Abstract
Recent applications of mass spectrometry technology have dramatically increased our understanding of the proteomic diversity of high density lipoproteins (HDL). Depending on the method of HDL isolation, upwards of 85 proteins have been identified, and the list continues to grow. In addition to proteins consistent with traditionally accepted roles in lipid transport, HDL carries surprising constituents, such as members of the complement pathway, protease inhibitors involved in hemostasis, acute-phase response proteins, immune function mediators, and even metal-binding proteins. This compositional diversity fits well with hundreds of studies demonstrating a wide functional pleiotrophy, including roles in lipid transport, oxidation, inflammation, hemostasis, and immunity. This review summarizes the progression of our understanding of HDL proteomic complexity and points out key experimental observations that reinforce the functional diversity of HDL. The possibility of specific HDL subspecies with distinct functions, the evidence supporting this concept, and some of the best examples of experimentally defined HDL subspecies are also discussed. Finally, key challenges facing the field are highlighted, particularly the need to identify and define the function of HDL subspecies to better inform attempts to pharmacologically manipulate HDL for the benefit of cardiovascular disease and possibly other maladies.
Keywords: mass spectrometry, function, apolipoproteins, complement, protease inhibition, inflammation, innate immunity, lipid metabolism, hemostasis
HDL PROTEIN HETEROGENEITY: A BRIEF HISTORY
Working at the Pasteur Institute in Paris in 1929, Michel Macheboeuf reported the first detailed isolation of a lipid-rich “α-globulin” from horse serum, a fraction that would later become known as high density lipoprotein (HDL) (1). Although relatively rapid progress was made at characterizing the lipid composition of this fraction - phospholipids (PL, ∼25%), cholesterol (∼4%), triglycerides (TG, ∼3%) and cholesteryl esters (CE,∼12%) - it took nearly 40 years to begin to sort out the complexities of the protein component. Part of the delay may have resulted from the widespread believe that “apo-HDL” was likely single protein entity, much like low-density lipoprotein (LDL), which was dominated by a single polypeptide later identified as apolipoprotein (apo)B. Using amino acid analyses, early electrophoresis methods, and a variety chromatographic techniques, the first HDL protein moiety was identified in the late 1960s. Variously referred to as α-protein, “R-Thr” peptide (2), fraction III (3), and fraction II peptide (4), it later became known as apoA-I (5). Working in parallel, these laboratories also first recognized the possibility for heterogeneity within the HDL protein complement. This led to the identification of a second protein later known as apoA-II (5). The significance of this observation was not lost on these early pioneers. For example, the Shores observed, “The existence of multiple forms of polypeptides may be of considerable significance in the physiological and biochemical functions of the lipoproteins….” (6).
Around the same time, the Alupovic laboratory documented still further complexity in the HDL proteome by identifying the first of the minor constituents, the apoC peptides (5). Soon after, Mahley and colleagues noted the presence of the arginine-rich apoE in light fractions of HDL in dogs (HDL-1) (7), while others found apoD in the more dense HDL fractions (8). ApoF was also identified (9). Improvements in SDS-PAGE technology in the early 1980s revealed serum amyloid A (SAA) (10) and apoA-IV (11) in human HDL. Immunological studies using antibodies raised against isolated HDL showed that paraoxonase 1 (PON1) coeluted with apoA-I upon gel filtration of plasma and that immunoabsorption of apoA-I removed 90% of PON1 from plasma (12). Similarly, immunoabsorption of clustrin (apoJ) pulled down apoA-I, indicating its presence on HDL. It also became clear that several plasma enzymatic activities were associated with HDL, at least transiently. Single-spin vertical ultracentrifugation studies showed that lecithin:cholesterol acyl transferase (LCAT) was present in the higher density subfractions of HDL and also in LDL (13). Other factors known to remodel HDL included cholesteryl ester transfer protein (CETP) (14), phospholipid transfer protein (PLTP) (15), and platelet-activating factor aryl hydrolase (PAF-AH) (16), also known as lipoprotein-associated phospholipase A2.
By the early 1990s, HDL was generally thought to contain somewhere around 15 proteins. Indeed, one of the first two-dimensional gel electrophoresis studies of human plasma HDL referred to a set of “established HDL proteins” that included apoA-I, A-II, A-IV, C-II, C-III, D, and E, with mention of about 8 others that “can associate” with HDL (17) (six other protein spots were observed but not identified). In general, these fell into four major functional groups: i) proteins associated with lipid transport or lipoprotein integrity, i.e., the “apos” ii) lipolytic enzymes, such as LCAT and PON; iii) lipid transfer proteins, such as CETP and PLTP; and iv) acute-phase response proteins, such as SAA and apoJ/clustrin. The presence of these proteins fit well into the general dogma of a primary HDL function as a lipid transport vehicle.
APPLICATIONS OF MODERN PROTEOMICS TO HDL
Classical biochemical and immunological approaches were typically hampered by sensitivity issues or the fact that one needed a preconceived notion of what one was looking for prior to the experiment. However, the development of soft ionization techniques, such as electrospray (18) and matrix-assisted laser desorption ionization (MALDI) (19), for introducing large molecules into a mass spectrometer (MS) allowed for the development of sensitive and unbiased tandem MS approaches capable of identifying components of complex protein mixtures. In general, ionized proteolytic peptides in the gas phase are sorted by mass and then subjected to a controlled fragmentation, typically cleaving at the peptide bonds. The fragments are then evaluated by a second mass analyzer, and the resulting patterns are bioinformatically compared with theoretical patterns for all known proteins, effectively sequencing the peptides and producing a redundant list of proteins present in the initial mixture. Given that modern MS instrumentation can identify proteins across a 10,000-fold difference in concentration with sensitivities down to the nano- to picomole range, it is not uncommon to identify hundreds of proteins in a given biological sample. Although strategies can vary significantly between laboratories, MS-based proteomic approaches applied to HDL fall into two rough categories. In the first, HDL proteins are first separated by gel electrophoresis either by size only (1D) or by charge and then size (2D). The resulting gel spots are excised, digested with a protease, such as trypsin, and then identified by tandem MS. The second approach, sometimes referred to as the “shotgun” technique, starts by trypsinizing all proteins together in solution. The peptides are then separated by HPLC (typically by reverse phase) and subjected to electrospray ionization (ESI) as they elute from the column and then analyzed by tandem MS. A more detailed description of proteomic technologies can be found in Ref. 20.
One of the first applications of these technologies toward the HDL proteome was pursued by Karlsson et al. (21). Using a 2D electrophoresis/tandem MS approach, they identified 13 proteins in HDL2 and HDL3 separated by density ultracentrifugation from healthy donors. Of these, 11 were previously suspected HDL components from the biochemical studies summarized above. In addition, this study highlighted a key advantage of the charge dimension of the electrophoretic approach in that multiple isoforms of apoA-I, apoA-II, apoC-III, apoE, apoM, SAA, and SAA-4 were identified that varied with respect to sequence differences or posttranslational modifications. The next year, Rezaee et al. (22) used a multipronged approach of 1D and 2D electrophoresis, shotgun proteomics, and immunological assays to study centrifugally isolated total HDL from normal donors. This study first identified key mediators of the complement system - C3, C1 inhibitor, and complement factor H - implicating HDL in innate immunity. Likely, the most high-profile proteomic analysis of HDL was performed by Vaisar et al. (23). Using centrifugally isolated total HDL or HDL3 from normal subjects, they identified 48 HDL proteins. These included additional members of the complement family, strengthening the argument for a role of HDL in innate immunity. Others, such as the serine protease inhibitor (SERPIN) family, showed a clear theme of protease inhibition, including those involved in hemostasis. Overall, the Rezaee and Vaisar studies clearly demonstrated that the proteins associated with HDL are not limited to those involved in lipid transport. These concepts drove tremendous interest in further exploring the HDL proteome.
