Abstract
Although HDL is inversely correlated with coronary heart disease, elevated HDL-cholesterol is not always protective. Additionally, HDL has biological functions that transcend any antiatherogenic role: shotgun proteomics show that HDL particles contain 84 proteins (latest count), many correlating with antioxidant and anti-inflammatory properties of HDL. ApoA-I has been suggested to serve as a platform for the assembly of these protein components on HDL with specific functions - the HDL proteome. However, the stoichiometry of apoA-I in HDL subspecies is poorly understood. Here we use a combination of immunoaffinity chromatography data and volumetric analysis to evaluate the size and stoichiometry of LpA-I and LpA-I,A-II particles. We conclude that there are three major LpA-I subspecies: two major particles, HDL[4] in the HDL3 size range (d = 85.0 ± 1.2 Å) and HDL[7] in the HDL2 size range (d = 108.5 ± 3.8 Å) with apoA-I stoichiometries of 3 and 4, respectively, and a small minor particle, HDL[1] (d = 73.8 ± 2.1Å) with an apoA-I stoichiometry of 2. Additionally, we conclude that the molar ratio of apolipoprotein to surface lipid is significantly higher in circulating HDL subspecies than in reconstituted spherical HDL particles, presumably reflecting a lack of phospholipid transfer protein in reconstitution protocols.
Keywords: apolipoprotein A-I; apolipoprotein A-II; LpA-I; LpA-I,A-II; HDL platform; HDL surface monolayer; reconstituted spheroidal HDL
High density lipoproteins (HDL) are a population of apoA-I-containing particles inversely correlated with the risk of coronary heart disease (CHD) (1); however, lower HDL-cholesterol levels are not uniformly associated with excess cardiovascular risk, nor do higher HDL-cholesterol levels always confer a protective benefit (2). Further, HDL has biological functions that transcend its antiatherogenic role. Shotgun proteomics has shown that HDL particles contain, at the latest count, 84 proteins (3), many correlating with the antioxidant, anti-inflammatory and antiatherogenic properties of HDL (4, 5). ApoA-I has been suggested to serve as a platform for the assembly of certain protein components on HDL with specific functions - the HDL proteome - creating the functional heterogeneity of HDL (4–6). The mechanisms driving HDL platform specificity are not known.
During assembly and remodeling of HDL, nascent discoidal HDL (dHDL) is converted to spheroidal HDL (sHDL), a particle containing a core of cholesteryl ester (CE) and varying amounts of triglyceride (TG), through the action of the enzyme, LCAT. Circulating sHDL contains 2–4 (perhaps more) apoA-I molecules of uncertain conformation on the particle surface (7). ApoA-I generally represents about 70–80% of HDL protein by weight, and apoA-II about 20%. Many other proteins in much smaller quantities are also associated with plasma sHDL (4, 5): some associate directly with the lipid via the amphipathic helical motif (8), and others associate with apoA-I through protein-protein interactions (9, 10).
The heterogeneous nature of human HDL (d 1.063 to 1.210 g/ml) with respect to size and density is well documented. Analytical ultracentrifugation has been used to measure levels of HDL2 and HDL3 as well as to elucidate the existence of other subspecies of HDL (11). Sequential isopycnic ultracentrifugation has been used to separate HDL2 and HDL3 (12). Zonal ultracentrifugation has been used to isolate HDL subspecies designated HDLl (d 1.080 to 1.090 g/ml), HDL2 (d 1.063 to 1.125 g/ml), and HDL3, (d 1.125 to 1.21 g/ml (13). The HDL subspecies HDL2 and HDL3 have also been shown to be heterogeneous. Five subspecies were identified by Blanche et al. (14) using nondenaturing gradient gel electrophoresis (NDGGE) analysis of HDL in the ultracentrifugal d < 1.20 g/ml fraction of human plasma and in HDL2b, HDL2a and HDL3 prepared by density gradient ultracentrifugation. The mean hydrated density and particle size of the five subspecies, from largest to smallest, were 1.085 g/ml and 10.57 nm in (HDL2b)gge; 1.115 g/ml and 9.16 nm in (HDL2a)gge; 1.136 g/ml and 8.44 nm in (HDL3a)gge; 1.154 g/ml and 7.97 nm in (HDL3b)gge; and 1.171 g/ml and 7.62 nm in (HDL3c)gge. This terminology represents a somewhat cumbersome choice of nomenclature.
Using immunoaffinity chromatography, two subsets of plasma sHDL particles were isolated. One subset contains both apoA-I and apoA-II (LpA-I,A-II). The other subset contains apoA-I but not apoA-II (LpA-I) (15, 16). Both LpA-I and LpA-I,A-II are heterogeneous in particle size. Attempts were made to assign the two major subspecies of LpA-I and the three major subspecies of LpA-I,A-II to the five NDGGE HDL subspecies, and the stoichiometry of apoA-I and apoA-II in these subspecies was determined using chemical crosslinking (17). Here, we apply volumetric analysis to further analyze the size and stoichiometry of LpA-I and LpA-I,A-II subspecies using recently reported lipid and protein compositions of these particles, and we define their upper and lower boundaries using an analytical method for volumetric limits.
METHODS
Immunoaffinity chromatography
In a previous study (16) we reported the characterization of HDL subspecies using a combination of apoA-I and A-II immunoaffinity chromatography (15) with single vertical spin (SVS) ultracentrifugation (18–20) and negative stain electron microscopy. The chief artifact of the immunoaffinity method used is elution with 0.1 M acetic acid, 1 mM EDTA, pH 3.0. The procedure appears to be minimally disruptive, since at least 98% of the total apoA-I is associated with HDL[1]–HDL[9] (15), in which the bands of free apolipoproteins are extremely minor compared with the total staining for HDL subspecies.
Ultracentrifugation by the SVS procedure has the advantage of short spin times (90–150 min), and thus less particle degradation than sequential flotation. Very little free apoA-I is produced by this procedure. Shorter ultracentrifugation spin times, such as with SVS or other approaches, appear to be minimally disruptive. After a 24 h single spin at 40,000 rpm to isolate HDL subspecies, Davidson and colleagues saw a small amount of what appears to be free apoA-I. But this may be an artifact of the NDGGE gels because if the same ultracentrifugation fractions are passed over gel filtration columns, no free apoA-I is seen (Davidson, personal communication).
Using this combination of techniques, we identified in this study a minimum of nine distinct subspecies of HDL. The vertical rotor procedure (SVS and vertical autoprofile, VAP) used for the analysis of HDL subspecies is a modification of one described earlier for single spin separation and analysis of the major classes of lipoproteins (21).
Calculations of the stoichiometry and dimensions of reconstituted HDL particles or isolated HDL fractions
These calculations require weight percentage measurements for the protein and lipid components of reconstituted HDL particles (22) or isolated whole-plasma fractions (23, 24). Using the molecular weight and volume of each molecule of apoA-I, apoA-II, phospholipid (PL), CE, unesterified cholesterol (UC), and TG, sequential permutations of protein stoichiometry per particle (e.g., two apoA-I, two apoA-I, and one apoA-II) allows calculation for that particular apolipoprotein stoichiometry of the total volume of the lipid core (CE and TG) and, by addition of the polar monolayer (apolipoprotein, PL and UC), the volume of the total HDL particle.
