Abstract
Atherosis of spiral arteries in uteroplacental beds from preeclamptic women resemble those of atherosclerosis, characterized by increased plasma lipids and lipoproteins. We hypothesized that: 1) lipoprotein receptors/transporters in the placenta would be upregulated in preeclampsia, associated with increased maternal and fetal lipoprotein concentrations; and 2) expression of these would be reduced in preeclamptic placentae from women delivering small-for-gestational-age (SGA) infants. Placental biopsies and maternal and umbilical serum samples were taken from 27 normotensive and 24 preeclamptic women. Maternal/umbilical cord serum LDL, HDL, total cholesterol, and triglycerides were measured. Placental mRNA expression of lipoprotein receptors/transporters were quantified using quantitative RT-PCR. Protein localization/expression of LDL receptor-related protein 1 (LRP-1) in the preeclamptic placentae with/without SGA was measured by immunohistochemistry. Placental mRNA expression of all genes except paraoxonase-1 (PON-1), microsomal triglyceride transfer protein (MTTP), and protein disulfide isomerase family A member 2 (PDIA2) were observed. No differences for any lipoprotein receptors/transporters were found between groups; however, in the preeclamptic group placental LRP-1 expression was lower in SGA delivering mothers (n = 7; P = 0.036). LRP-1 protein was localized around fetal vessels and Hofbauer cells. This is the first detailed study of maternal/fetal lipoprotein concentrations and placental lipoprotein receptor mRNA expression in normotensive and preeclamptic pregnancies. These findings do not support a role of altered lipid metabolism in preeclampsia, but may be involved in fetal growth.
Keywords: hypertension, lipids, low density liporotein cholesterol, high density lipoprotein cholesterol, triglycerides, ATP binding cassette transporter A1, LDL receptor-related protein 1, LDL receptor, scavenger receptor class B type 1, fetal growth
Gestational hyperlipidemia is a common factor in pregnancy in which the maternal circulating lipid profile changes from an anabolic to a catabolic state, increasing lipids, especially triglycerides (TGs) and lipoproteins (1). From the 12th week of gestation, phospholipids, total cholesterol (TC), LDL, HDL, and TGs increase in response to estrogen stimulation and insulin resistance (2). Thus, during the first two trimesters, it is common to see maternal fat accumulation; however, in the third trimester there is enhanced lipolytic activity and decreased lipoprotein lipase (LPL) activity in the adipose tissue, consequently, fat storage declines or ceases (3). Early in gestation, the lipids are required to develop the fetal brain and central nervous system (4), to build cell membranes, and as a precursor of bile acids and steroid hormones (2). However, a maternal source of lipids is still required until term, but in lower amounts, possibly due to some fetal-derived lipids from the lipogenic activity in the fetal liver, adrenal, and testes (2). Conversely, increased TGs during pregnancy have been shown to augment the risk of preeclampsia, preterm birth, and fetal growth restriction (FGR) (2, 5). The lipid metabolism and plasma levels are also affected by maternal factors such as body mass index (BMI), maternal weight gain, maternal nutrition, prepregnancy lipid levels, and various medical complications of pregnancy such as diabetes (6).
Complicating 2–8% of pregnancies, preeclampsia, along with the other hypertensive disorders of pregnancy, is one of the three leading causes of maternal morbidity and mortality worldwide (7). This disorder increases perinatal outcomes such as prematurity and FGR (8). Preeclampsia is generally defined as high blood pressure (systolic blood pressure of ≥140 mm Hg and/or diastolic blood pressure of ≥90 mm Hg) and proteinuria (≥300 mg/24 h) at or after 20 weeks gestation (9). The cause of preeclampsia remains unknown, but endothelial dysfunction, leading to compromised uteroplacental perfusion and reduced maternal-fetal transport of oxygen and nutrients is thought to be involved (10–13). Different lines of evidence indicate that abnormal lipid metabolism is involved in the pathogenesis of the disease, with acute atherosis seen in preeclamptic uteroplacental beds resembling atherosclerotic lesions of coronary arteries (14). The presence of lipoprotein receptors in placental syncytiotrophoblast, specifically LDL receptor (LDL-R), LDL receptor-related protein 1 (LRP-1), and scavenger receptor class B type 1 (SRB-1) in third trimester placentae, have previously been shown (15). Placental expression of some of these receptors from FGR pregnancies with (FGR-S) and without (FGR-M) fetal hemodynamic changes, based on results of the Doppler velocimetry of umbilical artery and pulsatility index and from non-FGR control pregnancies have been reported. LDL-R mRNA levels in FGR-M were similar to controls but lower in FGR-S. In contrast, LDL-R protein was higher in both FGR cases than in the control group. LRP-1 mRNA and protein levels were not altered in all FGR cases. SRB-1 mRNA was unchanged in FGR, but protein levels were lower in FGR-S than in the other groups. The authors concluded that LDL-R and SRB-1 levels are altered in FGR pregnancies and maternal plasma concentrations of LDL cholesterol are higher in the control group than in the FGR-S group (15). However, these have not previously been examined in relation to preeclampsia.