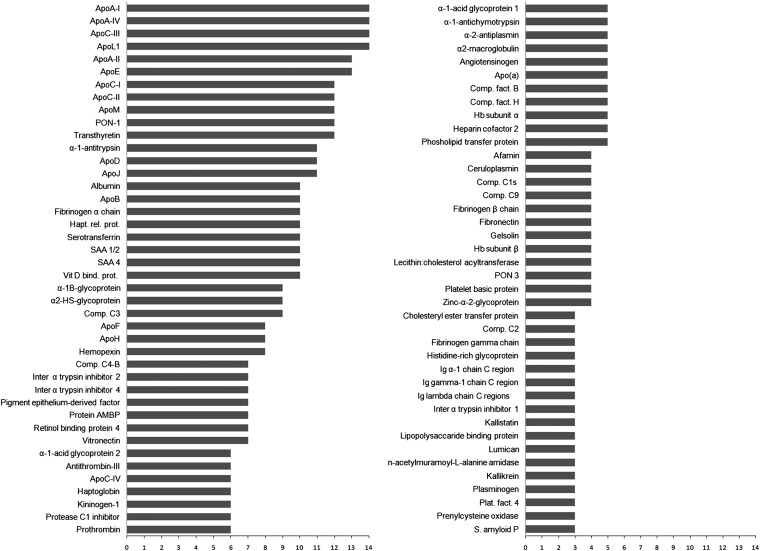
By the end of 2012, several laboratories had published some 14 studies that applied various MS techniques to understand the human HDL proteome. As sensitivity of the instrumentation has increased, so has the number of proteins that are proposed to be associated with HDL. These studies included HDL samples isolated by traditional density ultracentrifugation (21–31), immunoaffinity capture (32), size exclusion chromatography (33), and even anion-exchange and isoelectric focusing (S. M. Gordon and W. S. Davidson, unpublished observations). A summary of the key experimental details for each of these studies is given in Table 1. As a result of differences in instrumentation, sensitivity, HDL isolation technique, patient donors, and protein identification algorithms, the total list of putative HDL proteins can vary dramatically from study to study. Several estimates of the total number of HDL proteins have reached into the hundreds and have included hits that may induce skepticism. For example, certain intracellular and cell-surface proteins and even human skin keratin have been detected in HDL preparations. In an effort to manage this large amount of information and attempt to filter out instrument- or laboratory-specific artifacts, we have initiated the HDL Proteome Watch (http://homepages.uc.edu/~davidswm/HDLproteome.html or http://www.hdlforum.org/resources/links). This project tracks published reports that have used modern MS methodologies to study human HDL samples that have been physically separated in some way from plasma or serum. Currently, 204 individual proteins have been detected in human HDL samples. Of these, 85 proteins have appeared in at least three different studies (from independent laboratories), representing the best current estimate of the HDL proteome. These proteins are listed in Fig. 1.
TABLE 1.
Experimental details of recent MS-based proteomic studies of HDL
| Reference | Patient Population | HDL Type and Separation Technique | MS Approach | MS Database Searched | Gel Separation? |
| (21) | Pooled plasma from healthy adults (n = 4) | HDL2, HDL3 isolated by density gradient UC (KBr) | MALDI-TOF | NCBI and SwissProt | Yes |
| 2D gel | |||||
| (24) | Pool of plasma from >10,000 donors | Total HDL isolated by one-step density gradient UC (KBr) | MALDI-TOF | UniProt | Yes |
| 2D gel | |||||
| (25) | 1 healthy donor | Total HDL isolated by density gradient UC (KBr) | HPLC to separate peptides MALDI- TOF | Details not given | No |
| (22) | Healthy donors (n not reported) | Total HDL isolated by one-step density gradient UC (KBr) and immunoisolation | MALDI-TOF, isotope coded affinity tag plus Western blot analysis | Details not given | Yes |
| 1D and 2D gel | |||||
| (23) | Studies of total HDL: 20 males; studies of HDL3: 6 healthy males and 7 with CAD | Total HDL and HDL3 isolated by density gradient UC (KBr) | LC-ESI-MS/MS | International Protein Index | No |
| (26) | 9 healthy normolipidemic males and 3 samples, each consisting of a pool from 20 healthy normolipidemic males | HDL2b, 2a, 3a, 3b and 3c isolated by density gradient UC (KBr) | LC-ESI-MS/MS | SwissProt | No |
| (33) | 3 healthy normolipidemic males | “HDL” isolated by high resolution size exclusion chromatography followed by lipid removal agent | LC-ESI MS/MS | UniProt and SwissProt | No |
| (27) | 10 healthy adults, 10 with stable coronary disease and 10 with acute coronary syndrome. Age-matched males | Total HDL separated by density gradient UC (KBr) | LC-ESI MS/MS | UniProt | Yes |
| 1D gel | |||||
| (32) | Aged-matched females with rheumatoid arthritis: 4 with anti-inflammatory HDL index, and 4 with proinflammatory HDL index | Total HDL isolated by immunoaffinity capture | LC-ESI MS/MS | UniProt | Yes |
| IEF gel | |||||
| (28) | 19 healthy adults and 27 with end stage renal disease on hemodialysis. Males and females | Total HDL isolated by one-step density gradient UC (KBr) | LC-ESI-MS/MS | NCBI | No |
| (29) | 15 healthy adults and 15 with psoriasis. Males and females | Total HDL isolated by two-step density gradient UC (KBr) | LC-ESI-MS/MS | SwissProt | No |
| (30) | 10 healthy adults and 10 with end stage renal disease. Males and females | Total HDL isolated by density gradient UC (KBr) | LC-ESI-MS/MS | SwissProt | No |
| (31) | 30 healthy adults and 30 with end stage kidney disease on hemodialysis. Males and females | Total HDL isolated by three-step density gradient UC (KBr) | MALDI-TOF and iTRAQ labeling prior to LC-ESI MS/MS | UniProt | Yes |
| IEF gel |
IEF, isoelectric focusing; iTRAQ, multiplexed isobaric-tagged reagents produced by Sciex; UC, ultracentrifugation.
Fig. 1.
Frequency of detection of HDL-associated proteins in MS-based proteomic studies. By our count, there are currently 14 published studies that have used soft-ionization MS techniques on human plasma HDL in which an effort has been made to separate them from nonlipid-containing proteins. Proteins that have appeared in at least three of the studies (from different laboratories) are shown. The bar represents the number of studies in which the protein was observed. Although not quantitative by any means, proteins that are more frequently observed likely reflect the most abundant species in HDL.
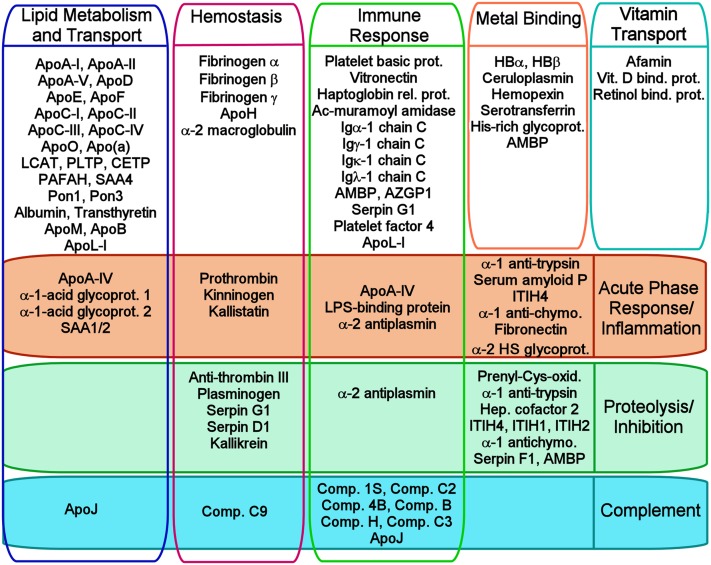
Figure 2 shows a gene ontology enrichment analysis showing the general functional classifications of the consensus proteins listed in Fig. 1. Although many HDL proteins fall within the general area of lipid metabolism, proteins with numerous other functions are also present, including proteins involved in hemostasis, such as fibrinogen, and several of the SERPINs involved in the clotting cascade. There are a striking number of HDL proteins involved in the inflammatory/immune response, including numerous members of the complement system and its associated proteolysis inhibitors, apoJ, and vitronectin. Also clearly represented are acute-phase response proteins, such as SAA and lipopolysaccharide (LPS)-binding protein (LPB). Surprisingly, there are also proteins involved in heme and iron metabolism, such as hemoglobin, transferrin, and hemopexin, as well as those with a host of additional and enigmatic functions, ranging from platelet regulation to vitamin binding and transport.