Assuming spherical symmetry (given the soft nature of HDL, a sphere is a reasonable approximation of the average shape of an HDL particle) the diameter of the core and total HDL particle can be calculated for each protein permutation. By difference, the thickness of the polar monolayer can also be calculated. As an example, for a given reconstituted HDL particle or isolated HDL fraction, as the total volume of the protein component increases, the number of each lipid moiety increases, and consequentially, the particle size increases; mathematical formulae can be derived that link protein stoichiometry to stoichiometry of each lipid class and HDL particle dimension. For each protein stoichiometry applied to a given particle or fraction, the calculated diameter is plotted and examined for best fits to diameters determined by NDGGE for HDL isolated by immunoaffinity chromatography (16).
Other apolipoproteins, such as the apoCs or apoE, because of their minor concentration compared with apoA-I and apoA-II, have been omitted from all but one of the calculations. Individual NDGGE subspecies bands, particularly those containing apoA-I but no apoA-II, are generally broad, suggesting a mixture of minor protein components. We included the effects of one molecule of apoC-III, the longest and most abundant of the apoCs, on calculated diameter of the 4:0 stoichiometry for the double-shell model; the resulting value stays well within the broad size distribution of HDL[7]. We also included the effects of one molecule of apoE on the calculated diameter of the 4:0 stoichiometry for the double-shell model; the resulting value falls just outside the broad size distribution of HDL[7].
RESULTS
Correspondence of HDL subspecies defined by immunoaffinity chromatography with subspecies defined by NDGGE
Immunoaffinity chromatography.
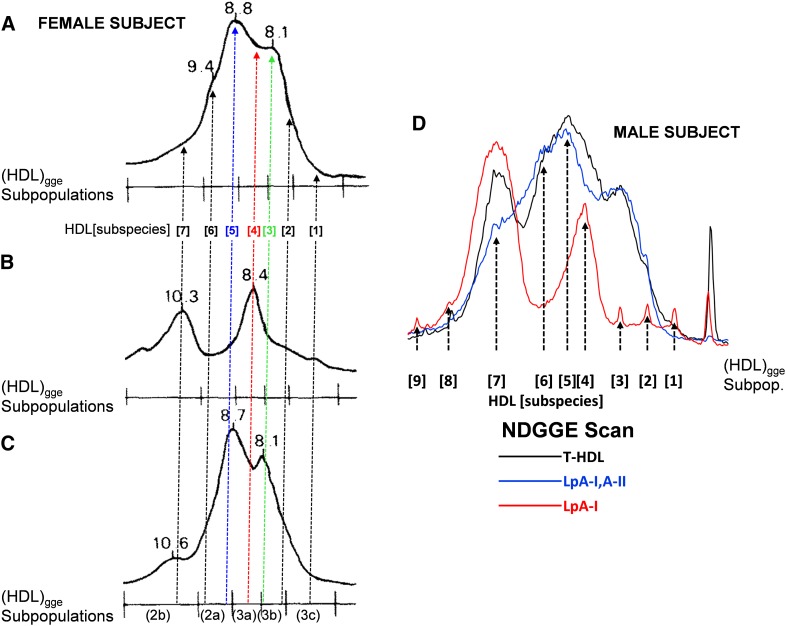
Fig. 1 summarizes the essential features of our previous characterization of nine LpA-I and LpA-I,A-II subspecies of HDL particles (15, 16). Fig. 1A represents NDGGE analysis of total HDL (T-HDL), LpA-I, and LpA-I,A-II, three HDL fractions isolated from a subject by immunoaffinity chromatography: i) T-HDL represents whole-plasma particles retained by and then eluted from an anti-apoA-I immunoaffinity column; ii) LpA-I,A-II represents plasma particles retained by and then eluted from the anti-apoA-II immunoaffinity column; and iii) LpA-I represents plasma particles not retained by an anti-apoA-II immunoaffinity column but retained by and eluted from an anti-apoA-I immunoaffinity column. Nine apoA-I-containing particles, designated HDL[1]–HDL[9], with mean diameter ranging from 76.4 to 131.8 Å (Table 1) were identified.
Fig. 1.
Characterization of HDL subspecies isolated by immunoaffinity chromatography and SVS ultracentrifugation. A. Nondenaturing gradient gel electrophoresis stained with Coomassie blue of the three immunoaffinity column chromatography-eluted fractions from a male subject. The positions of subspecies 1–9 are indicated to the left of the electrophoresis for each fraction, T-HDL, LpA-I,A-II, and LpA-I. Black triangles mark albumin and free apolipoprotein. Modified from Cheung, et al. (16). B. Nondenaturing gradient gel analysis of fractions 1–7 from HDL-SVS fractionation (2.5 h spin) of plasma from the male subject shown in (A) stained by Coomassie blue (lanes 2–7, respectively). The arrowhead to the right of lane 7 denotes HDL[7]. Black triangles mark albumin and free apolipoprotein. Modified from Cheung, et al. (16). C. HDL-VAP analysis (1.5 h spin) of T-HDL, LpA-I,A-II, and LpA-I isolated from whole plasma from a female subject. The estimated peak centers for the HDL subspecies are indicated by thin vertical lines. The LpA-I subspecies HDL[4] and HDL[7] are indicated by vertical arrows. Modified from Cheung, et al. (16).
TABLE 1.
Diameter by NDGGE
| HDL Subspecies | Mean Diameter in Å |
| 9 | 131.8 ± 1.1 (n = 3) |
| 8 | 121.4 ± 2.0 (n = 2) |
| 7 | 108.5 ± 3.8 (n = 4) |
| 6 | 95.3 ± 2.5 (n = 4) |
| 5 | 89.9 ± 2.4 (n = 4) |
| 4 | 85.0 ± 1.2 (n = 4) |
| 3 | 81.0 ± 0.9 (n = 4) |
| 2 | 78.2 ± 0.8 (n = 4) |
| 1 | 76.4 ± 0.8 (n = 4) |
Adapted from Cheung, et al. (15).
Fig. 1B represents NDGGE of seven fractions collected from a 2.5 h HDL SVS fractionation of whole plasma from the same subject analyzed in Fig. 1A (16). Although plasma protein bands contaminate all of the fractions, especially 1 and 2, it is reasonably clear that fractions 4–7 contain the four HDL subspecies referred to as HDL[4]–HDL[7]. Further, these fractions contain little free apoA-I (Fig. 1B, arrowhead). Finally, Fig. 1C (16) shows 1.5 h HDL-VAP analyses (25) of the three subspecies fractions (T-HDL, LpA-I and LpA-I,A-II) isolated by immunoaffinity chromatography as shown in Fig. 1A.
Matching of immunoaffinity-isolated LpA-I and LpA-I,A-II-containing particles to HDL isolated by ultracentrifugation and NDGGE.