Based on the literature relating to placental and liver cholesterol transport pathways, we chose to analyze the maternal and fetal lipoprotein concentrations in combination with placental mRNA expression of LRP-1, LDL-R, SRB-1, ATP-binding cassette transporter A1 (ABCA1), paraoxonase-1 (PON-1), microsomal triglyceride transfer protein (MTTP), and protein disulfide isomerase family A, member 2 (PDIA2).
We therefore hypothesized that the expression of those lipoprotein receptors involved in the cholesterol pathway in the liver, are upregulated in preeclamptic placentae compared with controls as a compensatory factor. In addition, the expression of some of these receptors may be reduced in preeclamptic placentae from women delivering small-for-gestational-age (SGA) infants versus appropriate-for-gestational-age (AGA) infants.
METHODS
Subjects and selection criteria
The study population consisted of two groups of white European women (27 normotensive, 24 with preeclampsia) (Table 1). Detailed demographics and outcome data have previously been published (16). The study was approved by the Hospital Ethics Committee of the Nottingham University Hospitals; written informed consent was obtained from each participant. Preeclampsia was stringently defined as stated in the International Society for the Study of Hypertension in Pregnancy guidelines (9). Medical and obstetric histories were obtained for each participant. The corrected birthweight percentile for each infant was computed, correcting for gestational age, gender, maternal parity, and BMI (17). SGA was defined as a centile below the 10th, and AGA as an individualized birthweight ratio between the 10th and 90th percentile (18). Table 2 provides demographic, obstetric, and pregnancy description data for the preeclamptic women delivering SGA and AGA infants.
TABLE 1.
Clinical and biochemical data of subject groups
| Parameter | NC (n = 27) | PE (n = 24) |
| Maternal age, years (mean ± SD) | 30 ± 6.9 | 31 ± 6.1 |
| Booking BMI, kg/m2 (mean ± SD) | 26.2 ± 5.4 | 26.6 ± 5.0 |
| Maximum systolic blood pressure outside labor, mm Hg (mean ± SD) | 116 ± 4.6 | 156 ± 7.1* |
| Maximum diastolic blood pressure outside labor, mm Hg (mean ± SD) | 76.0 ± 3.0 | 98.0 ± 4.5* |
| Proteinuria, g/l (median [IQR]) | — | 1.0 [0.3, 11.5] |
| Gestational age at delivery, weeks (mean ± SD) | 40 ± 1.1 | 36.7 ± 3.8* |
| Birth weight, kg (median [IQR]) | 3.5 [3.3, 3.7] | 2.9 [2.0, 3.4]* |
| Birthweight centile (median [IQR]) | 45 [23, 62] | 13 [1, 82] |
| SGA infants [n (%)] | 1 (3.7) | 7 (29.2) |
| Preterm deliveries, ≤37 weeks gestation [n (%)] | 0 | 14 (58.3) |
NC, normotensive controls; PE, preeclampsia; IQR, interquartile range. *P < 0.05 between normotensive controls and women with preeclampsia.
TABLE 2.
Clinical and biochemical data of the preeclamptic women delivering AGA and SGA infants
| Parameter | PE AGA (n = 17) | PE SGA (n = 7) |
| Maternal age, years (mean ± SD) | 32 ± 7.8 | 32 ± 2.9 |
| Booking BMI, kg/m2 (mean ± SD) | 27.2 ± 4.3 | 27.1 ± 6.8 |
| Gestational age at delivery, weeks (mean ± SD) | 38 ± 2.5 | 35 ± 5 |
| Birth weight, kg (median [IQR]) | 3.2 [3, 3.5] | 2 [1.6, 2.4]** |
| Birth weight centile (median [IQR]) | 47.1 [12.6, 69.4] | 0.7 [0.3, 1.9]*** |
PE, preeclampsia; IQR, interquartile range. **P = 0.001 and ***P < 0.0001 between PE AGA and PE AGA groups.
Sample collection and measurements
Before delivery, venous blood samples were taken from mothers and immediately after placental delivery, where possible, umbilical cord venous blood was collected. Venous samples were allowed to clot prior to centrifugation at 1,400 g for 10 min at 4°C. Serum samples were stored at −80°C prior to analysis. The number of fetal serum samples missing was 1 in the control (n = 26) and 10 in the preeclamptic (n = 14) group. All women who took part in this study were laboring and either delivered vaginally or by emergency Caesarean section.
The lipoproteins (LDL, HDL, TC, and TG) were measured using MicroSlide technology on the Vitros Fusion 5.1 Chemistry System (New York, NY) following the manufacturer's instructions. Briefly, 200 μl of each sample were uniformly distributed over the entire slide area that contained all the reagents for the selected assays to allow larger molecules to be broken up and penetrate into the reagent layer. All samples were analyzed in triplicate, with the inter-assay variation being less than 5% and the intra-assay variation less than 10%.