Fig. 2.
General functional relationships of the HDL proteins in Fig. 1. We performed a gene ontology (GO) search for every protein shown in Fig. 1 as listed in UniProt (http://www.uniprot.org). We determined the eight most frequently reoccurring biological process or molecular function annotations and distributed the proteins among these as appropriate in the figure. Note that most proteins had a large number of GO annotations, and this figure is not meant to be a comprehensive representation of all potential functions for a given protein. Six proteins (α-1B glycoprotein, haptoglobin, angiotensinogen, gelsolin, platelet basic protein, and lumican) are not listed because their entries did not include these top eight annotations, but they are associated with additional entries that further attest to the functional diversity of HDL.
HDL FUNCTIONAL DIVERSITY MATCHES ITS PROTEOMIC DIVERSITY
HDL is most widely recognized for its ability to shuttle cholesterol from the periphery to the liver for catabolism/excretion during the process of reverse cholesterol transport (RCT). Numerous studies have shown that HDL and most of its apolipoproteins can promote lipid efflux from cells via a number of mechanisms (34) and can deliver cholesteryl esters to the liver in the process of selective uptake (35). The importance of this process has been demonstrated by in vivo models of RCT showing that genetic lowering of plasma HDL decreases the appearance of macrophage-derived cholesterol in the feces (36). HDL also has well documented antioxidative properties and has been shown to prevent oxidative modification of LDL, thus reducing macrophage foam-cell generation in the vessel wall (37). Aside from lipid transport functions, HDL has clear anti-inflammatory traits (reviewed in Ref. 38). It can inhibit the expression of cell-adhesion molecules on endothelial cells that sequester circulating monocytes during injury (39). HDL can also reduce the activity of macrophage chemotactic factor 1, which signals the infiltration of surface-adhered monocytes into the vessel wall (40). In rabbits fitted with carotid periarterial collars, HDL administration resulted in 40% reductions in VCAM-1 expression and monocyte infiltration within one week (41). As an in depth discussion of the well-studied lipid transport, antioxidative, and anti-inflammatory functions of HDL is beyond the scope of this review, readers interested in more detail are directed to the excellent recent treatise on HDL by Kontush and Chapman (42).
Under conditions of infection, inflammation, or tissue injury, the acute-phase response (APR) is triggered, causing huge alterations in liver protein synthesis patterns that translate to remarkable changes in HDL protein composition. Negative APR proteins (i.e., those decreasing in APR) include apoA-I, transthyretin, and retinol-binding protein. Their rapid reduction may quickly release “acute-booster reactant” or free ligands, such as metal ions and vitamin A, needed for repair processes (43). These proteins tend to be replaced in HDL by positive APR proteins, which include SAA, apoJ/clustrin, and lipopolysaccharide-binding protein (LBP). SAA can increase in expression up to 1000-fold within 24 h of onset of APR in certain animal models (44). SAA appears to work toward preserving the cholesterol content of peripheral tissues by reducing HDL's ability to promote cholesterol efflux (45) and whole-body transfer of macrophage cholesterol to the feces (46). This may enhance local repair processes. However, SAA increases both holoparticle uptake of HDL (46) and selective uptake of cholesteryl ester by the liver and adrenals via SR-BI (47). Because the adrenals rely heavily on HDL-derived cholesterol for steroid hormone production, enhanced cholesterol delivery from HDL may allow the adrenals to better produce anti-inflammatory glucocorticoids in response to stress. As it is known to bind vascular proteoglycans (48), SAA may also tether HDL in the vascular matrix, thereby preventing RCT and inhibiting oxidation (49). ApoJ/clustrin is predominantly found in HDL3 (26). It may prevent rampant tissue injury by inhibiting complement activation (50). LBP, as its name suggests, is an avid LPS-binding factor. It is known to increase host-cell sensitivity to LPS by presenting it to its cell-surface receptor, CD14 (51). Thus, positive and negative APR proteins residing in HDL appear to coordinate short-term inflammatory and repair processes. (For a more complete review of HDL protein changes in the ARP, see Ref. 52).
HDL is also known to play important roles in hemostasis (for a recent review, see Ref. 53). Several studies have shown that HDL-C levels correlate inversely with different pathological modes of thrombosis (54, 55). HDL can oppose LDL induction of platelet aggregation, serotonin release, and thromboxane B2 production (56), and it can block ox-LDL inhibition of nitric oxide synthase (57). Griffin et al. (58) showed that HDL, but not LDL, could inhibit the activation of coagulation factor Va by activated protein C, an important early step of the clotting cascade, although this activity did not appear to coelute with the majority of apoA-I upon gel filtration separation (59). Recent evidence has also revealed that the cholesterol efflux functions of HDL and apoA-I may significantly impact platelet function. Mice lacking the SR-BI receptor, known for mediating lipid efflux, among other functions, produce platelets that aggregate poorly due to high levels of membrane cholesterol (60). In humans, when type II diabetes patients were infused with reconstituted HDL preparations, they experienced a 50% decrease in platelet aggregation versus controls (61). Overall, these effects on hemostasis are consistent with the discovery of HDL protein groups involved in clotting, such as thrombin, PAF-AH, and clot propagating/inhibiting proteases, although much more work is needed to understand the precise roles they play.
Intriguingly and somewhat underappreciated in the lipoprotein field, HDL also plays important roles in host defense. It has been shown to be a major bacteriocidic factor in several species of fish (62) as well as humans (63, 64). HDL can also deactivate particular oxidized phospholipids accumulating in macrophages that have been infected with Mycobacterium leprae, the organism that causes leprosy (65). Similarly, HDL components can neutralize toxins released during infection, including enterohemolysin (66), LPS, and lipoteichoic acid (67–71). This sequestration prevents the activation of toll-like receptors (TLR) on macrophages and their subsequent secretion of proinflammatory cytokines (for a more in-depth review on the role of HDL in innate immunity, see Ref. 72). Recent work has also demonstrated that specific components of HDL can play a highly intriguing role in neutralizing the protozoan Trypanosoma brucei (T. brucei) (more on this below). In fact, the importance of HDL in host defense is highlighted by the fact that some invading pathogens have evolved offensive capabilities that specifically target HDL. For example, Streptococcus pyogenes, a group A streptococcal bacterium responsible for tonsillitis, pharyngitis, and toxic shock syndrome, secretes a protein called serum opacity factor (SOF). SOF specifically targets sHDL particles by binding to apoA-I and apoA-II, causing a dramatic redistribution of the HDL neutral lipid cargo into large protein-poor micro-emulsions (73). Thus, SOF might have evolved as a virulence factor designed to subvert the antibacterial properties of intact HDL particles. Given the tight linkage between infection and inflammation, the ability of HDL to regulate the amplitude of the inflammatory response may work in conjunction with these host-defense effects to resolve infections. Indeed, since cardiovascular disease (CVD) and other chronic derangements of lipid metabolism typically affect mortality at postreproductive ages, one can argue that selective pressures related to host defense and inflammation were likely the dominant evolutionary factors that have shaped HDL composition and function.