In a study carried out to match the subspecies of LpA-I and LpA-I,A-II to the subspecies of HDL prepared by ultracentrifugation (17), we found that the two distinct subspecies of LpA-I corresponding to HDL[4] and HDL[7] in Fig. 1A, fall into the (HDL3a)gge and (HDL2b)gge size intervals, respectively (align Fig. 2B with Fig. 2A). In LpA-I,A-II, the subspecies corresponding to HDL[3] and HDL[5] appear to be a mixture of (HDL3a and HDL3b)gge, and a mixture of (HDL3a and HDL2a)gge, respectively, while LpA-I,A-II subspecies HDL[6] falls mostly in the (HDL2a)gge size interval (align Fig. 2C with Fig. 2A). Thus, density cuts or size exclusion chromatography to isolate the HDL particles in the size interval of (HDL3a)gge will not be able to resolve HDL[3], HDL[4], and HDL[5] and will contain a mixture of LpA-I and LpA-I,A-II. This conclusion is confirmed by Fig. 2D, an overlay of densitometry scans of the three immunoaffinity isolated fractions, T-HDL, LpA-I,A-II and LpA-I, subjected to NDGGE in Fig. 1A.
Fig. 2.
NDGGE patterns of LpA-I,A-II, LpA-I, and d < 1.20 g/ml fractions isolated from plasma of normal subjects. A. LpA-I,A-II. B. LpA-I. C. d < 1.20 g/ml. All from a female subject. Particle sizes of subpopulations determined at peak maxima are indicated (in nm). Scale at the bottom corresponds to size intervals of major HDL subpopulations identified by NDGGE in plasma of normal subjects. The positions of HDL[1–7] subspecies are indicated by dotted vertical arrows: HDL[5], blue; HDL[4], red; HDL[3], green. Modified from Cheung, et al. (17). D. Densitometry scans of the three lanes in Fig. 1A. T-HDL is black; LpA-I,A-II is blue; and LpA-I is red. The positions of the nine HDL subspecies are indicated with vertical dotted arrows.
The HDL[1] and HDL[2] subspecies with mean diameters of 76.4 Å and 78.2 Å fall into the 7.2–7.8 nm size interval of (HDL3c)gge, whereas HDL[8] and HDL[9] with mean diameters of 12.4 Å and 131.8 Å are larger than the average HDL particles in (HDL2b)gge (Table 1 and Fig. 2D) (17).
Volumetric analyses of the stoichiometry of HDL subspecies
Stoichiometry of reconstituted particles with well-defined protein and lipid compositions.
We developed a volumetric assay for varying the apolipoprotein and lipid stoichiometries of native HDL subspecies to determine best fits to known particle diameters. Sequential permutations of protein stoichiometry (e.g., two apoA-I, two apoA-I, and one apoA-II) were used to convert the weight percentage of each lipid component (PL, UC, CE, and TG) to molecules per particle (see Table 2 footnotes for details). Using known volumes for each molecular component, we plotted and examined the results for best fits to diameters determined by NDGGE for HDL isolated by immunoaffinity chromatography (16).
TABLE 2.
Calculation of size and composition of S93 and S80 by volumetric analysis
| In Vitro Particle (Ref.) | Method | A-I | A-I | A-II | A-II | (Ref.) | PL | PL | CE | CE | UC | UC | TG | TG |
| MW | Vol (Å) | MW | Vol (Å) | MW | Vol (Å) | MW | Vol (Å) | MW | Vol (Å) | MW | Vol (Å) | |||
| S93 (Silva, personal communication) | LCAT | 28016 | 33899 | 17414 | 21071 | (27) | 787 | 1307 | 650 | 1179 | 387 | 610 | 850 | 1575 |
| S80 (22) | LCAT+ CETP | 28016 | 33899 | 17414 | 21071 | (27) | 787 | 1307 | 650 | 1179 | 387 | 610 | 850 | 1575 |
| A-I per Particle | A-II per Particle | PL% | PL per Particle | CE% | CE per Particle | UC% | UC per Particle | TG% | TG per Particle |
| 3 | 0 | 40.8 | 96.6 a | 12.9 | 37.0 a | 1.2 | 5.7 a | 0.0 | 0.0 a |
| 2 | 0 | 86 | 12 | 6 | 10 |
| Core Vol (CE+TG) (Å3) b | Core Diameter (Å3) c | Total HDL Vol (Å3) b | HDL Diameter (Å) c | HDL Diameter with Single Hydrated Layer (+5.6 Å) | HDL Diameter With Double Hydrated Layer (+11.2 Å) |
| 43605 | 45 | 275103 | 81 | 86 | 93 |
| 29898 | 39 | 213759 | 74 | 80 | 86 |
Calculation of the number nL of each type of lipid L (PL, CE, UC, TG) per HDL particle either experimentally determined or by examining different combinations of the number nA of each type of apolipoprotein A (A-I, A-II…) per HDL particle is as follows: nL = (MWP/ MWL) × (%L/ %P), where MWP = total molecular weight of protein = (A-I MW × nA-I) + (A-II MW × nA-II) + …), where MWL = molecular weight of single lipid L; %L = weight percentage of L; %P = weight percentage of total apolipoprotein = (1 − ∑%L).
Calculation of core volume (VC) = (nCE × VCE) + (nTG × VTG), where VL is volume of each L. Calculation of the total HDL volume (VHDL) = ∑ (nL × VL) + VP, where VP is total volume of apolipoprotein.
Calculation of core and HDL diameters = 2r; r = (3V/(4π))1/3 assuming a sphere.
Table 2 represents an example of the application of this volumetric method to the well-characterized S80 and S93 sHDL particles reconstituted in synthetico by Silva et al. (22). S80 and S93 were shown to contain two and three apoA-I, respectively, and the weight percentage for each lipid component was determined for each particle (22).
In developing this assay, we assumed that the protein components were unhydrated and their volume could be calculated by multiplying their molecular weights by a factor of 1.21 Å3/Da (26). Volumes for the lipid components were derived from McNamara et al. (27). Assuming a regular spherical shape (28), by sequentially varying the number of apoA-I and apoA-II per particle and using the weight percentage for each lipid component, a sequence of particle diameters can be calculated for each sHDL particle.
Plugging the known apoA-I stoichiometries and lipid weight percentage into Table 2, particle diameters of 74.2 and 80.7 Å are calculated for S80 and S93; the Stokes diameters reported for S80 and S93 are 80 Å and 93 Å, respectively (22), larger by 6–12 Å than diameters calculated by the volumetric method. It is known that hydrated protein (26, 29, 30) and lipid (31, 32) are surrounded by one to three hydration shells of approximately 3 Å/shell. A publication by Atmeh and Elrazeq (28) shows that the Stokes diameters of sHDL determined by NDGGE are larger than expected, on the basis of which they suggest that sHDL particles are surrounded by approximately two hydration layers that affect electrophoretic mobility on NDGGE. However, the precise number of hydration shells for any molecule is not at all clear. Certain ions have one or even one-and-one-half hydration shells (33). Protein (29) likely is less hydrated that lipid (32), and its hydration is affected by the presence of associated lipid (32). Finally, the presence of ions affects the hydration shells of lipoproteins (34), and the unusually high fraction of charged residues in apoA-I (∼30%) compared with typical globular proteins (∼15%) may contribute to stronger hydration. Thus, while HDL is certainly hydrated, the precise number of hydration shells is not clear. Here, we assume that the number of hydration shells for HDL ranges between one and two.