Full depth placental tissue samples were collected within 10 min of the placental delivery from halfway between the cord insertion and the periphery of the placentae, avoiding infarcts. The samples were immediately rinsed in ice-cold phosphate buffered saline, and the membranes were removed and snap-frozen in liquid nitrogen for mRNA analysis. A second sample was fixed in formalin for immunohistochemistry analysis. All samples were then stored at −80°C until analysis.
RNA extraction and cDNA synthesis
Total RNA was extracted from a known amount of placental tissue (∼100 mg) using QIAzollysis reagent (Qiagen, Crawley, UK). RNA concentration and the quality of each gene were verified spectrophotometrically using the Nanodrop ND-1000 (Nanodrop Technologies, Labtech, Ringmer, UK); all of the samples had an A260/A280 ratio >1.96 and were stored at −80°C. RNA (1 μg) was then reverse transcribed using the QuantiTect Reverse Transcription kit containing a mix of random primers and oligo-dT (Qiagen) in a Primus 96 advanced gradient thermocycler (Peqlab Ltd., Fareham, UK).
Quantitative real-time PCR
Real-time PCR was carried out with the use of SYBR Green chemistry (2× QuantiFast SYBR Green, Qiagen) on a RotorGene 6000 (Corbett Research, Sydney, Australia) using the primers detailed in Table 3, following our previous protocol (19). Briefly, a pre-PCR cycle was run for 5 min at 95°C followed by 45 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 20 s. Melt-curve analysis was performed to confirm the presence of one single product and nontemplate controls run to assess contamination. Cycle threshold values were used for analysis, and abundance data were obtained by the use of quantified cDNA to generate a standard curve. Standards were quantified using densitometry and 10-fold serial dilutions (108 to 101 copies) run in parallel with the samples. Abundance data for the genes of interest were normalized to GAPDH, a stably expressed housekeeping gene suitable for human placental samples (20).
TABLE 3.
Details the forward and reverse primer sequences and BLAST sequences
| Gene | BLAST Sequence Accession Number | Primer | Length (bp) |
| LDL-R | NM_000527.3 | 5′-aggacggctacagctaccc-3′ | 73 |
| 5′- ctccaggcagatgttcacg-3′ | |||
| LRP-1 | NM_002332.2 | 5′-ggtgtcacccacctcaacat-3′ | 88 |
| 5′- agtcggtccagtacacgtttc-3′ | |||
| SRB-1 | NM_005505.4 | 5′-catcaagcagcaggtcctta-3′ | 95 |
| 5′-cggagagatagaaggggatagg-3′ | |||
| PON-1 | NM_000446.5 | 5′-actatagtccaagtgaagttcgagtg-3′ | 110 |
| 5′-atgagccagcaactcagctat-3′ | |||
| ABCA1 | NM_005502.2 | 5′-tgctgcatagtcttgggactc-3′ | 76 |
| 5′-atcacctcctgtcgcatgt-3′ | |||
| MTTP | NM_000253.2 | 5′-ggctggtcttcacggtagc-3′ | 88 |
| 5′-gttctcctccccctcgtc-3′ | |||
| PDIA2 | NM_006849.2 | 5′-ctccaagttcctggacaacg-3′ | 104 |
| 5′-tggaccccatagtggagttg-3′ |
Immunohistochemical staining of LRP-1
LRP-1 protein expression for the 17 AGA and 7 SGA preeclamptic placentae from this cohort were analyzed by immunohistochemistry. Serial sections of placental tissue were cut (5 μm) in the same orientation from paraffin-embedded tissue blocks (Sledge Microtome, Anglia Scientific, Norwich, UK) and mounted onto SuperFrost Plus glass microscope slides (Menzel-Glaser, Braunschweig, Germany). Before use, sections were dewaxed by immersion in xylene followed by rehydration in descending concentrations of alcohol (3 min each).
Immunohistochemical staining was performed using the Dako Envision staining kits (Dako Ltd., Germany). LRP-1 rabbit polyclonal antibody (ARP58562_P050; Aviva Systems Biology, USA) was used for immunostaining of paraffin-embedded placental sections; the optimal dilution (1 in 500) was optimized. Heat-induced epitope retrieval was achieved by heating in a citrate buffer (pH 6.0) using a microwave oven for 15 min, followed by incubation for 30 min in normal rabbit serum (Sigma-Aldrich, UK) to block nonspecific binding; slides were then incubated with anti-LRP-1 overnight at 4°C. A negative control was performed for each test section by incubation with rabbit IgG. Sections were dehydrated and cleared in ascending concentrations of alcohol and xylene before mounting in DPX (BDH, Poole, UK).