Overall, the huge amount of work on HDL has produced a diverse functional portfolio that closely mirrors the known functions of its recently identified protein constituents. It follows that the tremendous functional heterogeneity inherent to HDL is driven in large part by its compositional heterogeneity. This raises an important question: Does the HDL proteome undergo changes in the face of disease?
ALTERATIONS IN THE HDL PROTEOME IN DISEASE STATES
With the complexity of the HDL proteome largely established, investigations have begun to focus on monitoring its changes in various disease states. Vaisar et al. was the first to compare HDL proteomic profiles between normolipidemic subjects and patients with documented coronary artery disease (CAD) (23, 74, 75). They identified several proteins that were enriched in the CAD patients, including apoE, apoC-IV, PON1, complement C3, and apoA-IV, all involved in vascular inflammation. Moreover, pattern recognition analyses showed a promising ability to differentiate mass signatures from normal and CAD subjects, particularly from mass markers found in apoA-I, apoC-III, and apoC-I (74). Alwaili et al. (27) performed a proteomic comparison in control, stable CAD, and acute coronary syndrome (ACS) subjects. Here, significant differences in SAA, apoA-IV, and complement C3 levels were noted in the ACS patients, indicating a shift to an inflammatory profile. These studies illustrate the promise of HDL proteomics for deriving new biomarkers for CAD diagnosis and, perhaps more importantly, for ways to measure the effectiveness of current and future treatment regimens. Indeed, Vaisar et al. compared the HDL3 proteome of subjects with stenotic lesions verified by angiography before and one year after combination treatment with a statin and the HDL-raising drug niacin (75). The treatment was found to reduce apoE levels while increasing apoF and PLTP, partially remodeling the stenotic proteomic profile toward that of control subjects. Alterations in the HDL proteome were also noted after treatment with the CETP inhibitor anacetrapib, though the impact of these changes on CVD is unclear (76).
The state of the HDL proteome has been evaluated in other conditions as well. Asking similar questions in the context of type II diabetes, Hoofnagle et al. looked at the levels of five proteins previously implicated in cardioprotective effects of HDL (77). They found that clusterin concentration in HDL was negatively associated with insulin resistance, potentially implicating it in HDL cardioprotection. Because renal disease is associated with low HDL-C, two laboratories have monitored the HDL proteome in response to chronic dialysis. Holzer et al. (29) found that dialysis patients have increased levels of the acute-phase inflammatory proteins SAA, PAF-AH, and apoC-III in HDL along with decreases in phospholipid and increases in triglyceride content. These changes corresponded with impaired cholesterol efflux function. Weichart et al. (30) showed that HDL from advanced renal disease patients lacked normal anti-inflammatory properties and correlated this with HDL enrichment of several proteins, including SAA. These studies suggest a link between HDL dysfunction and increased risk of CAD in renal disease.
Turning to other chronic inflammatory diseases, Holzer et al. investigated the HDL proteome from a cohort of patients suffering from psoriasis, an inflammatory skin disease (28). These patients exhibited a reduction in apoA-I levels relative to controls, but they had increased levels of apoA-II and proteins involved in APR. Interestingly, the ability of HDL to promote cholesterol efflux from macrophages was negatively correlated with psoriasis severity.
Sex steroid withdrawal in men was shown to increase HDL-associated levels of clusterin (apoJ) while increasing the capacity of HDL to promote cholesterol efflux from macrophages (78). A follow-up study showed that testosterone replacement in hypogonadal men promoted significant increases in PON1 and fibrinogen α-chain while lowering apoA-IV, but it had no effect on HDL-C levels or cholesterol efflux functionality (79).
Overall, these studies indicate that the HDL proteome can change in a variety of disease states and that these changes are often related to at least in vitro measures of HDL function. However, it remains to be seen whether these changes are secondary to other processes occurring during disease progression or whether the HDL particles themselves contribute to the disease etiology. This will be an important question to pursue going forward.
EVIDENCE FOR HDL PROTEIN SUBSPECIATION AND COOPERATIVE PROTEIN FUNCTION
When it was becoming clear that HDL contained more than a single protein constituent, Scanu and colleagues asked as early as 1969 “whether HDL represents a single lipoprotein species having distinct polypeptide chains or whether HDL, as we prepare it in the ultracentrifuge, is a mixture of HDL species each with distinct peptide moieties….” (3). Is HDL essentially a single entity with numerous interchanging protein components, or is it a collection of individualized species with distinct functionalities that happen to have similar physicochemical properties that are easily isolated together? This is a critical question with respect to pharmacological approaches to altering HDL function. Under the single entity scenario, it may be possible to raise HDL levels generically and thereby achieve improvements on a majority of its functions. Alternatively, if individual species perform distinct functions, it may be most advantageous to pharmacologically raise only certain ones to achieve benefits, particularly if altering other subspecies might have deleterious effects on other important functions.
Careful in vitro studies have documented the movement of apolipoproteins between VLDL and HDL (80). In fact, many of the apolipoproteins associated with HDL have been termed “exchangeable” due to their ability to exist in a soluble lipid-poor state that allows them to ping-pong between different lipoprotein species. Therefore, one school of thought views HDL as a transient ensemble of randomly exchanging proteins. This view is reflected in the clinic where the HDL-C measurement is commonly taken as a surrogate for the totality of HDL species and function. However, there is significant evidence that many apolipoproteins do in fact segregate into compositionally stable particles. Asztalos and colleagues used antibodies to visualize individual protein migration patterns in a native 2D gel electrophoresis system. They found apoA-I in 11 distinct spots representing variously charged and sized species (81). However, apoA-II associated with apoA-I only in the α2 and α3 species but not in the others. ApoE was found on larger particles that did not completely overlap with apoA-I, and apoA-IV was also found in distinct locations (82). Similarly, our group applied a shotgun ESI-MS/MS approach to learn about the distribution of proteins across five density subfractions (HDL2b,a and HDL3a,b,c) isolated from humans by density-gradient ultracentrifugation (26). Using an abundance pattern analysis, we categorized different HDL proteins into five groups based on their distribution. Some proteins preferred small, dense particles whereas others preferred large, light ones. In a follow-up study, we separated human lipoproteins into 17 individual fractions by size exclusion chromatography and again saw highly distinct elution patterns for individual proteins (33). In more recent work, we separated human plasma using three different separation techniques (gel filtration, ion exchange chromatography, and isoelectric focusing) and tracked protein elution patterns across them. The results show that numerous pairs of proteins tend to comigrate across the different separation techniques (83), suggesting the existence of discrete and stable subparticles. These reports indicate that, although some apolipoproteins and HDL-associated lipid remodeling factors undoubtedly exchange between HDL particles, others clearly do not. The mechanisms driving such segregation are as yet unknown but likely involve some combination of the following: a) the affinity of a given protein to a particular lipid composition; b) the affinity for a certain degree of surface curvature on different-sized HDL particles; or c) specific protein-protein interactions on the particle surface that promote and maintain protein segregation.
The idea of protein-protein interactions or at least protein colocalization within specific HDL particles opens the possibility that some proteins have cooperative functions. In fact, there are several examples of cofactor interactions within the HDL proteome. On its own, LCAT is inefficient in mediating cholesterol esterification in lipoproteins, but in the presence of apoA-I, LCAT activity is stimulated by several orders of magnitude (84). ApoC-II is an important cofactor for efficient lipolysis by lipoprotein lipase (85). One role of apoA-II may be to modulate endothelial lipase (EL), an important enzyme for the physiological regulation of HDL-C levels, perhaps via effects on apoA-I conformation (86). In terms of reciprocal regulation, ApoF (also known as lipid transport inhibitor protein) can inhibit the CETP-mediated exchange of CE between HDL and TG-rich LPs, possibly by modulating CETP's affinity for the HDL particle surface (87). There is also emerging evidence that HDL may rely on cooperative interactions to carry out antioxidative functions. HDL can prevent LDL oxidation (88–90) via associated PON1 (91, 92), a calcium-dependent esterase that closely associates with apoA-I and is thought to prevent LDL oxidation by hydrolyzing oxidized phospholipids (90) and cholesteryl linoleate hydroperoxides (93). Recent work by Hine et al. (94) demonstrates PON1 may interact with apoA-I and LCAT to inhibit LDL oxidation, with the combination preventing LCAT inactivation.