Using the diameter of a single water molecule, 2.8 Å, for the thickness of a single hydration layer, a range of diameters assuming one or two hydration shells was calculated for S80 and S93 (Table 2). One and two hydration layers give particle diameters of 79.8 and 85.4 Å for S80 and 86.3 and 92.7 Å for S93. This volumetric method can also be used to calculate the monolayer thickness (Tm) of surface components (apolipoprotein + PL + UC). Using the formula, Tm = (particle diameter − core diameter) / 2, Tm is calculated to be 17.8 Å for both S80 and S93 (Table 2).
Stoichiometry of circulating HDL subspecies.
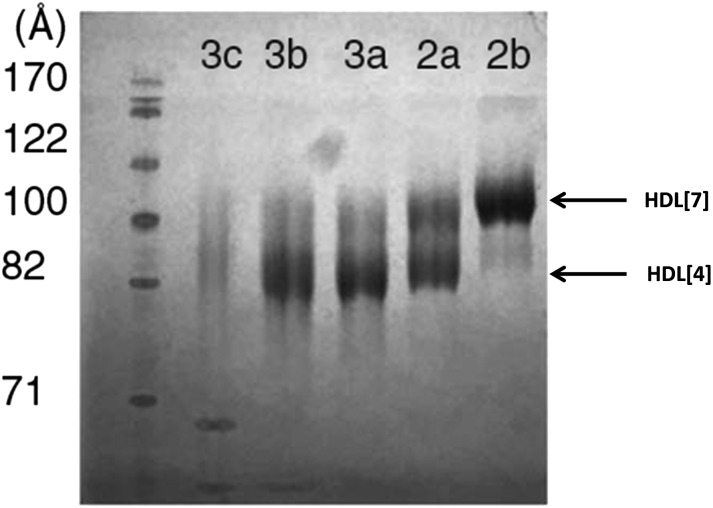
Huang et al. (24) reported the weight percentage for the protein and lipid of five HDL fractions HDL(2a–3c) isolated by a one-step gradient ultracentrifugation method (supplementary Table I). The mean densities of their five HDL subfractions are comparable to the corresponding five gradient gel size subfractions of Blanche et al. (14). In a somewhat unusual twist, the HDL was first partially depleted of apoA-II using sulfhydryl covalent chromatography to produce an HDL containing only about 20–30% of its original apoA-II content. Figure 3 represents NDGGE analyses of the five resulting density cut fractions. It is of interest that there are essentially two major protein bands in each of the five fractions (Fig. 3) that correspond in diameter to the two major LpA-I subspecies, HDL[4] and HDL[7].
Fig. 3.
NDGGE analysis of LpA-I HDL subfractions isolated by sulfhydryl chromatography. The gel was stained with Coomassie blue. The positions of HDL[4] and HDL[7] are indicated with arrows. Modified from Huang et al. (24).
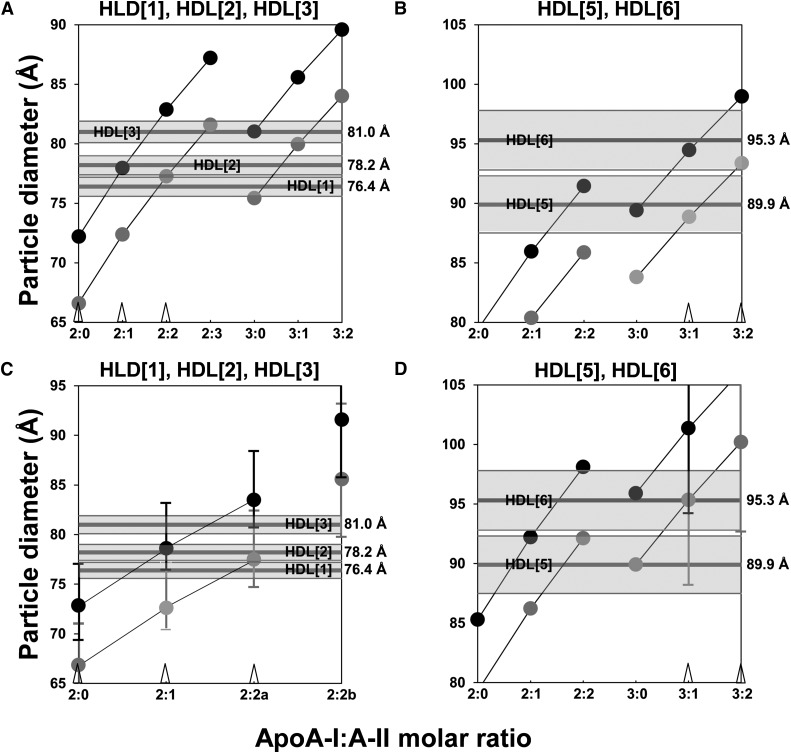
Given the prominence of these two subspecies, we plugged the compositional data in supplementary Table I into our volumetric method for determining the best fit of the LpA-I subspecies HDL[4] and HDL[7] (16). The volumetric plots are shown in Fig. 4A, B. Since Fig. 2 shows that HDL[4] lies halfway between the (HDL3a)gge and (HDL3b)gge subspecies, in our volumetric analysis we entered compositional data that represented the average of HDL3a and HDL3b (supplementary Table I). From Fig. 4A, assuming a regular spherical shape (28), by sequentially varying the number of apoA-I and apoA-II per particle and using the weight percentage for each lipid component (PL, CE, UC, and TG) determined for the five different HDL subspecies (supplementary Table I), a range of particle diameters can be calculated. Knowing that HDL[4] contains no apoA-II, two apoA-I alone predicts a diameter of 72–78 Å and four apoA-I alone predicts a diameter of 90–95 Å, both off by 5–10 Å from the measured diameter of 85.0 Å (Table 1). The best fit is to a protein stoichiometry of three apoA-I (and no apoA-II) and two layers of hydration, resulting in a calculated diameter of 85.2 Å (Fig. 4A, black circle above 3:0), virtually identical to the measured diameter of 85.0 ± 1.2 Å (Table 1).
Fig. 4.
Best-fit volumetric analyses of apoA-I-alone circulating HDL subspecies HDL[4] and HDL[7] quantified by density cut ultracentrifugation. The apoA-I:A-II stoichiometries for each calculation are denoted on the x axis, and the diameters calculated at each stoichiometry for the particle containing one (lower gray point) and two (upper black point) hydration shells are plotted. The mean and standard deviation measured by NDGGE for each subspecies are denoted by narrow and shaded horizontal bands, respectively. A, B. Best-fit volumetric analyses for the 85 Å diameter LpA-I HDL[4] and the 108.5 Å diameter LpA-I HDL[7] particles, respectively, using compositional data from Huang, et al. (24). Points for particles containing the same number of apoA-I and hydration shells are linked by thin lines. The best-fit points for HDL[4] and HDL[7] - both with two hydration shells - are denoted by open arrowheads on the x axis above their apoA-I:A-II stoichiometries. The increase in the diameter of the 4:0 particle with two hydration shells by addition of one molecule of apoC-III is denoted by a black point with C inside, and the increase by addition of one molecule of apoE is denoted by a black point with E inside. C, D. Best-fit volumetric analyses for the 85 Å diameter apoA-I-alone HDL[4] and the 108.5 Å diameter apoA-I-alone HDL[7] particles, respectively, using compositional data from Kontush, et al. (35). Error bars show variation in the data. There are two alternatives for the 3:0 particle: 3:0a is derived from the HDL3c fraction and 3:0b is derived from the HDL3b fraction. The best-fit points for HDL[4] and HDL[7] - both with single hydration shells - are denoted by open arrowheads on the x axis above their apoA-I:A-II stoichiometries.