All of the slides were assessed by the same observer, blinded to pregnancy outcome. For analysis of placental sections, digital images of five randomly selected, high-power (×400 magnification) fields were captured on NIS-Elements F2.20 microscope (Nikon United Kingdom Ltd., Surrey, UK). Quantification of LRP-1 was performed as described previously (19, 21) using the Positive Pixel Algorithm of Aperio ImageScope software. This software is able to discriminate between positive- and negative-stained pixels and combines the number of positive pixels stained with the intensity of these same pixels to produce the value “positivity.” A visual check was also performed to ensure accurate discrimination of immunolabeled regions.
Statistical analysis
All tests were performed using SPSS for Windows version 19. Summary data are presented as means ± standard deviation (SD) or median and interquartile range as appropriate, the Student's t-test or Mann-Whitney U test were used depending on the distribution of the data, after testing using the Kolmogorov-Smirnov test. Correlations between the parameters were tested with a Spearman's rank test. The null hypothesis was rejected where P < 0.05.
RESULTS
Subjects
Demographic, obstetric, and pregnancy description data of the 51 participants are shown in Table 1; clinical descriptions have previously been published (16). All patients carried singleton pregnancies and the women with preeclampsia all had moderate-to-severe disease, without HELLP syndrome. The neonates from both pregnancy groups survived. The number of fetal serum samples that were missing was 1 in the control and 10 in the preeclamptic groups. Within the preeclamptic group, 7 women delivered SGA infants and 17 delivered AGA infants (Table 2); only 1 woman in the normotensive control group delivered an SGA infant, and thus was excluded in this analysis.
Biochemical measurements
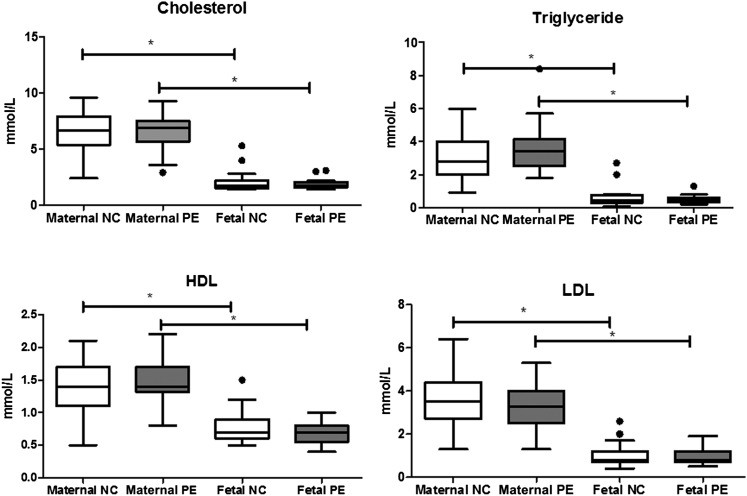
For all maternal and umbilical serum lipoproteins, there were no significant differences (P > 0.05) between normotensive and preeclamptic samples. However, umbilical venous samples had significantly lower lipid and lipoprotein concentrations compared with maternal concentrations in both groups; TG (normal and preeclampsia: P < 0.0001), TC (normal and preeclampsia: P < 0.001), LDL (normal: P = 0.003; preeclampsia: P = 0.01), and HDL (normal and preeclampsia: P = 0.001) (Fig. 1).
Fig. 1.
Serum lipoprotein concentrations in maternal and fetal circulation, from normotensive controls and preeclampsia. The number of fetal serum samples that were missing were 1 in the control (n = 26) and 10 in the preeclamptic (n = 14). NC, normotensive control; PE, preeclampsia. Data presented as median [interquartile range]. * P < 0.05 between maternal and umbilical samples for all lipoproteins.
Placental lipoprotein receptors, transporters, and enzyme expression
The mRNA expression of LDL-R, LRP-1, SRB-1, ABCA1, PON-1, MTTP, and PDIA2 in placental tissue from normotensive control and women with preeclampsia are shown in Table 4. LDL-R, LRP-1, SRB-1, and ABCA1 were all found to be expressed in placenta tissue, but there was no expression of PON-1, MTTP, and PDIA2. The placental mRNA expression of the receptors did not significantly differ between groups (P > 0.05 for all).
TABLE 4.
Placental lipoprotein mRNA expression from normotensive controls and preeclampsia
| Placenta Normalized mRNA Expression | NC (n = 27) | PE (n = 24) | P |
| LDL-R (median [IQR]) | 0.05 [0.03, 0.07] | 0.06 [0.045, 0.11] | 0.107 |
| LRP-1 (median [IQR]) | 7.0 [5.04, 17.33] | 12.3 [5.23, 23.89] | 0.503 |
| SRB-1 (median [IQR]) | 0.34 [0.21, 0.51] | 0.32 [ 0.24, 0.56] | 0.605 |
| ABCA1 (median [IQR]) | 31.38 [20.8, 73.55] | 28.8 [19.32, 82.81] | 0.942 |
NC, normotensive control; PE, preeclampsia; IQR, interquartile range.
