Abstract
Hepatic VLDL overproduction is a characteristic feature of diabetes and an important contributor to diabetic dyslipidemia. Hepatic sortilin 1 (Sort1), a cellular trafficking receptor, is a novel regulator of plasma lipid metabolism and reduces plasma cholesterol and triglycerides by inhibiting hepatic apolipoprotein B production. Elevated circulating free fatty acids play key roles in hepatic VLDL overproduction and the development of dyslipidemia. This study investigated the regulation of hepatic Sort1 in obesity and diabetes and the potential implications in diabetic dyslipidemia. Results showed that hepatic Sort1 protein was markedly decreased in mouse models of type I and type II diabetes and in human individuals with obesity and liver steatosis, whereas increasing hepatic Sort1 expression reduced plasma cholesterol and triglycerides in mice. Mechanistic studies showed that the saturated fatty acid palmitate activated extracellular signal-regulated kinase (ERK) and inhibited Sort1 protein by mechanisms involving Sort1 protein ubiquitination and degradation. Consistently, hepatic ERK signaling was activated in diabetic mice, whereas blocking ERK signaling by an ERK inhibitor increased hepatic Sort1 protein in mice. These results suggest that increased saturated fatty acids downregulate liver Sort1 protein, which may contribute to the development of dyslipidemia in obesity and diabetes.
Keywords: extracellular signal-regulated kinase, obesity, diabetes, fatty liver, lipid metabolism, mitogen-activated protein kinase
Diabetes is closely associated with dyslipidemia characterized by hypertriglyceridemia, reduced high density lipoprotein (HDL), and abnormal low density lipoprotein (LDL) metabolism, which significantly increases the risk of cardiovascular disease (CVD), the leading cause of morbidity and mortality in type II diabetes (1). Increased hepatic very low density lipoprotein (VLDL)/apolipoprotein B (apoB) secretion is a characteristic feature of type II diabetes and a major cause of diabetic dyslipidemia (2, 3), but molecular mechanisms linking diabetes to hepatic apoB overproduction are only partially understood. In obesity and diabetes, circulating free fatty acids (FFA) are often elevated mainly due to abnormal adipocyte lipolysis (3). Recent studies showed that elevated plasma and tissue FFAs are critically involved in the development of hyperlipidemia. Current evidence supports that circulating FFA level is an important CVD risk factor in diabetes (4, 5). In addition, metabolic profiling studies also showed that the abundant saturated fatty acid palmitate in the plasma is a reliable biomarker for type II diabetes (6). At the molecular level, increased hepatic FFA uptake provides substrates for hepatic triglyceride synthesis, leading to apoB lipidation and VLDL synthesis and secretion. In addition, elevated FFAs and their lipid intermediates, namely, ceramides and diacylglycerols, cause abnormal activation of protein kinase C (PKC) isoforms and mitogen-activated protein kinases (MAPK), which results in inflammation and insulin resistance (7, 8). Although it is well known that activation of inflammatory signaling and impairment of insulin signaling contribute significantly to apoB overproduction and hyperlipidemia in diabetes, the downstream mechanisms are still not fully clear (9, 10).
Sortilin 1 (Sort1), a transmembrane-sorting receptor (11), has recently been identified as a novel regulator of hepatic apoB metabolism and plasma LDL-cholesterol (LDL-C) concentrations (12, 13). A number of genome-wide association studies (GWAS) first identified a strong association between LDL-C and SNPs at the 1p13.3 locus containing SORT1 and several other genes (12). It was further shown that the homozygous causal minor allele was associated with increased hepatic Sort1 mRNA and lower plasma LDL-C, which translated into significant reduction of myocardial infarction risk in humans (12). Sort1 mainly localizes in the trans-Golgi network, and it is involved in trafficking of various target proteins to the secretory pathway or to endosome/lysosome compartment (11). A small amount of Sort1 (∼10%) also localizes on the cell membrane and is involved in ligand internalization or intracellular signaling (11). Mechanistic studies in mice suggest that liver Sort1 may reduce plasma LDL-C via modulating apoB metabolism: first, intracellular Sort1 recognizes apoB as a ligand and directs newly synthesized apoB for lysosomal degradation, leading to reduced hepatic apoB secretion; second, cell surface Sort1 may facilitate extracellular apoB-containing LDL internalization for subsequent lysosomal degradation (12, 14, 15).