Since Fig. 3 shows that HDL[7] falls precisely in the HDL2b subspecies density cut, in our volumetric analysis we entered the compositional data for (HDL2b)gge (supplementary Table I). From Fig. 4B, knowing that HDL[7] contains no apoA-II, three apoA-I (3:0) predicts a diameter of 95–100 Å, smaller by about 8–13 Å than the 108.5 Å measured by NDGGE. Five apoA-I (5:0) predicts a diameter of 112–117 Å, values that partially fall within the rather broad NDGGE band for HDL[7] (Fig. 4B). The best fit is to a protein stoichiometry of four apoA-I (4:0); the double-shell model gives a diameter of 109.6 Å (Fig. 4B, black circle above 4:0), close to 108.5 Å measured by NDGGE (16), while the single-shell model gives a diameter of 104.0 Å (gray circle above 4:0), at the lower edge of the HDL[7] band. Given the overlap of 5:0 with the HDL[7] band, it is possible that HDL[7] is a mixture of both 4:0 and 5:0.
Addition of one apoC-III molecule to the double-shell 4:0 model results in an HDL particle with a diameter of 111.5 Å, well within the HDL[7] band (Fig. 4B, black circle with C). Addition of one apoE molecule to the double-shell 4:0 model produces an HDL particle with a diameter of 116.4 Å, slightly outside the HDL[7] band (Fig. 4B, black circle with E) but within the HDL[7] band if added to the single-shell model.
We also tested the compositional data of Kontush et al. (35) using our volumetric method. These investigators also calculated the weight percentage for the protein and lipid of the five HDL fractions, HDL(2a–3c), isolated by the same density cuts used by Huang et al. (24) but without removal of apoA-II (supplementary Table II). These data, unlike the Huang et al. (24) data, provides error bars that have been incorporated into the analyses. Fig. 4C, D show the resulting volumetric plots for HDL[4] and HDL[7] using their data with error bars. In spite of the fact that the Kontush fractions (35) were not enriched in apoA-I versus apoA-II like the data of Huang et al. (24), the best fits for HDL[4] and HDL[7] are also to apoA-I-alone particles 3:0 and 4:0, respectively. However, error bars for the adjacent stoichiometries of LpA-I particles overlap the size range for HDL[4] (2:0) and HLD[7] (both 3:0 and 5:0).
The volumetric method, using either Huang et al. (24) or Kontush et al. (35) compositional data, was then fit to five other immunoaffinity-isolated particles: HDL[1], HDL[2], and HDL[3] (Fig. 5A, C), and HDL[5] and HDL[6] (Fig. 5B, D). Of these five particles, only HDL[1] is predominantly LpA-I (Figs. 1 and 2). Both compositional data give similar results for all five particles, HDL[1], HDL[2], HDL[3] (Fig. 5A versus Fig. 5C) and HDL[5] and HDL[6] (Fig. 5B versus Fig. 5D). The best-fit protein and lipid stoichiometries for HDL[1]–HDL[7] derived from the Huang et al. (24) data are shown in Table 3.
Fig. 5.
Best-fit volumetric analyses of LpA-I,A-II HDL subspecies quantified by density cut ultracentrifugation. Data plotted as in Fig. 4. A. Volumetric analyses for the HDL[1], HDL[2], and HDL[3] subspecies, respectively, using compositional data from Huang, et al. (24). The best-fit points for the three particles are denoted by three open arrowheads on the x axis above their apoA-I:A-II stoichiometries. B. Volumetric analyses for the HDL[5] and HDL[6] subspecies, respectively, using compositional data from Huang, et al. (24). The best-fit points for the two particles are denoted by two open arrowheads on the x axis above their apoA-I:A-II stoichiometries. C. Volumetric analyses for the HDL[1], HDL[2], and HDL[3] subspecies, respectively, using compositional data from Kontush, et al. (35). Error bars show variation in the data. There are two alternatives for the 2:2 particle: 2:2a is derived from the HDL3c fraction and 2:2b is derived from the HDL3b fraction. The best-fit points for the three particles are denoted by three open arrowheads on the x axis above their apoA-I:A-II stoichiometries. D. Volumetric analyses for the HDL[5] and HDL[6] subspecies, respectively, using compositional data from Kontush, et al. (35). The best-fit points for the two particles are denoted by two open arrowheads on the x axis above their apoA-I:A-II stoichiometries.
TABLE 3.
Best-fit volumetric analysis of HDL subspecies
| Circulating HDL Subspecies | ||||||
| Stoichiometry |
Diameter |
Monolayer Thickness | ||||
| Object | Origin | Lipid (PC:CE:UC:TG) | Apo (A-I:A-II) | NDGGE (Å) | Calculated (Å) | Calculated (Å) |
| HDL[1] | Immunoaffinity | 18:15:4:4 | 2:0 | 76.4 ± 0.8 | 66.6-72.2 | 12.4 |
| HDL[2] | Immunoaffinity | 24:20:6:5 | 2:1 | 78.2 ± 0.8 | 72.4-78.0 | 13.6 |
| HDL[3] | Immunoaffinity | 40:25:7:7 | 2:2 | 81.0 ± 0.9 | 77.3-82.9 | 14.6 |
| HDL[4] | Immunoaffinity | 41:34:9:7 | 3:0 | 85.0 ± 1.2 | 79.6-85.2 | 13.9 |
| HDL[5] | Immunoaffinity | 67:55:17:13 | 3:1 | 89.9 ± 2.4 | 88.5-94.5 | 14.6 |
| HDL[6] | Immunoaffinity | 78:66:17:13 | 3:2 | 95.3 ± 2.5 | 93.4-99.0 | 15.4 |
| HDL[7] | Immunoaffinity | 133:114:43:19 | 4:0 | 108.5 ± 3.8 | 104.0-109.6 | 15.3 |
Outer monolayer thickness of circulating HDL subspecies.
We calculated the thickness of the surface lipid monolayer for all seven HDL subspecies in Table 3. In Fig. 6, we plot HDL subspecies monolayer thicknesses versus surface-to-volume ratios (S/V = 3/radius) for six native sHDL particles: HDL[2], HDL[3], HDL[4], HDL[5], HDL[6], and HDL[7] (16). These six sHDL particles fit a straight line, y = −0.01x + 0.2128, with a correlation coefficient of R2 = 0.8545. The x intercept represents the limiting monolayer thickness of an infinitely large sHDL, a planar monolayer, as the apoA-I/polar lipid ratio approaches zero. Mathematically, the larger the radius (r), the less curved the surface monolayer. Since S/V = 3/r, as r → ∞, S/V → 0 (i.e., the monolayer → planarity). The magnitude of this intercept, 21.3 Å, is significant because the monolayer thickness of all-atom and coarse-grained planar POPC bilayers using the MARTINI force field for coarse-grained lipid (36) are 20.9 Å and 21.1 Å, respectively.