Although the numbers were small, we additionally analyzed the 10 late-onset preeclampsia women (>34 weeks gestation) separately against the control group to test for any gestational-specific differences. The same nonsignificant results were seen for all the maternal and fetal lipoprotein concentrations and for the placental expression of the receptors, transporters, and enzymes (P > 0.1 for all).
Placental lipoprotein receptor expression and SGA
In the preeclamptic group only, we found that those delivering SGA infants had significantly lower LRP-1 expression compared with the AGA group (shown as median [IQR]) (SGA: 4.72 [3.6, 8.7]; AGA: 20.2 [13.5, 27.1]; P = 0.036). All the other genes did not show any differences (P > 0.05).
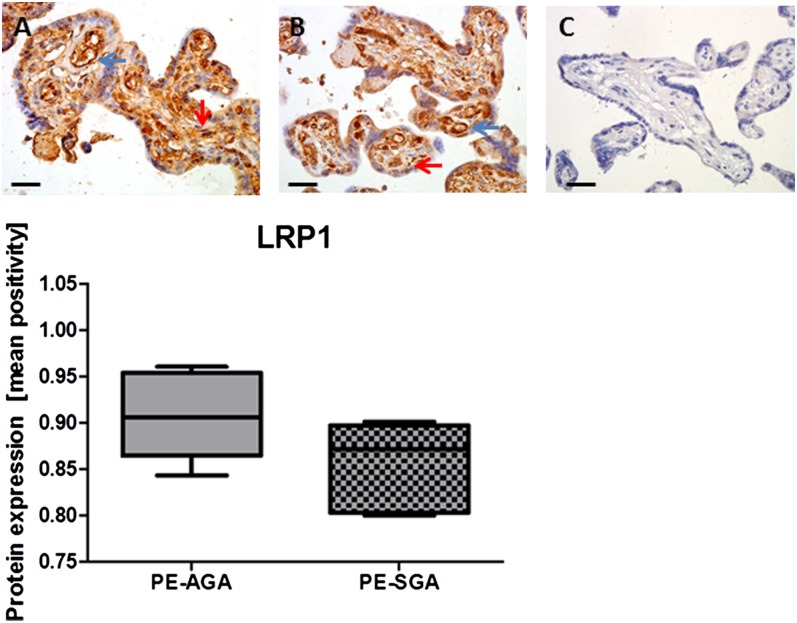
Protein expression of LRP-1 was localized mainly around fetal vessels and Hofbauer cells (Fig. 2). Visual inspection suggests lower protein expression in the preelcamptic placentae delivering SGA infants compared with the AGA group, although this was not significant when analyzed semi-quantitatively, possibly due to small sample size (median [IQR]: 0.91 [0.86, 0.95] vs. 0.87 [0.81, 0.89]; P > 0.05; Fig. 2).
Fig. 2.
LRP-1 immunostaining in preeclamptic (PE) placentae from AGA delivering mothers (n = 17) (A), SGA delivering mothers (n = 7) (B), and IgG negative control (C). In photomicrographs, positive cells appear in brown; magnification ×400; scale bar = 100 μm. Protein expression was localized to Hofbauer cells (red arrows) and fetal vessels (blue arrows). In the graph, data is represented as median [interquartile range].
Maternal serum, umbilical cord serum, and placental tissue
When maternal and umbilical cord serum lipoprotein concentrations were correlated with placental mRNA expression of the lipoprotein receptors, no difference between groups was found (P > 0.05). In addition, no associations between serum lipoproteins or receptors with birth weights or gestational age at delivery were seen (P > 0.05 for both).
DISCUSSION
In the present study, we have analyzed the lipid profiles in women with preeclampsia and normotensive pregnant women. To the best of our knowledge, this is the first study that has concurrently investigated the placental, maternal, and fetal lipoprotein system comprehensively in normotensive and preeclamptic pregnancies, and associated these results with SGA infants in the third trimester of pregnancy.
Previous studies have reported that hyperlipidemia is enhanced in preeclampsia (22, 23) and has a negative impact on fetal lipid profiles (24, 25). It has been suggested that dyslipidemia may contribute to the increased oxidative stress and endothelial dysfunction, and possibly insulin resistance, which causes a compensatory increase in insulin concentration, decreased LPL activity, and increased TGs. However, factors that influence preeclampsia, such as chronic hypertension, obesity, and insulin resistance, share common features with dyslipidemia related to oxidative stress and altered vascular function. Our data showed no significant differences in maternal and fetal lipoprotein concentrations between preeclampsia and normotensive controls, which is in line with previous data on maternal levels (26). In addition, in the current study the mean BMI was 26 kg/m2 in both groups (Table 1), suggesting that the abnormal lipid profile could be associated with obesity (27), and not necessarily with preeclampsia. This could also be a reason why some previous studies have reported increased lipoprotein concentrations in preeclampsia, because they may not have controlled for this. Thus, the results from this study do not suggest that changes in the lipoprotein concentrations play a direct key role in preeclampsia pathology.