Because abnormal hepatic apoB metabolism critically contributes to dyslipidemia and because the molecular mechanisms for altered apoB metabolism in obesity and diabetes are still not fully understood, we asked whether hepatic Sort1 expression is changed in obesity and diabetes, and if so, what the impact of altered hepatic Sort1 levels on the development of diabetic dyslipidemia is. We first found that hepatic Sort1 protein was significantly reduced in obese and diabetic mice and in steatotic livers of obese humans. We then provided in vitro and in vivo evidence demonstrating that elevated FFA-mediated MAPK activation results in posttranslational Sort1 degradation, which provides a novel molecular basis for hepatic apoB/VLDL overproduction in diabetes. In addition, we showed that overexpression of Sort1 in the liver was sufficient to reduce plasma cholesterol and triglycerides in obese and diabetic mice, suggesting that therapeutic approaches that can either restore or further raise liver Sort1 levels may provide benefits in improving plasma lipid homeostasis in obesity and diabetes.
MATERIALS AND METHODS
Reagents
Anti-Sort1 (ab16640) and anti-apoB were purchased from Abcam (Cambridge, MA). Antibodies against phosphor-ERK (4736 and 9106), total ERK (4695), histone 3 (9717) and HA (3724), the ERK inhibitor U0126 and the JNK inhibitor SP600125 were purchased from Cell Signaling Technology (Danvers, MA). Anti-flag (M2) antibody, palmitate, stearic acid, oleate, cyclohexamide and the PKC inhibitor Go6983 were purchased from Sigma (St. Louis, MO). Ceramide C6 was from Santa Cruz Biotechnology (Dallas, TX). The proteasome inhibitor MG132 was from EMD Millipore (Billerica, MA).
Animals
Male wild-type (WT) C57BL/6J mice and ob/ob mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained on a standard chow diet and water ad libitum and were housed in a room with a 12 h light (6 AM to 6 PM) and 12 h dark (6 PM to 6 AM) cycle. WT mice were fed either a standard chow diet or a Western diet (Harlan Teklad TD.88137, 42% fat calories, 0.2% cholesterol) for four months. For U0126 injection, mice were fasted for 16 h and given a single dose of U0126 (5 mg/kg, in sterile saline containing 1% DMSO) via tail vein. Control mice received vehicle alone. Insulin-deficient C57BL/6 mice were generated via intraperitoneal injection with 7.5 mg/kg streptozocin (STZ) once daily for five consecutive days. Control mice were injected with vehicle only (sodium citrate buffer, pH 4.5). One week after the last injection, mice with hyperglycemia (nonfasting blood glucose > 400 mg/dl) were used. All studies were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Human liver samples
Human liver samples were obtained from the University of Kansas Liver Center Tissue Bank. Liver samples used as controls were from individuals who were without the presence of significant liver steatosis by histological analysis and who had a body mass index (BMI) ≤ 25. Steatotic liver samples were selected based on the presence of steatosis ≥ 20% without evidence of fibrosing steatohepatitis or cirrhosis by histology and BMI ≥ 30. Histology was read by multiple board-certified pathologists.
Cell culture and fatty acid treatment
The human hepatoma cell line HepG2 was obtained from the American Type Culture Collection (Manassas, VA). Primary human hepatocytes were obtained from the Liver Tissue and Cell Distribution System of the National Institutes of Health (S. Strom, University of Pittsburgh, PA) or from the Biospecimen Core facility, University of Kansas Medical Center (KUMC). Primary mouse hepatocytes were obtained from the Biospecimen Core facility at KUMC. Palmitate (50 mM) or oleate (50 mM) was first dissolved in 100% ethanol by heating at 80°C to yield a clear solution, which was then added to serum-free medium containing 10% fatty acid-free BSA (BSA) and incubated at 37°C for 2 h to obtain a final concentration of palmitate at 5 mM. For cell treatment, the 5 mM palmitate solution was further diluted in serum-free culture medium containing 1% BSA and incubated at 37°C for 2 h before addition to cells. Control cells were treated with vehicle prepared using the same procedure.