Fig. 6.
Linear regression plots of monolayer thickness measured by volumetric analysis versus surface-to-volume ratios. The analyses assume spherical shapes for all six native subspecies. Filled circles, HDL[2], HDL[3], HDL[4], HDL[5], HDL[6], and HDL[7]. Open circles, HDL[4] and HDL[7] alone; Open circles upper (d = 131.8 Å, 3/r = 6/131.8 = 0.0455) and lower (r = 13.5 Å) limits (labeled MAX and MIN, respectively) of monolayer thickness plotted along the linear regression line (solid diagonal). The surface-to-volume ratio (S/V = (4πr2)/(4/3πr3) = 3/radius) varies inversely with particle diameter and is a measure of the ratio of surface moieties (protein and polar lipid) to core lipid. The x intercept of the linear regression line (equation and R2 shown in black in the lower left-hand corner) to the six native HDL subspecies are indicated. The dotted diagonal line is the regression line to HDL[4] and HDL[7] alone whose equation and intercept are shown in open (white) figures.
All evidence suggests that the monolayer thickness in HDL subspecies is related to two factors: i) the acyl chain tilt with decreasing radius and predominantly, ii) the well-known wedge effect of amphipathic helixes on monolayers to produce increased bilayer curvature (37) and thinning (38). Monolayer packing in small, protein-rich HDL appears to exist but is reduced to only two or three phospholipids between adjacent helixes (39).
Clearly, analysis of the five more complicated particles is less robust than for HDL[4] and HDL[7] because i) the HDL[4] and HDL[7] unequivocally do not include apoA-II and ii) the HDL fractions isolated by Huang et al. (24) have been partially depleted of apoA-II. Nonetheless, our general compositional models for HDL subspecies (Table 3) showing sequential changes in the stoichiometry of apoA-I and apoA-II fit the general features of the models (dimer, trimer, tetramer) we previously proposed based on chemical crosslinking studies (17), and they are consistent with the results of a recent study by Gauthamadasa et al. (7) using a combination of chemical crosslinking and MALDI-MS analysis.
Although complicated by effects of hydration shells, size determination of HDL subspecies is less of an issue than stoichiometry. Although the immunoaffinity method we used to isolate LpA-I and LpA-I,A-II from plasma does not isolate individually sized subspecies (15, 16), NDGGE, when performed under conditions that allow all particles to reach their equilibrium positions, provides reasonably accurate estimate of particle sizes (most measured in quadruplet, Table 1).
DISCUSSION
The results described here have similarities to a recent publication by Gauthamadasa et al. (7) in suggesting that in the LpA-I subspecies the number of apoA-I molecules increased from two to three to four with an increase in the LpA-I particle size. Gauthamadasa et al. (7) results differ from ours in that they suggest that the entire population of LpA-I,A-II demonstrated the presence of only two proximal apoA-I molecules per particle, and we suggest two or three. In only slight disagreement, they suggest that the number of apoA-II molecules in the LpA-I,A-II particles vary from one dimeric apoA-II to three, whereas we suggest that the upper limit is two apoA-II. However, due to the uncertainties alluded regarding our analyses of LpA-I,A-II particles, three apoA-II is possible.
A publication of 1977 by Shen et al. (40) used many of the approaches and assumptions we used here. They used compositional analyses and geometric molecular arguments to infer the structure of HDL (and, in fact, all lipoprotein classes). Many of their conclusions and approaches were unique for the time. In particular, although proposed less rigorously before by, among others, Verdery and Nichols (41), they suggested the presence of a hydrophobic core surrounded by an amphipathic monolayer composed of protein, PL, and UC. There were, however, several questionable assumptions on their part: i) they proposed that only PL and UC covered the core, not protein, whereas our MD simulation shows that protein makes contact with core molecules (39); ii) in spite of the knowledge of the amphipathic helix at the time (42), they proposed that the protein of HDL was largely unfolded; iii) they postulated that the protein covers the polar OH groups of UC, whereas our MD simulation study shows that this is clearly not the case; and iv) they posited a sharp boundary between the core and the PL surface. Although for our geometric calculations we also assumed a sharp boundary, on average, between core and surface, our MD simulations show that there is considerable interdigitation between core molecules and the surface PL and, to a lesser extent, UC (39).
A recent comprehensive review of HDL subspecies and their relationship to CHD (43) is relevant to this article and its conclusions. The review covers most of the various methods for isolation and analysis of HDL subspecies: flotation ultracentrifugation, zonal ultracentrifugation, density gradient ultracentrifugation (including VAP), precipitation, ion mobility, NDGGE, and 2D gel electrophoresis.
Unfortunately, although mentioned in a table, immunoaffinity chromatography is not discussed. This technique provides the core basis for our present HDL subspecies analyses. By omitting immunoaffinity chromatography, four of the nine HDL subspecies considered in our paper, HDL[1], HDL[4], HDL[8], and HDL[9], are missed. The most import of these, the LpA-I particle HDL[4], is lumped in with HDL[5] (their medium HDL or HDL3a) and HDL[3] (their small HDL or HDL3b). The very small HDL[1] and the very large HDL[8] and HDL[9] are missed entirely.
It is also worth mentioning that the single spin density gradient ultracentrifugation method described involves a rather long spin time (40,000 rpm × 48 h.) and, on the basis of NDGGE of the five isolated fractions, results in isolation of an HDL[2] particle (their very small HDL or HDL3c) that is 84.7 Å in diameter, a particle much too large to be HDL[2]. This size suggests that either the particle is damaged or contaminated with larger HDL subspecies. Therefore, conclusions about the large proteome associated with this subspecies must be suspect.
Figure 6 provides support for the calculated stoichiometries of the six circulating HDL subspecies, HDL[2]–HDL[7], shown in Table 3. When monolayer thickness is plotted against S/V ratio, the resulting points fit a linear regression line with a high correlation coefficient. The x intercept of the regression line, representing the limiting monolayer thickness as the S/V ratio approaches zero (i.e., a planar lipid bilayer) is equivalent to the thickness of a planar lipid monolayer. Since the HDL[4] and HDL[7] subspecies are based upon the most solid numbers - the monolayer thickness for the remaining four HDL subspecies containing apoA-II might be biased by removal of most of the apoA-II (14) - we plotted a regression line for HDL[4] and HDL[7] alone. This regression line (dotted line in Fig. 6) is virtually identical to the regression line for all six subspecies; the x intercept is 20.4 Å. All HDL subspecies fall between the minimal monolayer thickness (13.5 Å) and the S/V ratio of the largest subspecies, HDL[9], whose diameter is 131.8 Å (16), limits that are plotted as large open circles and labeled MIN and MAX in Fig. 6.