However, the most striking and best documented example of HDL protein-protein cooperation relates to HDL's role in innate immunity. Dense fractions of HDL are well known to mediate the lysis of T. brucei, a trypanosome related to the one that causes African sleeping sickness in humans. This activity was termed trypanosome lytic factor (TLF) (95, 96). Immunoprecipitation studies demonstrated that TLF is a distinct and minor HDL particle that contains apoA-I, apoL-I, and haptoglobin-related protein (HRP) (97). The current model for TLF lysis of T. brucei holds that the HDL particle is taken up by the trypanosome in a receptor-mediated pathway, possibly via the HRP moiety. The complex is then targeted to the lysosome where apoL-I, via a colicin-like pore-forming domain, permeates the organelle to kill the organism (98). TLF's unique composition is the strongest evidence yet that distinct particles within classically defined HDL exist and perform highly specialized functions that are quite distinct from traditional lipid transport roles (99).
There are also examples of HDL-like particles that have been isolated without a specifically known function. Chueng et al. used immunoaffinity techniques to isolate particles that contain PLTP (100). MS analysis showed that they contained numerous proteins, including clusterin, coagulation factors, complement factors, and apoA-I. Interestingly, these particles were extremely lipid-poor, with only 3% of the mass attributed to phospholipid. Given the composition, the authors proposed that these particles may play roles in host defense and inflammation. This illustrates that the composition of some HDL particles may differ significantly from commonly envisioned lipid-dominated particles and that protein-protein interactions may play important roles in HDL subspeciation.
CONCLUSIONS AND CHALLENGES
The tremendous leaps that have been made in characterizing the HDL proteome have left little doubt as to the role of HDL in a range of processes, ranging from lipid metabolism to inflammation to host defense. Further work will undoubtedly confirm and expand the list of proteins known to associate with extracellular phospholipid in the plasma. Already new technologies are being developed, such as selected reaction monitoring (SRM), that allow not only the identification of HDL protein constituents but also their quantitation with precision approaching traditional immunological methods (30, 101). These technologies will be critical for determining alterations in the HDL proteome in the face of disease states such as CAD and diabetes. As stated above, these studies will be useful for identifying new biomarkers for early diagnosis of disease, pinpointing new pathways for therapeutic intervention, and assessing the effectiveness of current and new therapeutics.
Along with the promises of this field, there are also several challenges that need to be met. First, in terms of characterizing the HDL proteome, it will be important to distinguish artifacts from truly functioning HDL proteins. As MS technology continues to improve, it is certain that more and more proteins will be identified in HDL preparations. However, the sensitivity of MS has likely already outstripped the fidelity of the various HDL isolation strategies used to generate the samples. Thus, it is increasingly likely that minor non-HDL-associated contaminants, often resulting from highly abundant soluble proteins in plasma, will be reproducibly detected as HDL constituents. It will be important to recognize these possibilities of contamination and develop better HDL isolation techniques as well as to cross-check results with different methodologies. It should also be kept in mind that HDL may be a repository for fragments of proteins that have been degraded in plasma (25). Therefore, the detection of peptides from some proteins may not reflect an intact and functioning protein in HDL. MS protein identifications should be backed up with immunodetection methods, such as Western blotting, that are capable of assessing whether the intact protein is present. Second, it will be important to determine the plasma distribution of many of these HDL factors. For example, complement C3 is consistently detected as an HDL constituent; however, it is also a highly abundant soluble entity in plasma. How much of plasma C3 is associated with HDL? Does HDL association make it functionally different from the soluble form? These are important questions that will need to be asked for many of the newly identified HDL proteins. Third, given the large number of proteins associated with HDL and the relatively few protein structural and functional interactions that we currently understand, it is easy to imagine that many more HDL subparticles await discovery and characterization. These particles may underlie a huge amount of unknown biology relating to a range of processes and diseases. Thus, it would seem prudent to devote resources to identifying these complexes and correlating them with specific functions. We may find that the levels of a given protein may not differ in a given disease state but that its distribution across HDL subspecies or its association with other proteins may change significantly. Put another way, particular HDL complexes could turn out to be better biomarkers than individual proteins. This principle was elegantly illustrated by the recent work of Jensen et al. (102). Using immunoaffinity separations, they separated HDL from two large, prospective CVD studies and quantified the cholesterol content of the HDL particles that contained apoC-III and those that lacked it. Strikingly, they found that apoC-III-containing HDL was associated with increased CVD risk, whereas the apoC-III-lacking fraction was associated with cardioprotection.
The recent failure of niacin (103) and two different CETP inhibitors (104, 105), all capable of significantly raising HDL-C, in cardioprotection has cast some doubt on whether HDL plays an truly protective role in CVD or represents a readout of other more directly operational processes. Indeed, the “HDL-cholesterol hypothesis” has begun to be replaced by the “HDL function/flux hypothesis” (106), reflecting the growing consensus that measurement of one minor HDL component (i.e., cholesterol) is not sufficient to capture the cardioprotective potential of HDL. Recent studies have clearly shown that specific functional readouts, such as capacity for cholesterol efflux (107) or anti-inflammatory index (108), may be better indicators than the HDL-C number for predicting CAD risk in an individual. Given the huge functional and compositional pleotrophy in HDL, individuals likely have a “portfolio” of HDL subspecies that are individually tasked to different functions across lipid metabolism, inflammation, antioxidation, and host defense. The HDL-C number may represent these particles as a group, but it probably does not represent those subfractions that play the most important roles in cardioprotection. If this is correct, then it becomes extremely important to identify those subspecies that are most relevant to CVD protection and focus on therapeutics that specifically target those species. Raising HDL-C in the generic sense without such targeting may not elevate the truly cardioprotective species. Importantly, such treatments might even have deleterious effects on subspecies that are important for other pathways, such as host defense. Indeed, an underappreciated fact from the failed ILLUMINATE trial (104) is that a major cause of death in the torcetrapib treatment group, aside from the drug's off-target effect that contributed to increased CAD (109), was sepsis.