Measurement of Sort1 ubiquitination
HepG2 cells were transfected with an HA-tagged ubiquitin expression plasmid (PRK5-HA-Ubiquitin-WT, Addgene plasmid 17608) that was deposited by Dr. Ted M. Dawson (Johns Hopkins University School of Medicine) (16). On the same day, HepG2 cells were then infected with Adenovirus expressing a C-terminal flag-tagged Sort1 at multiplicity of infection of 1. After 24 h, Flag-tagged Sort1 was immunoprecipitated with anti-flag antibody (M2) conjugated to magnetic beads (Sigma). Ubiquitinated Sort1 was detected with anti-HA antibody in Western blot. HepG2 cells that did not express Flag-Sort1 or HA-Ubiquitin were used as a negative control.
Recombinant adenovirus
Adeno-GFP was a gift from Dr. Li Wang (University of Utah, Salt Lake City, UT). Adenovirus-expressing human full length Sortilin 1 was a generous gift from Dr. Anders Nykjaer (Lundbeck Foundation Research Center MIND, Department of Medical Biochemistry, Aarhus University, Denmark). Adenovirus expressing a C-terminal flag-tagged human Sortilin1 was generated using AdEasy Adenoviral Vector System (Agilent Technologies, Santa Clara, CA). Adenovirus was purified from HEK293A cells by CsCl centrifugation. Adenovirus titer was determined with an Adeno-X rapid titer kit from Clontech (Mountain View, CA). Adenovirus was administered at 1 × 109 pfu/mouse via tail vein. Experiments were carried out seven days after injection.
Measurement of apoB secretion
Measurement of apoB secretion into culture medium was performed essentially the same as previously published (17), with minor modifications. Briefly, primary mouse hepatocytes or HepG2 cells were infected with Ad-GFP or Ad-Sort1 at MOI of ∼5 for 24 h. Cells were then cultured in methionine/cysteine-free medium (Sigma, St. Louis, MO) for 15 min, followed by incubation with medium containing 100 μCi S-35 methionine/cysteine (EasyTag protein labeling mix, Perkin Elmer, Waltham, MA) for 20 min. Cells were washed three times with culture medium and chased for an additional 90 min, after which apoB in the culture medium was immunoprecipitated with apoB antibody and resolved on a 4–15% SDS-PAGE.
Dried gels were exposed to a phosphoimager, and labeled apoB was detected with a Typhoon imaging system. The apoB band intensity was normalized to total cellular protein. Each assay was performed in duplicate.
RNA isolation and quantitative real-time PCR
Total RNA was purified with Tri-reagent (Sigma). Real-time PCR assays were performed with SYBR primers or Taqman primers/probe (Applied Biosystems, Foster City, CA) on an Applied Biosystems 7300 real-time PCR system. Amplification of mt18s or Ubiquitin C (UBC) was used as an internal control. Relative mRNA expression was quantified using the comparative CT (Ct) method and expressed as 2−ΔΔCt.
Immunoblot
Cell or tissue protein samples were prepared in RIPA buffer followed by brief sonication. Protein concentrations were determined by a BCA assay kit (Rockford, IL). Equal amount of protein was used for SDS-PAGE and Western blotting.
Lipid analysis
Cholesterol, triglyceride, and free fatty acids were measured with colorimetric assay kits (Biovision, Milpitas, CA) as described previously (18).