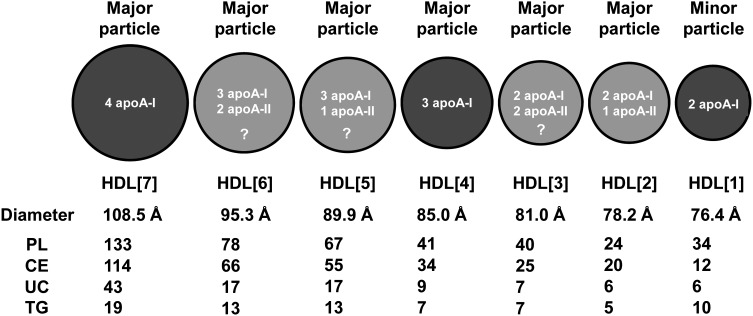
Figure 7 is a schematic diagram that summarizes the basic conclusions of this article regarding size; apoA-I, apoA-II, and lipid stoichiometries; and relative abundance (major or minor) of the seven HDL subspecies HDL[1]–HDL[7]. The other two subspecies discussed earlier, HDL[8] and HDL[9], because of uncertainties in stoichiometries and because they represent minor particles, are not included in the diagram. Apolipoprotein stoichiometries for the LpA-I particles (HDL[4] and HDL[7] and, to a lesser degree, HDL[1]) are the most definitive. Apolipoprotein stoichiometries for the LpA-I,A-II particles (HDL[2], HDL[3], HDL[5], and HDL[6]) are less certain both because of their increased complexity and because of uncertainty about their lipid compositions (Fig. 5).
Fig. 7.
Schematic illustration of the best-fit apoA-I:apoA-II stoichiometries for each of the HDL[1]–HDL[7] subspecies of plasma LpA-I and LpA-I,A-II isolated by immunoaffinity chromatography and analyzed by NDGGE. Relative diameters in the diagram are proportional to the measured diameter of each subspecies. The three distinct LpA-I subspecies are in dark gray, and the four LpA-I,A-II subspecies are in light gray. All are considered major bands except HDL[1], which is usually found in small quantities in normolipidemic plasma but whose level is increased in hyperlipidemic plasma and the plasma of individuals with coronary disease (45). The subspecies with the least certain apolipoprotein stoichiometries are indicated by question marks. Diameters of each particle and lipid composition by volumetric analysis are also shown.
Understanding the biological role of the various HDL subspecies is currently in its infancy. The two major LpA-I particles, HDL[7] and HDL[4], particularly HDL[7], have been suggested to be atheroprotective. Because the existence of HDL[4] is generally not recognized, its role remains unclear. Cheung and Albers in their original description of immunoaffinity-isolated particles (15) showed isoelectric-focusing gels that seemed to imply that the majority of the members of the HDL proteome might be associated with the LpA-I particles, suggesting that apoA-II might regulate the binding of certain members of the HDL proteome. One step to further the understanding of the role of the diverse HDL subspecies will be to noninvasively isolate and determine the proteome content of individual HDL subspecies. Functional assays of individual HDL subspecies would also be helpful.
An additional conclusion from this article is that the thickness of the polar monolayer - a composite of apolipoproteins, PL, and UC - is directly proportional to the particle diameter. This raises the question of the biological significance of this direct correlation of monolayer thickness with particle size. There as several possibilities: i) the very small particles possess very little contiguous polar lipid surface area and this could affect the binding of remodeling proteins, such as LCAT, CEPT, or phospholipid transfer protein (PLTP) or many members of the more diverse HDL proteome; ii) even those members of the HDL proteome that have binding sites on apoA-I, such as LCAT, may be affected because of conformational changes induced in apoA-I by a thinned polar lipid monolayer; and iii) the thinning polar lipid monolayer might be involved in increasing, decreasing, or keeping constant the lipid surface pressure between HDL subspecies. As we demonstrated previously, LDL has mechanisms built into apoB to dampen changes in surface pressure between different-sized subspecies (44).
A corollary of this conclusion is that the apolipoprotein-to-lipid molar ratio is significantly higher for circulating HDL subspecies compared with reconstituted sHDL particles S80 and S93 (22). This issue is considered in more detail in a publication (39) in which molecular dynamics simulations are used to examine reconstituted and circulating sHDL particles at the molecular level.
Supplementary Material
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CHD
- coronary heart disease
- dHDL
- discoidal HDL
- LpA-I
- HDL containing apoA-I without apoA-II
- LpA-I
- A-II, HDL containing apoA-I and apoA-II
- NDGGE
- nondenaturing gradient gel electrophoresis
- PL
- phospholipid
- PLTP
- phospholipid transfer protein
- POPC
- palmitoyloleoylphosphatidylcholine
- sHDL
- spheroidal HDL
- SVS
- single vertical spin
- T-HDL
- total HDL particles that are retained by an apoA-I immunoaffinity column
- TG
- triglyceride
- UC
- unesterified cholesterol
- VAP
- vertical auto profile
This work was supported by the National Institutes of Health Grants HL-34343 and HL-102515.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Linsel-Nitschke P., Tall A. R. 2005. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4: 193–205 [DOI] [PubMed] [Google Scholar]
- 2.Mahdy Ali K., Wonnerth A., Huber K., Wojta J. 2012. Cardiovascular disease risk reduction by raising HDL cholesterol - current therapies and future opportunities. Br. J. Pharmacol. 167: 1177–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah A. S., Tan L., Lu Long J., Davidson W. S. The proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res.Epub ahead of print. February 24, 2013; 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaisar T., Pennathur S., Green P. S., Gharib S. A., Hoofnagle A. N., Cheung M. C., Byun J., Vuletic S., Kassim S., Singh P., et al. 2007. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 117: 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson W. S., Silva R. A., Chantepie S., Lagor W. R., Chapman M. J., Kontush A. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiflett A. M., Bishop J. R., Pahwa A., Hajduk S. L. 2005. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J. Biol. Chem. 280: 32578–32585 [DOI] [PubMed] [Google Scholar]
- 7.Gauthamadasa K., Rosales C., Pownall H. J., Macha S., Jerome W. G., Huang R., Silva R. A. G. D. 2010. Speciated human high-density lipoprotein protein proximity profiles. Biochemistry. 49: 10656–10665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segrest J. P., Garber D. W., Brouillette C. G., Harvey S. C., Anantharamaiah G. M. 1994. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 45: 303–369 [DOI] [PubMed] [Google Scholar]
- 9.Roosbeek S., Vanloo B., Duverger N., Caster H., Breyne J., De Beun I., Patel H., Vandekerckhove J., Shoulders C., Rosseneu M., et al. 2001. Three arginine residues in apolipoprotein A-I are critical for activation of lecithin:cholesterol acyltransferase. J. Lipid Res. 42: 31–40 [PubMed] [Google Scholar]
- 10.Cheung M. C., Vaisar T., Han X. L., Heinecke J. W., Albers J. J. 2010. Phospholipid transfer protein in human plasma associates with proteins linked to immunity and inflammation. Biochemistry. 49: 7314–7322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gofman J. W., Lindgren F. T., Elliot H. 1949. Ultracentrifugal studies of lipoproteins of human serum. J. Biol. Chem. 179: 973–979 [PubMed] [Google Scholar]