Footnotes
Abbreviations:
- APR
- acute-phase response
- CAD
- coronary artery disease
- CE
- cholesteryl ester
- CETP
- CE transfer protein
- CVD
- cardiovascular disease
- LBP
- LPS-binding protein
- LPS
- lipopolysaccharide
- PAF-AH
- platelet-activating factor aryl hydrolase
- PL
- phospholipid
- PLTP
- PL transfer protein
- PON1
- paraoxonase 1
- RCT
- reverse cholesterol transport
- SAA
- serum amyloid A
- SERPIN
- serine protease inhibitor
- TG
- triglyceride
REFERENCES
- 1.Macheboeuf M. 1929. Recherches sur les phosphoaminolipides du sérum sanguin. Nature des phospholipides liés aux albumines du sérum de Cheval à l’état de cenapses acido-précipitables. Bull. Soc. Chim. Biol. (Paris). 11: 485–503 [Google Scholar]
- 2.Shore B., Shore V. 1968. Heterogeneity in protein subunits of human serum high-density lipoproteins. Biochemistry. 7: 2773–2777 [DOI] [PubMed] [Google Scholar]
- 3.Scanu A., Toth J., Edelstein C., Koga S., Stiller E. 1969. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 8: 3309–3316 [DOI] [PubMed] [Google Scholar]
- 4.Rudman D., Garcia L. A., Howard C. H. 1970. A new method for isolating the nonidentical protein subunits of human plasma alpha-lipoprotein. J. Clin. Invest. 49: 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostner G., Alaupovic P. 1971. Studies of the composition and structure of plasma lipoproteins. C- and N-terminal amino acids of the two nonidentical polypeptides of human plasma apolipoprotein A. FEBS Lett. 15: 320–324 [DOI] [PubMed] [Google Scholar]
- 6.Shore B., Shore V. 1969. Isolation and characterization of polypeptides of human serum lipoproteins. Biochemistry. 8: 4510–4516 [DOI] [PubMed] [Google Scholar]
- 7.Mahley R. W., Innerarity T. L. 1977. Interaction of canine and swine lipoproteins with the low density lipoprotein receptor of fibroblasts as correlated with heparin/manganese precipitability. J. Biol. Chem. 252: 3980–3986 [PubMed] [Google Scholar]
- 8.Ayrault-Jarrier M., Alix J. F., Polonovski J. 1980. Presence and isolation of 2 lipoproteins immunologically related to apolipoprotein A I in human serum [article in French]. Biochimie. 62: 51–59 [DOI] [PubMed] [Google Scholar]
- 9.Olofsson S. O., McConathy W. J., Alaupovic P. 1978. Isolation and partial characterization of a new acidic apolipoprotein (apolipoprotein F) from high density lipoproteins of human plasma. Biochemistry. 17: 1032–1036 [DOI] [PubMed] [Google Scholar]
- 10.Bausserman L. L., Herbert P. N., McAdam K. P. 1980. Heterogeneity of human serum amyloid A proteins. J. Exp. Med. 152: 641–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vézina C. A., Milne R. W., Weech P. K., Marcel Y. L. 1988. Apolipoprotein distribution in human lipoproteins separated by polyacrylamide gradient gel electrophoresis. J. Lipid Res. 29: 573–585 [PubMed] [Google Scholar]
- 12.Blatter M. C., James R. W., Messmer S., Barja F., Pometta D. 1993. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur. J. Biochem. 211: 871–879 [DOI] [PubMed] [Google Scholar]
- 13.Campos E., McConathy W. J. 1986. Distribution of lipids and apolipoproteins in human plasma by vertical spin ultracentrifugation. Arch. Biochem. Biophys. 249: 455–463 [DOI] [PubMed] [Google Scholar]
- 14.Marcel Y. L., Vezina C., Teng B., Sniderman A. 1980. Transfer of cholesterol esters between human high density lipoproteins and triglyceride-rich lipoproteins controlled by a plasma protein factor. Atherosclerosis. 35: 127–133 [DOI] [PubMed] [Google Scholar]
- 15.Tall A. R., Krumholz S., Olivecrona T., Deckelbaum R. J. 1985. Plasma phospholipid transfer protein enhances transfer and exchange of phospholipids between very low density lipoproteins and high density lipoproteins during lipolysis. J. Lipid Res. 26: 842–851 [PubMed] [Google Scholar]
- 16.Stafforini D. M., McIntyre T. M., Carter M. E., Prescott S. M. 1987. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J. Biol. Chem. 262: 4215–4222 [PubMed] [Google Scholar]
- 17.James R. W., Hochstrasser D., Tissot J. D., Funk M., Appel R., Barja F., Pellegrini C., Muller A. F., Pometta D. 1988. Protein heterogeneity of lipoprotein particles containing apolipoprotein A-I without apolipoprotein A-II and apolipoprotein A-I with apolipoprotein A-II isolated from human plasma. J. Lipid Res. 29: 1557–1571 [PubMed] [Google Scholar]
- 18.Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. 1989. Electrospray ionization for mass spectrometry of large biomolecules. Science. 246: 64–71 [DOI] [PubMed] [Google Scholar]
- 19.Hillenkamp F., Karas M., Beavis R. C., Chait B. T. 1991. Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal. Chem. 63: 1193A–1203A [DOI] [PubMed] [Google Scholar]
- 20.Cañas B., López-Ferrer D., Ramos-Fernández A., Camafeita E., Calvo E. 2006. Mass spectrometry technologies for proteomics. Brief Funct. Genomic Proteomic. 4: 295–320 [DOI] [PubMed] [Google Scholar]
- 21.Karlsson H., Leanderson P., Tagesson C., Lindahl M. 2005. Lipoproteomics II: mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 5: 1431–1445 [DOI] [PubMed] [Google Scholar]
- 22.Rezaee F., Casetta B., Levels J. H., Speijer D., Meijers J. C. 2006. Proteomic analysis of high-density lipoprotein. Proteomics. 6: 721–730 [DOI] [PubMed] [Google Scholar]
- 23.Vaisar T., Pennathur S., Green P. S., Gharib S. A., Hoofnagle A. N., Cheung M. C., Byun J., Vuletic S., Kassim S., Singh P., et al. 2007. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 117: 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller M., Stalder D., Schlappritzi E., Hayn G., Matter U., Haeberli A. 2005. Mass spectrometry-based analytical tools for the molecular protein characterization of human plasma lipoproteins. Proteomics. 5: 2619–2630 [DOI] [PubMed] [Google Scholar]
- 25.Hortin G. L., Shen R. F., Martin B. M., Remaley A. T. 2006. Diverse range of small peptides associated with high-density lipoprotein. Biochem. Biophys. Res. Commun. 340: 909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson W. S., Silva R. A., Chantepie S., Lagor W. R., Chapman M. J., Kontush A. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alwaili K., Bailey D., Awan Z., Bailey S. D., Ruel I., Hafiane A., Krimbou L., Laboissiere S., Genest J. 2012. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim. Biophys. Acta. 1821: 405–415 [DOI] [PubMed] [Google Scholar]
- 28.Holzer M., Wolf P., Curcic S., Birner-Gruenberger R., Weger W., Inzinger M., El-Gamal D., Wadsack C., Heinemann A., Marsche G. 2012. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 53: 1618–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzer M., Birner-Gruenberger R., Stojakovic T., El-Gamal D., Binder V., Wadsack C., Heinemann A., Marsche G. 2011. Uremia alters HDL composition and function. J. Am. Soc. Nephrol. 22: 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weichhart T., Kopecky C., Kubicek M., Haidinger M., Doller D., Katholnig K., Suarna C., Eller P., Tolle M., Gerner C., et al. 2012. Serum amyloid A in uremic HDL promotes inflammation. J. Am. Soc. Nephrol. 23: 934–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangé A., Goux A., Badiou S., Patrier L., Canaud B., Maudelonde T., Cristol J. P., Solassol J. 2012. HDL proteome in hemodialysis patients: a quantitative nanoflow liquid chromatography-tandem mass spectrometry approach. PLoS ONE. 7: e34107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe J., Charles-Schoeman C., Miao Y., Elashoff D., Lee Y. Y., Katselis G., Lee T. D., Reddy S. T. 2012. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 64: 1828–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon S. M., Deng J., Lu L. J., Davidson W. S. 2010. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J. Proteome Res. 9: 5239–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., Rothblat G. H. 2007. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48: 2453–2462 [DOI] [PubMed] [Google Scholar]
- 35.Gu X., Trigatti B., Xu S., Acton S., Babitt J., Krieger M. 1998. The efficient cellular uptake of high density lipoprotein lipids via scavenger receptor class B type I requires not only receptor-mediated surface binding but also receptor-specific lipid transfer mediated by its extracellular domain. J. Biol. Chem. 273: 26338–26348 [Erratum. 1998. J. Biol. Chem. 273: 35388.] [DOI] [PubMed] [Google Scholar]