Statistical analysis
Results were expressed as mean ± SE unless noted. Statistical analysis was performed by Student t-test or ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Hepatic Sort1 levels are markedly decreased in obese mice and steatotic livers from obese individuals
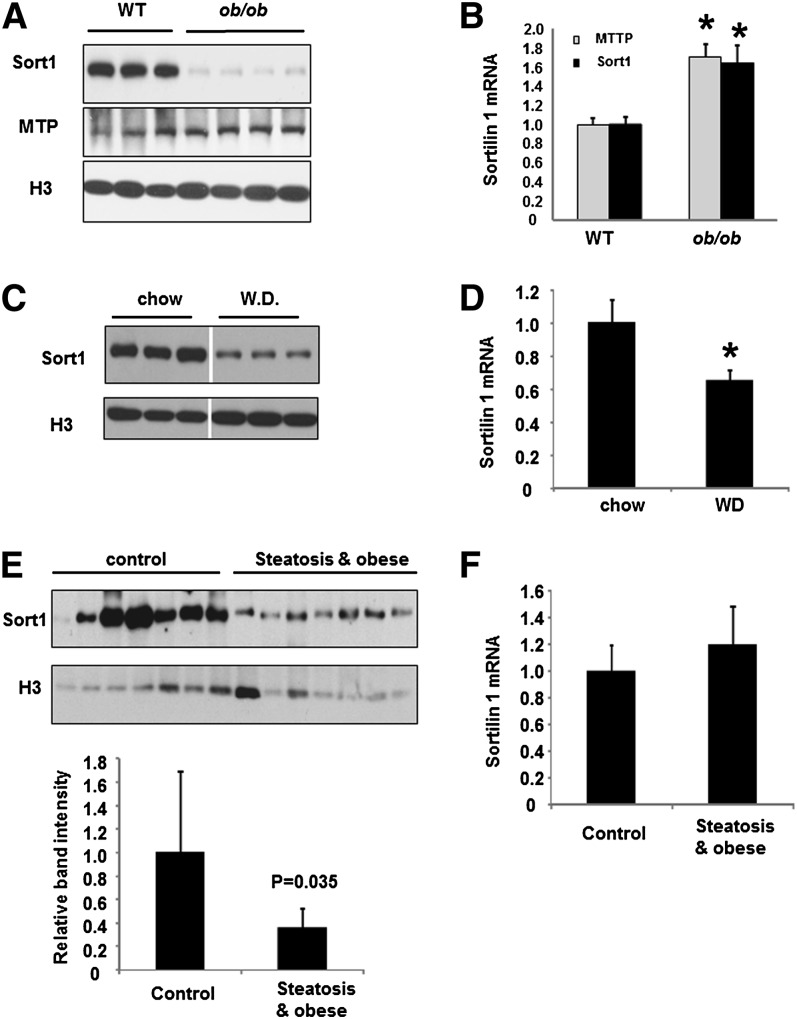
To investigate whether obesity, diabetes, and hepatic steatosis are associated with altered hepatic Sort1, we first measured liver Sort1 expression in genetic obese ob/ob mice. Hepatic Sort1 protein levels were decreased by more than 90% in ob/ob mice as estimated by densitometry (Fig. 1A). Unexpectedly, Sort1 mRNA was modestly elevated in ob/ob mice (Fig. 1B). As controls, the mRNA and protein of microsome triglyceride transfer protein (MTTP) were higher in ob/ob mice (Fig. 1A, B), consistent with a previous report (19). In mice fed a high saturated fat-containing Western diet, a model of diet-induced obesity, hepatic Sort1 protein was also reduced by more than 80%, but hepatic Sort1 mRNA was only reduced by ∼35% (Fig. 1C, D). To find whether obesity and fatty liver is associated with decreased Sort1 expression in humans, we compared Sort1 levels in human liver samples from individuals with steatosis and obesity to that of controls. Results showed that Sort1 protein levels, but not mRNA, were markedly lower in the steatosis/obese group (Fig. 1E, F).
Fig. 1.
Hepatic Sort1 is downregulated in obese and diabetes. (A, B) Sort1 and MTTP protein (A) and mRNA (B) in the liver of WT and ob/ob mice were measured by Western blot or real-time PCR, respectively. H3, histone 3 as loading control. (C, D) Mice were fed a Western diet (WD) or a chow diet for four months. Liver Sort1 protein and mRNA expression were measured. Results are plotted as mean ± SE; n = 4. *P ≤ 0.05 versus WT. (E) In upper panel, Western blot analysis of Sort1 protein in human liver tissues. In lower panel, Sort1 band intensity was quantified using ImageJ software and normalized to the intensity of H3. (F) Sort1 mRNA levels in the human liver tissues were measured by real-time PCR. Results are plotted as mean ± SE; n = 7. *P ≤ 0.05 versus controls.
It was shown that increasing hepatic Sort1 expression in apobec1−/−;apoB transgenic mice that had elevated LDL-C significantly reduced plasma total cholesterol, LDL-C and triglycerides, while siRNA-mediated knockdown of hepatic Sort1 in these mice raised plasma cholesterol and triglycerides (12). To test whether decreased hepatic Sort1 under obese and diabetic conditions might play a causative role in the development of hyperlipidemia, we increased hepatic Sort1 expression in both WT and ob/ob mice by adenovirus-mediated gene delivery. Interestingly, liver-specific Sort1 overexpression significantly lowered plasma triglyceride and cholesterol levels in WT mice (Table 1). In addition, increasing hepatic Sort1 levels in ob/ob mice was able to reduce plasma cholesterol and triglycerides to levels seen in WT control mice (Table 1). We also found that Sort1 overexpression reduced apoB secretion from primary mouse hepatocytes and HepG2 cells (supplementary Fig. I), consistent with previous studies (12). Taken together, these results suggest that obesity and diabetes are associated with impaired hepatic Sort1 function, which may contribute to the development of diabetic hyperlipidemia.