All samples were collected at delivery and in the third trimester of pregnancy. Although not matched for gestation, lipoprotein concentrations are increased between the first two trimesters and last trimester, but are then stable (28); therefore these could be compared between groups even if the gestational age was not exactly the same. Furthermore, the same nonsignificant results were seen when only the term preeclamptic samples were compared with controls.
The highest mRNA expression was ABCA1, followed by LRP-1. A previous study also reported that ABCA1 is highly expressed in human placenta (29). LRP-1 is the direct lipoprotein receptor of intermediate density lipoprotein, a precursor of LDL (15). However SRB-1 and LDL-R, the main cholesterol transporters (30), had very low expression in third trimester placental tissue, which may suggest that the function of LDL-R and SRB-1 may not have an important role toward term, which may reflect a downregulation due to lipids derived from the fetus (2). A previous study at term concluded that SRB-1 does not play a critical role in controlling plasma cholesterol concentration, even in pregnant women with high or low levels of cholesterol (30). Our findings are in agreement with others who have reported a marked ABCA1 expression level in diverse placental cell types (31), and the presence of LDL-R, LRP-1, and SRB-1 in placental tissues (15). However, in contrast to the current data, Wadsack et al. showed lower mRNA expression of LDL-R in FGR-S, and Stepan et al. (32) reported higher. Different criteria in selecting the study population may account for these differences (32); contrasting protein values have also been observed in several studies. In our study, we examined LRP-1 protein localization and expression due to the differences seen in the preeclamptic plus SGA group. These data suggest lower expression in the SGA group, although this didn't reach significance, possibly due to small numbers. LRP-1 is highly expressed in macrophages, and therefore it is unsurprising that the expression is localized to the macrophages of the placentae (Hofbauer cells).
It must be noted that cholesterol is not only transported by the receptors; experimental evidence suggests that trophoblast cells efflux cholesterol from cells like any other polarized cells (33). Three different mechanisms for cholesterol transportation from the circulation and peripheral cells to target tissues have been proposed: 1) aqueous diffusion, a protein-independent pathway based on concentration gradients; 2) the SRB-1 mediated bidirectional transport between cells and extracellular acceptors; and 3) ABCA1-mediated unidirectional efflux to lipid-free apolipoproteins (34, 35). The physiological consequences of the regulation of receptors for placental function and fetal development are unclear. A yet undefined proportion of cholesterol uptake by the placenta may be released into the fetal circulation. The remaining placental cholesterol, however, is used for sterol synthesis, principally of progesterone.
When we analyzed the relationship between placental genes and SGA, we observed that LRP-1 was significantly lower in placentae from SGA infants in the preeclamptic group. A possible mechanism for this could be through the matrix metalloproteinase (MMP) family. Membrane-type MMPs can degrade LRP-1 into low molecular-mass fragments. Intact soluble LRP-1 α-chain is shed into human plasma and has been identified at the blood-brain barrier following ischemia. Proteins of the MMP family are involved in the breakdown of the extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodelling, as well as in diseases processes, such as arthritis and metastasis (36). Increased MMP-2 activity (LRP-1 mediates its internalization) may contribute to the endothelial dysfunction that is central to the pathophysiology of preeclampsia and/or SGA.
Further analysis is necessary to understand the cholesterol pathway, especially in relation to fetal growth, in particular alterations in FGR pregnancies and gestational diabetes mellitus, which can result in extremely high birth weight infants (1). It would also be interesting to study the expression of the proprotein convertase subtillisin/kexin type 9 (PCSK9) gene, as it regulates cholesterol metabolism via degradation of LDL-R (37).
This is a preliminary study that contributes to the current knowledge with the need for future research. It would also be desirable to examine changes at both mRNA and protein levels of all the lipoprotein receptors, transporters, and enzymes. Another limitation of the current study is that we did not have details of exposure to antenatal steroids and other medications, surgery, or timing of the last meal, which may influence some of these alterations. Much has to be done to elucidate the placental transport in pregnancy pathologies, as the transport mechanism for lipids and lipoproteins is far from being understood.
The findings of this study do not support a direct contribution of lipid metabolism in the pathogenesis of preeclampsia. However, this is the first study that simultaneously analyzed seven lipoprotein receptors, transporters, and enzymes related to the cholesterol pathway, with the lipids and lipoprotein levels (LDL, HDL, TC, and TG) in maternal and umbilical cord serum, and in relation to fetal growth.
Acknowledgments
The authors thank all the women who participated in the study and the midwives and doctors whose support made this study possible. The authors are also grateful to Professor Fiona Broughton Pipkin (University of Nottingham) for help and advice regarding statistical analysis.