- 36.Moore R. E., Navab M., Millar J. S., Zimetti F., Hama S., Rothblat G. H., Rader D. J. 2005. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ. Res. 97: 763–771 [DOI] [PubMed] [Google Scholar]
- 37.Navab M., Hama S. Y., Anantharamaiah G. M., Hassan K., Hough G. P., Watson A. D., Reddy S. T., Sevanian A., Fonarow G. C., Fogelman A. M. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein. Steps 2 and 3. J. Lipid Res. 41: 1495–1508 [PubMed] [Google Scholar]
- 38.Rye K. A., Bursill C. A., Lambert G., Tabet F., Barter P. J. 2009. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 50(Suppl.): S195–S200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barter P. J., Rye K. A. 1996. High density lipoproteins and coronary heart disease. Atherosclerosis. 121: 1–12 [DOI] [PubMed] [Google Scholar]
- 40.Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. 1991. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88: 2039–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puranik R., Bao S., Nobecourt E., Nicholls S. J., Dusting G. J., Barter P. J., Celermajer D. S., Rye K. A. 2008. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis. 196: 240–247 [DOI] [PubMed] [Google Scholar]
- 42.Kontush A., Chapman M. J. 2012. High-Density Lipoproteins: Structure, Metabolism, Function and Therapeutics. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 43.Ingenbleek Y., Young V. 1994. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu. Rev. Nutr. 14: 495–533 [DOI] [PubMed] [Google Scholar]
- 44.McAdam K. P., Sipe J. D. 1976. Murine model for human secondary amyloidosis: genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. J. Exp. Med. 144: 1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artl A., Marsche G., Lestavel S., Sattler W., Malle E. 2000. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler. Thromb. Vasc. Biol. 20: 763–772 [DOI] [PubMed] [Google Scholar]
- 46.Annema W., Nijstad N., Tolle M., de Boer J. F., Buijs R. V., Heeringa P., van der Giet M., Tietge U. J. 2010. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A(2). J. Lipid Res. 51: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai L., Ji A., de Beer F. C., Tannock L. R., van der Westhuyzen D. R. 2008. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Invest. 118: 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ancsin J. B., Kisilevsky R. 1999. The heparin/heparan sulfate-binding site on apo-serum amyloid A. Implications for the therapeutic intervention of amyloidosis. J. Biol. Chem. 274: 7172–7181 [DOI] [PubMed] [Google Scholar]
- 49.Chait A., Han C. Y., Oram J. F., Heinecke J. W. 2005. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J. Lipid Res. 46: 389–403 [DOI] [PubMed] [Google Scholar]
- 50.Rosenfeld S. I., Packman C. H., Leddy J. P. 1983. Inhibition of the lytic action of cell-bound terminal complement components by human high density lipoproteins and apoproteins. J. Clin. Invest. 71: 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hailman E., Lichenstein H. S., Wurfel M. M., Miller D. S., Johnson D. A., Kelley M., Busse L. A., Zukowski M. M., Wright S. D. 1994. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 179: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahangiri A. 2010. High-density lipoprotein and the acute phase response. Curr. Opin. Endocrinol. Diabetes Obes. 17: 156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nofer J. R., Brodde M. F., Kehrel B. E. 2010. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin. Exp. Pharmacol. Physiol. 37: 726–735 [DOI] [PubMed] [Google Scholar]
- 54.Wang P., Wang Y., Ma W., Li H., Chen H. 2013. High-density lipoprotein cholesterol and intracoronary thrombosis burden. Coron. Artery Dis. 24: 1–5 [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Wang P., Li H. 2010. Correlation study of pulmonary embolism and high-density lipoprotein cholesterol. Clin. Cardiol. 33: 72–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aviram M., Sirtori C. R., Colli S., Maderna P., Morazzoni G., Tremoli E. 1985. Plasma lipoproteins affect platelet malondialdehyde and thromboxane B2 production. Biochem. Med. 34: 29–36 [DOI] [PubMed] [Google Scholar]
- 57.Mehta J. L., Chen L. Y. 1996. Reversal by high-density lipoprotein of the effect of oxidized low-density lipoprotein on nitric oxide synthase protein expression in human platelets. J. Lab. Clin. Med. 127: 287–295 [DOI] [PubMed] [Google Scholar]
- 58.Griffin J. H., Kojima K., Banka C. L., Curtiss L. K., Fernandez J. A. 1999. High-density lipoprotein enhancement of anticoagulant activities of plasma protein S and activated protein C. J. Clin. Invest. 103: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oslakovic C., Norstrom E., Dahlback B. 2010. Reevaluation of the role of HDL in the anticoagulant activated protein C system in humans. J. Clin. Invest. 120: 1396–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dole V. S., Matuskova J., Vasile E., Yesilaltay A., Bergmeier W., Bernimoulin M., Wagner D. D., Krieger M. 2008. Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28: 1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calkin A. C., Drew B. G., Ono A., Duffy S. J., Gordon M. V., Schoenwaelder S. M., Sviridov D., Cooper M. E., Kingwell B. A., Jackson S. P. 2009. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation. 120: 2095–2104 [DOI] [PubMed] [Google Scholar]
- 62.Concha M. I., Smith V. J., Castro K., Bastias A., Romero A., Amthauer R. J. 2004. Apolipoproteins A-I and A-II are potentially important effectors of innate immunity in the teleost fish Cyprinus carpio. Eur. J. Biochem. 271: 2984–2990 [DOI] [PubMed] [Google Scholar]
- 63.Villarroel F., Bastias A., Casado A., Amthauer R., Concha M. I. 2007. Apolipoprotein A-I, an antimicrobial protein in Oncorhynchus mykiss: evaluation of its expression in primary defence barriers and plasma levels in sick and healthy fish. Fish Shellfish Immunol. 23: 197–209 [DOI] [PubMed] [Google Scholar]
- 64.Johnston L. D., Brown G., Gauthier D., Reece K., Kator H., Van V. P. 2008. Apolipoprotein A-I from striped bass (Morone saxatilis) demonstrates antibacterial activity in vitro. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 151: 167–175 [DOI] [PubMed] [Google Scholar]
- 65.Cruz D., Watson A. D., Miller C. S., Montoya D., Ochoa M. T., Sieling P. A., Gutierrez M. A., Navab M., Reddy S. T., Witztum J. L., et al. 2008. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J. Clin. Invest. 118: 2917–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Figueirêdo P. M., Catani C. F., Yano T. 2003. Serum high-density lipoprotein (HDL) inhibits in vitro enterohemolysin (EHly) activity produced by enteropathogenic Escherichia coli. FEMS Immunol. Med. Microbiol. 38: 53–57 [DOI] [PubMed] [Google Scholar]
- 67.Grunfeld C., Marshall M., Shigenaga J. K., Moser A. H., Tobias P., Feingold K. R. 1999. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J. Lipid Res. 40: 245–252 [PubMed] [Google Scholar]
- 68.Ma J., Liao X. L., Lou B., Wu M. P. 2004. Role of apolipoprotein A-I in protecting against endotoxin toxicity. Acta Biochim. Biophys. Sin. (Shanghai). 36: 419–424 [DOI] [PubMed] [Google Scholar]
- 69.Parker T. S., Levine D. M., Chang J. C., Laxer J., Coffin C. C., Rubin A. L. 1995. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect. Immun. 63: 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiao Y. L., Wu M. P. 2008. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine. 43: 83–87 [DOI] [PubMed] [Google Scholar]
- 71.Li Y., Dong J. B., Wu M. P. 2008. Human ApoA-I overexpression diminishes LPS-induced systemic inflammation and multiple organ damage in mice. Eur. J. Pharmacol. 590: 417–422 [DOI] [PubMed] [Google Scholar]