- 12.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patsch W., Schonfeld G., Gotto A. M., Jr, Patsch J. R. 1980. Characterization of human high density lipoproteins by zonal ultracentrifugation. J. Biol. Chem. 255: 3178–3185 [PubMed] [Google Scholar]
- 14.Blanche P. J., Gong E. L., Forte T. M., Nichols A. V. 1981. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim. Biophys. Acta. 665: 408–419 [DOI] [PubMed] [Google Scholar]
- 15.Cheung M. C., Albers J. J. 1984. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J. Biol. Chem. 259: 12201–12209 [PubMed] [Google Scholar]
- 16.Cheung M. C., Segrest J. P., Albers J. J., Cone J. T., Brouillette C. G., Chung B. H., Kashyap M., Glasscock M. A., Anantharamaiah G. M. 1987. Characterization of high density lipoprotein subspecies: structural studies by single vertical spin ultracentrifugation and immunoaffinity chromatography. J. Lipid Res. 28: 913–929 [PubMed] [Google Scholar]
- 17.Cheung M. C., Nichols A. V., Blanche P. J., Gong E. L., Franceschini G., Sirtori C. R. 1988. Characterization of A-I-containing lipoproteins in subjects with A-I Milano variant. Biochim. Biophys. Acta. 960: 73–82 [DOI] [PubMed] [Google Scholar]
- 18.Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. 1980. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J. Lipid Res. 21: 284–291 [PubMed] [Google Scholar]
- 19.Chung B. H., Segrest J. P., Cone J. T., Pfau J., Geer J. C., Duncan L. A. 1981. High resolution plasma lipoprotein cholesterol profiles by a rapid, high volume semi-automated method. J. Lipid Res. 22: 1003–1014 [PubMed] [Google Scholar]
- 20.Cone J. T., Segrest J. P., Chung B. H., Ragland J. B., Sabesin S. M., Glasscock A. 1982. Computerized rapid high resolution quantitative analysis of plasma lipoproteins based upon single vertical spin centrifugation. J. Lipid Res. 23: 923–935 [PubMed] [Google Scholar]
- 21.Chung B. H., Segrest J. P., Ray M. J., Brunzell J. D., Hokanson J. E., Krauss R. M., Beaudrie K., Cone J. T. 1986. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 128: 181–209 [DOI] [PubMed] [Google Scholar]
- 22.Silva R. A., Huang R., Morris J., Fang J., Gracheva E. O., Ren G., Kontush A., Jerome W. G., Rye K. A., Davidson W. S. 2008. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. USA. 105: 12176–12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontush A., Chapman M. J. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58: 342–374 [DOI] [PubMed] [Google Scholar]
- 24.Huang R., Silva R. A., Jerome W. G., Kontush A., Chapman M. J., Curtiss L. K., Hodges T. J., Davidson W. S. 2011. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat. Struct. Mol. Biol. 18: 416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni K. R., Marcovina S. M., Krauss R. M., Garber D. W., Glasscock A. M., Segrest J. P. 1997. Quantification of HDL2 and HDL3 cholesterol by the Vertical Auto Profile-II (VAP-II) methodology. J. Lipid Res. 38: 2353–2364 [PubMed] [Google Scholar]
- 26.Harpaz Y., Gerstein M., Chothia C. 1994. Volume changes on protein folding. Structure. 2: 641–649 [DOI] [PubMed] [Google Scholar]
- 27.McNamara J. R., Small D. M., Li Z., Schaefer E. J. 1996. Differences in LDL subspecies involve alterations in lipid composition and conformational changes in apolipoprotein B. J. Lipid Res. 37: 1924–1935 [PubMed] [Google Scholar]
- 28.Atmeh R. F., Abd Elrazeq I. O. 2005. Small high density lipoprotein subclasses: some of their physico-chemical properties and stability in solution. Acta Biochim. Pol. 52: 515–525 [PubMed] [Google Scholar]
- 29.Svergun D. I., Richard S., Koch M. H., Sayers Z., Kuprin S., Zaccai G. 1998. Protein hydration in solution: experimental observation by x-ray and neutron scattering. Proc. Natl. Acad. Sci. USA. 95: 2267–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durchschlag H., Zipper P. 2003. Modeling the hydration of proteins: prediction of structural and hydrodynamic parameters from X-ray diffraction and scattering data. Eur. Biophys. J. 32: 487–502 [DOI] [PubMed] [Google Scholar]
- 31.Perera L., Essmann U., Berkowitz M. L. 1996. Role of water in the hydration force acting between lipid bilayers. Langmuir. 12: 2625–2629 [Google Scholar]
- 32.Wood K., Plazanet M., Gabel F., Kessler B., Oesterhelt D., Tobias D. J., Zaccai G., Weik M. 2007. Coupling of protein and hydration-water dynamics in biological membranes. Proc. Natl. Acad. Sci. USA. 104: 18049–18054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waluyo I., Huang C. C., Nordlund D., Bergmann U., Weiss T. M., Pettersson L. G. M., Nilsson A. 2011. The structure of water in the hydration shell of cations from x-ray Raman and small angle x-ray scattering measurements. J. Chem. Phys. 134: 064513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corradini D., Rovere M., Gallo P. 2011. Structural properties of high and low density water in a supercooled aqueous solution of salt. J. Phys. Chem. B. 115: 1461–1468 [DOI] [PubMed] [Google Scholar]
- 35.Kontush A., Chantepie S., Chapman M. J. 2003. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 1881–1888 [DOI] [PubMed] [Google Scholar]
- 36.Marrink S. J., Risselada H. J., Yefimov S., Tieleman D. P., de Vries A. H. 2007. The MARTINI force field: coarse grained model for biomolecular simulations. J. Phys. Chem. B. 111: 7812–7824 [DOI] [PubMed] [Google Scholar]
- 37.Campelo F., McMahon H. T., Kozlov M. M. 2008. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J. 95: 2325–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hristova K., Wimley W. C., Mishra V. K., Anantharamiah G. M., Segrest J. P., White S. H. 1999. An amphipathic alpha-helix at a membrane interface: a structural study using a novel X-ray diffraction method. J. Mol. Biol. 290: 99–117 [DOI] [PubMed] [Google Scholar]
- 39.Segrest J. P., Jones M. K., Catte A. MD simulations suggest important surface differences between reconstituted and circulating spherical HDL. J. Lipid Res. 54:2718–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen B. W., Scanu A. M., Kezdy F. J. 1977. Structure of human serum lipoproteins inferred from compositional analysis. Proc. Natl. Acad. Sci. USA. 74: 837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdery R. B., Nichols A. V. 1975. Arrangement of lipid and protein in human serum high density lipoproteins: a proposed model. Chem. Phys. Lipids. 14: 123–134 [DOI] [PubMed] [Google Scholar]
- 42.Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr 1974. A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 38: 247–258 [DOI] [PubMed] [Google Scholar]
- 43.Rosenson R. S., Brewer H. B., Jr, Chapman M. J., Fazio S., Hussain M. M., Kontush A., Krauss R. M., Otvos J. D., Remaley A. T., Schaefer E. J. 2011. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 57: 392–410 [DOI] [PubMed] [Google Scholar]
- 44.Segrest J. P., Jones M. K., De Loof H., Dashti N. 2001. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 42: 1346–1367 [PubMed] [Google Scholar]
- 45.Cheung M. C., Brown B. G., Wolf A. C., Albers J. J. 1991. Altered particle size distribution of apolipoprotein A-I-containing lipoproteins in subjects with coronary artery disease. J. Lipid Res. 32: 383–394 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.