. doi: 10.1016/j.micinf.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain chedid. Journal of Bacteriology. 1995;177(7):1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu K, Li N, Yan J, et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. Journal of Bacteriology. 2009;191(14):4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeowell HN, White JR. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrobial Agents and Chemotherapy. 1982;22(6):961–968. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keynan Y, Rubinstein E. The changing face of Klebsiella pneumoniae infections in the community. International Journal of Antimicrobial Agents. 2007;30(5):385–389. doi: 10.1016/j.ijantimicag.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Colodner R, Raz R, Chazan B, Sakran W. Susceptibility pattern of extended-spectrum β-lactamase producing bacteria isolated from inpatients to five antimicrobial drugs in a community hospital in Northern Israel. International Journal of Antimicrobial Agents. 2004;24(4):409–410. doi: 10.1016/j.ijantimicag.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. Journal of Antimicrobial Chemotherapy. 2010;65(6):1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 47.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. The Lancet Infectious Diseases. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. The Lancet Infectious Diseases. 2009;9(4):228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 49.Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-β-lactamase gene, bla NDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrobial Agents and Chemotherapy. 2009;53(12):5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regueiro V, Campos MA, Pons J, Albertí S, Bengoechea JA. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology. 2006;152(part 2):555–566. doi: 10.1099/mic.0.28285-0. [DOI] [PubMed] [Google Scholar]
- 51.Nassif X, Honoré N, Vasselon T, Cole ST, Sansonetti PJ. Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence-plasmid genes of Klebsiella pneumoniae . Molecular Microbiology. 1989;3(10):1349–1359. doi: 10.1111/j.1365-2958.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 52.Nassif X, Sansonetti PJ. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infection and Immunity. 1986;54(3):603–608. doi: 10.1128/iai.54.3.603-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizuta K, Ohta M, Mori M. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infection and Immunity. 1983;40(1):56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ofek I, Kabha K, Athamna A, et al. Genetic exchange of determinants for capsular polysaccharide biosynthesis between Klebsiella pneumoniae strains expressing serotypes K2 and K21a. Infection and Immunity. 1993;61(10):4208–4216. doi: 10.1128/iai.61.10.4208-4216.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simoons-Smit AM, Verwey-van Vught AMJJ, Kanis IYR, MacLaren DM. Virulence of Klebsiella strains in experimentally induced skin lesions in the mouse. Journal of Medical Microbiology. 1984;17(1):67–77. doi: 10.1099/00222615-17-1-67. [DOI] [PubMed] [Google Scholar]
- 56.Lee C, Leu H, Wu T, Su L, Liu J. Risk factors for spontaneous rupture of liver abscess caused by Klebsiella pneumoniae . Diagnostic Microbiology and Infectious Disease. 2005;52(2):79–84. doi: 10.1016/j.diagmicrobio.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 57.De Champs C, Sauvant MP, Chanal C, et al. Prospective survey of colonization and infection caused by expanded-spectrum-β-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. Journal of Clinical Microbiology. 1989;27(12):2887–2890. doi: 10.1128/jcm.27.12.2887-2890.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markowitz SM, Veazey JM, Jr., Macrina FL. Sequential outbreaks of infection due to Klebsiella pneumoniae in a neonatal intensive care unit: implication of a conjugative R plasmid. Journal of Infectious Diseases. 1980;142(1):106–112. doi: 10.1093/infdis/142.1.106. [DOI] [PubMed] [Google Scholar]
- 59.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annual Review of Biochemistry. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 60.Fuqua C, Greenberg EP. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Current Opinion in Microbiology. 1998;1(2):183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 61.Romby P, Vandenesch F, Wagner EGH. The role of RNAs in the regulation of virulence-gene expression. Current Opinion in Microbiology. 2006;9(2):229–236. doi: 10.1016/j.mib.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Current Opinion in Microbiology. 1999;2(6):582–587. doi: 10.1016/s1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 63.Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. Journal of Bacteriology. 2010;192(12):3144–3158. doi: 10.1128/JB.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Touati D. Iron and oxidative stress in bacteria. Archives of Biochemistry and Biophysics. 2000;373(1):1–6. doi: 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]