- 72.Feingold K. R., Grunfeld C. 2011. The role of HDL in innate immunity. J. Lipid Res. 52: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Courtney H. S., Zhang Y. M., Frank M. W., Rock C. O. 2006. Serum opacity factor, a streptococcal virulence factor that binds to apolipoproteins A-I and A-II and disrupts high density lipoprotein structure. J. Biol. Chem. 281: 5515–5521 [DOI] [PubMed] [Google Scholar]
- 74.Vaisar T., Mayer P., Nilsson E., Zhao X. Q., Knopp R., Prazen B. J. 2010. HDL in humans with cardiovascular disease exhibits a proteomic signature. Clin. Chim. Acta. 411: 972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green P. S., Vaisar T., Pennathur S., Kulstad J. J., Moore A. B., Marcovina S., Brunzell J., Knopp R. H., Zhao X. Q., Heinecke J. W. 2008. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 118: 1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krauss R. M., Wojnooski K., Orr J., Geaney J. C., Pinto C. A., Liu Y., Wagner J. A., Luk J. M., Johnson-Levonas A. O., Anderson M. S., et al. 2012. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J. Lipid Res. 53: 540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoofnagle A. N., Wu M., Gosmanova A. K., Becker J. O., Wijsman E. M., Brunzell J. D., Kahn S. E., Knopp R. H., Lyons T. J., Heinecke J. W. 2010. Low clusterin levels in high-density lipoprotein associate with insulin resistance, obesity, and dyslipoproteinemia. Arterioscler. Thromb. Vasc. Biol. 30: 2528–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubinow K. B., Tang C., Hoofnagle A. N., Snyder C. N., Amory J. K., Heinecke J. W., Page S. T. 2012. Acute sex steroid withdrawal increases cholesterol efflux capacity and HDL-associated clusterin in men. Steroids. 77: 454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rubinow K. B., Vaisar T., Tang C., Matsumoto A. M., Heinecke J. W., Page S. T. 2012. Testosterone replacement in hypogonadal men alters the HDL proteome but not HDL cholesterol efflux capacity. J. Lipid Res. 53: 1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyle K. E., Phillips M. C., Lund-Katz S. 1999. Kinetics and mechanism of exchange of apolipoprotein C-III molecules from very low density lipoprotein particles. Biochim. Biophys. Acta. 1430: 302–312 [DOI] [PubMed] [Google Scholar]
- 81.Asztalos B. F., Schaefer E. J. 2003. High-density lipoprotein subpopulations in pathologic conditions. Am. J. Cardiol. 91: 12E–17E [DOI] [PubMed] [Google Scholar]
- 82.Santos R. D., Schaefer E. J., Asztalos B. F., Polisecki E., Wang J., Hegele R. A., Martinez L. R., Miname M. H., Rochitte C. E., Da Luz P. L., et al. 2008. Characterization of high density lipoprotein particles in familial apolipoprotein A-I deficiency. J. Lipid Res. 49: 349–357 [DOI] [PubMed] [Google Scholar]
- 83.Gordon S. M., Deng J., Tomann A. B., Shah A. S., Lu L. J., Davidson W. S. 2013. Multi-dimensional co-separation analysis reveals protein:protein interactions defining plasma lipoprotein subspecies. Mol Cell Proteomics. Epub ahead of print.Epub ahead of print. July 23, 2013; 10.1074/mcp.M113.028134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jonas A. 1991. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim. Biophys. Acta. 1084: 205–220 [DOI] [PubMed] [Google Scholar]
- 85.Kinnunen P. K., Jackson R. L., Smith L. C., Gotto A. M., Jr., Sparrow J. T. 1977. Activation of lipoprotein lipase by native and synthetic fragments of human plasma apolipoprotein C-II. Proc. Natl. Acad. Sci. USA. 74: 4848–4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jahangiri A., Rader D. J., Marchadier D., Curtiss L. K., Bonnet D. J., Rye K. A. 2005. Evidence that endothelial lipase remodels high density lipoproteins without mediating the dissociation of apolipoprotein A-I. J. Lipid Res. 46: 896–903 [DOI] [PubMed] [Google Scholar]
- 87.Wang X., Driscoll D. M., Morton R. E. 1999. Molecular cloning and expression of lipid transfer inhibitor protein reveals its identity with apolipoprotein F. J. Biol. Chem. 274: 1814–1820 [DOI] [PubMed] [Google Scholar]
- 88.Mackness M. I., Arrol S., Durrington P. N. 1991. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 286: 152–154 [DOI] [PubMed] [Google Scholar]
- 89.Aviram M., Billecke S., Sorenson R., Bisgaier C., Newton R., Rosenblat M., Erogul J., Hsu C., Dunlop C., La Du B. 1998. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler. Thromb. Vasc. Biol. 18: 1617–1624 [DOI] [PubMed] [Google Scholar]
- 90.Watson A. D., Berliner J. A., Hama S. Y., La Du B. N., Faull K. F., Fogelman A. M., Navab M. 1995. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Invest. 96: 2882–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mackness M. I., Durrington P. N. 1995. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis. 115: 243–253 [DOI] [PubMed] [Google Scholar]
- 92.Loued S., Isabelle M., Berrougui H., Khalil A. 2012. The anti-inflammatory effect of paraoxonase 1 against oxidized lipids depends on its association with high density lipoproteins. Life Sci. 90: 82–88 [DOI] [PubMed] [Google Scholar]
- 93.Aviram M., Rosenblat M., Bisgaier C. L., Newton R. S., Primo-Parmo S. L., La Du B. N. 1998. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Invest. 101: 1581–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hine D., Mackness B., Mackness M. 2012. Coincubation of PON1, APO A1, and LCAT increases the time HDL is able to prevent LDL oxidation. IUBMB Life. 64: 157–161 [DOI] [PubMed] [Google Scholar]
- 95.Rifkin M. R. 1978. Identification of the trypanocidal factor in normal human serum: high density lipoprotein. Proc. Natl. Acad. Sci. USA. 75: 3450–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hajduk S. L., Moore D. R., Vasudevacharya J., Siqueira H., Torri A. F., Tytler E. M., Esko J. D. 1989. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J. Biol. Chem. 264: 5210–5217 [PubMed] [Google Scholar]
- 97.Vanhamme L., Paturiaux-Hanocq F., Poelvoorde P., Nolan D. P., Lins L., Van den Abbeele J., Pays A., Tebabi P., Van X. H., Jacquet A., et al. 2003. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature. 422: 83–87 [DOI] [PubMed] [Google Scholar]
- 98.Vanhollebeke B., Nielsen M. J., Watanabe Y., Truc P., Vanhamme L., Nakajima K., Moestrup S. K., Pays E. 2007. Distinct roles of haptoglobin-related protein and apolipoprotein L-I in trypanolysis by human serum. Proc. Natl. Acad. Sci. USA. 104: 4118–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shiflett A. M., Bishop J. R., Pahwa A., Hajduk S. L. 2005. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J. Biol. Chem. 280: 32578–32585 [DOI] [PubMed] [Google Scholar]
- 100.Cheung M. C., Vaisar T., Han X., Heinecke J. W., Albers J. J. 2010. Phospholipid transfer protein in human plasma associates with proteins linked to immunity and inflammation. Biochemistry. 49: 7314–7322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoofnagle A. N., Becker J. O., Oda M. N., Cavigiolio G., Mayer P., Vaisar T. 2012. Multiple-reaction monitoring-mass spectrometric assays can accurately measure the relative protein abundance in complex mixtures. Clin. Chem. 58: 777–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jensen M. K., Rimm E. B., Furtado J. D., Sacks F. M. 2012. Apolipoprotein C-III as a potential modulator of the association between HDL-cholesterol and incident coronary heart disease. J. Am. Heart Assoc. 1: jah3–e000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267 [DOI] [PubMed] [Google Scholar]
- 104.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122 [DOI] [PubMed] [Google Scholar]
- 105.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099 [DOI] [PubMed] [Google Scholar]
- 106.Larach D. B., Degoma E. M., Rader D. J. 2012. Targeting high density lipoproteins in the prevention of cardiovascular disease? Curr. Cardiol. Rep. 14: 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khera A. V., Cuchel M., Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., et al. 2003. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756 [DOI] [PubMed] [Google Scholar]
- 109.Vergeer M., Bots M. L., van Leuven S. I., Basart D. C., Sijbrands E. J., Evans G. W., Grobbee D. E., Visseren F. L., Stalenhoef A. F., Stroes E. S., et al. 2008. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation. 118: 2515–2522 [DOI] [PubMed] [Google Scholar]