Footnotes
Abbreviations:
- AGA
- adequate-for-gestational-age
- BMI
- body mass index
- FGR
- fetal growth restriction
- FGR-M
- fetal growth restriction without hemodynamic changes
- FGR-S
- fetal growth restriction with hemodynamic changes
- IQR
- interquartile range
- LDL-R
- LDL receptor
- LRP-1
- LDL receptor-related protein 1
- MMP
- matrix metalloproteinase
- MTTP
- microsomal triglyceride transfer protein
- PDIA2
- protein disulfide isomerase family A member 2
- PON-1
- paraoxonase-1
- SGA
- small-for-gestational-age
- SRB-1
- scavenger receptor class B type 1
- TC
- total cholesterol
- TG
- triglyceride
This work was supported by Tommy's Charity (charity number: 1060508) and CAPES/CNPq, Brazil.
REFERENCES
- 1.Desoye G., Gauster M., Wadsack C. 2011. Placental transport in pregnancy pathologies. Am. J. Clin. Nutr. 94(6 Suppl.): 1896S–1902S [DOI] [PubMed] [Google Scholar]
- 2.Ghio A., Bertolotto A., Resi V., Volpe L., Di Cianni G. 2011. Triglyceride metabolism in pregnancy. Adv. Clin. Chem. 55: 133–153 [DOI] [PubMed] [Google Scholar]
- 3.Vrijkotte T. G., Krukziener N., Hutten B. A., Vollebregt K. C., van Eijsden M., Twickler M. B. 2012. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J. Clin. Endocrinol. Metab. 97: 3917–3925 [DOI] [PubMed] [Google Scholar]
- 4.Carr B. R., Simpson E. R. 1982. Cholesterol synthesis in human fetal tissues. J. Clin. Endocrinol. Metab. 55: 447–452 [DOI] [PubMed] [Google Scholar]
- 5.Pecks U., Brieger M., Schiessl B., Bauerschlag D. O., Piroth D., Bruno B., Fitzner C., Orlikowsky T., Maass N., Rath W. 2012. Maternal and fetal cord blood lipids in intrauterine growth restriction. J. Perinat. Med. 40: 287–296 [DOI] [PubMed] [Google Scholar]
- 6.Alvarez J. J., Montelongo A., Iglesias A., Lasuncion M. A., Herrera E. 1996. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J. Lipid Res. 37: 299–308 [PubMed] [Google Scholar]
- 7.Ghulmiyyah L., Sibai B. 2012. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 36: 56–59 [DOI] [PubMed] [Google Scholar]
- 8.Duley L. 2009. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33: 130–137 [DOI] [PubMed] [Google Scholar]
- 9.Brown M. A., Lindheimer M. D., de Swiet M., Van Assche A., Moutquin J. M. 2001. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens. Pregnancy. 20: IX–XIV [DOI] [PubMed] [Google Scholar]
- 10.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. 2000. Am. J. Obstet. Gynecol. 183: S1–S22 [PubMed] [Google Scholar]
- 11.Lim K. H., Zhou Y., Janatpour M., McMaster M., Bass K., Chun S. H., Fisher S. J. 1997. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 151: 1809–1818 [PMC free article] [PubMed] [Google Scholar]
- 12.Huppertz B., Peeters L. L. 2005. Vascular biology in implantation and placentation. Angiogenesis. 8: 157–167 [DOI] [PubMed] [Google Scholar]
- 13.Campos B., Chames M., Lantry J. M., Bill J. P., Eis A., Brockman D., Neil J., Tischner E., Barton J., Wong C., et al. 2006. Determination of non-bilayer phospholipid arrangements and their antibodies in placentae and sera of patients with hypertensive disorders of pregnancy. Placenta. 27: 215–224 [DOI] [PubMed] [Google Scholar]
- 14.Hubel C. A. 1999. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 222: 222–235 [DOI] [PubMed] [Google Scholar]
- 15.Wadsack C., Tabano S., Maier A., Hiden U., Alvino G., Cozzi V., Hüttinger M., Schneider W. J., Lang U., Cetin I., et al. 2007. Intrauterine growth restriction is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition. Am. J. Physiol. Endocrinol. Metab. 292: E476–E484 [DOI] [PubMed] [Google Scholar]
- 16.Mistry H. D., Wilson V., Ramsay M. M., Symonds M. E., Broughton Pipkin F. 2008. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension. 52: 881–888 [DOI] [PubMed] [Google Scholar]
- 17.Gardosi J. 2012. Customised centile calculator. Accessed March 26, 2012, at www.gestation.net
- 18.Cetin I., Foidart J. M., Miozzo M., Raun T., Jansson T., Tsatsaris V., Reik W., Hauguel-de-Mouzon S., Illsley N., Kingdom J., et al. 2004. Fetal growth restriction: a workshop report. Placenta. 25: 753–757 [DOI] [PubMed] [Google Scholar]
- 19.Mistry H. D., McCallum L. A., Kurlak L. O., Greenwood I. A., Broughton Pipkin F., Tribe R. M. 2011. Novel expression and regulation of voltage-dependent potassium channels in placentas from women with preeclampsia. Hypertension. 58: 497–504 [DOI] [PubMed] [Google Scholar]
- 20.Murthi P., Fitzpatrick E., Borg A. J., Donath S., Brennecke S. P., Kalionis B. 2008. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta. 29: 798–801 [DOI] [PubMed] [Google Scholar]
- 21.Williams P. J., Mistry H. D., Innes B. A., Bulmer J. N., Broughton Pipkin F. 2010. Expression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the human. Placenta. 31: 448–455 [DOI] [PubMed] [Google Scholar]
- 22.Ray J. G., Diamond P., Singh G., Bell C. M. 2006. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG. 113: 379–386 [DOI] [PubMed] [Google Scholar]
- 23.Lorentzen B., Endresen M. J., Clausen T., Hendriksen T. 1994. Fasting serum free fatty acids and triglycerides are increased before 20 weeks of gestation in women who later develop preeclampsia. Hypertens. Pregnancy. 13: 103–109 [Google Scholar]
- 24.Belo L., Caslake M., Gaffney D., Santos-Silva A., Pereira-Leite L., Quintanilha A., Rebelo I. 2002. Changes in LDL size and HDL concentration in normal and preeclamptic pregnancies. Atherosclerosis. 162: 425–432 [DOI] [PubMed] [Google Scholar]
- 25.Catarino C., Rebelo I., Belo L., Rocha-Pereira P., Rocha S., Castro E. B., Patrício B., Quintanilha A., Santos-Silva A. 2008. Fetal lipoprotein changes in pre-eclampsia. Acta Obstet. Gynecol. Scand. 87: 628–634 [DOI] [PubMed] [Google Scholar]
- 26.Emet T., Ustüner I., Güven S. G., Balık G., Ural U. M., Tekin Y. B., Sentürk S., Sahin F. K., Avşar A. F. 2013. Plasma lipids and lipoproteins during pregnancy and related pregnancy outcomes. Arch. Gynecol. Obstet. 288: 49–55 [DOI] [PubMed] [Google Scholar]
- 27.Catapano A. L., Reiner Z., De Backer G., Graham I., Taskinen M. R., Wiklund O., Agewall S., Alegria E., Chapman M., Durrington P., et al. 2011. ESC/EAS guidelines for the management of dyslipidaemias the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 217: 3–46 [DOI] [PubMed] [Google Scholar]
- 28.Herrera E., Ortega H., Alvino G., Giovannini N., Amusquivar E., Cetin I. 2004. Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. Eur. J. Clin. Nutr. 58: 1231–1238 [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharjee J., Ietta F., Giacomello E., Bechi N., Romagnoli R., Fava A., Paulesu L. 2010. Expression and localization of ATP binding cassette transporter A1 (ABCA1) in first trimester and term human placenta. Placenta. 31: 423–430 [DOI] [PubMed] [Google Scholar]
- 30.Ethier-Chiasson M., Duchesne A., Forest J. C., Giguere Y., Masse A., Mounier C., Lafond J. 2007. Influence of maternal lipid profile on placental protein expression of LDLr and SR-BI. Biochem. Biophys. Res. Commun. 359: 8–14 [DOI] [PubMed] [Google Scholar]
- 31.Nikitina L., Wenger F., Baumann M., Surbek D., Korner M., Albrecht C. 2011. Expression and localization pattern of ABCA1 in diverse human placental primary cells and tissues. Placenta. 32: 420–430 [DOI] [PubMed] [Google Scholar]
- 32.Stepan H., Faber R., Walther T. 1999. Expression of low density lipoprotein receptor messenger ribonucleic acid in placentas from pregnancies with intrauterine growth retardation. Br. J. Obstet. Gynaecol. 106: 1221–1222 [DOI] [PubMed] [Google Scholar]
- 33.Woollett L. A. 2005. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am. J. Clin. Nutr. 82: 1155–1161 [DOI] [PubMed] [Google Scholar]
- 34.Rothblat G. H., de la Llera-Moya M., Atger V., Kellner-Weibel G., Williams D. L., Phillips M. C. 1999. Cell cholesterol efflux: integration of old and new observations provides new insights. J. Lipid Res. 40: 781–796 [PubMed] [Google Scholar]
- 35.Yancey P. G., Bortnick A. E., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Rothblat G. H. 2003. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23: 712–719 [DOI] [PubMed] [Google Scholar]
- 36.Selvais C., D'Auria L., Tyteca D., Perrot G., Lemoine P., Troeberg L., Dedieu S., Noël A., Nagase H., Henriet P., et al. 2011. Cell cholesterol modulates metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 (LRP-1) and clearance function. FASEB J. 25: 2770–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy E., Ben Djoudi Ouadda A., Spahis S., Sane A. T., Garofalo C., Grenier É., Emonnot L., Yara S., Couture P., Beaulieu J. F., et al. 2013. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis. 227: 297–306 [DOI] [PubMed] [Google Scholar]