TABLE 1.
Effect of hepatic-specific Sort1 overexpression on serum lipid parameters in mice
| WT |
ob/ob |
|||
| Ad-GFP | Ad-Sort1 | Ad-GFP | Ad-Sort1 | |
| Serum triglyceride (mg/dl) | 129.2 ± 15 | 42.5 ± 7.9 * | 186.9 ± 3.2 * | 107.9 ± 10 */# |
| Serum cholesterol (mg/dl) | 128.3 ± 6.8 | 96.4 ± 12 * | 340.1 ± 23 * | 125.3 ± 17 */# |
n = 5–6. *P < 0.05 versus Ad-GFP WT mice; #P < 0.05 versus Ad-GFP ob/ob mice.
Saturated fatty acid represses Sort1 protein, but not mRNA, in HepG2 cells and primary human hepatocytes
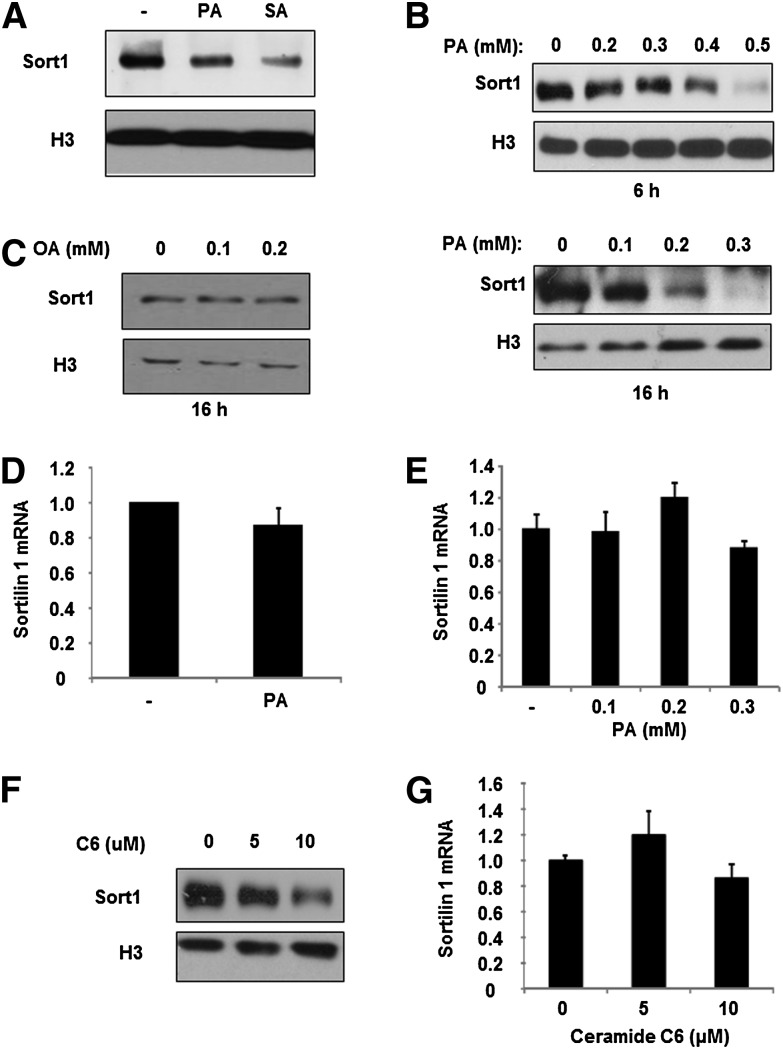
The differential expression of liver Sort1 mRNA and protein observed in obese mice and humans implies potential posttranslational downregulation of hepatic Sort1 protein. Increased circulating and tissue-saturated FFAs are thought to play critical roles in hepatic VLDL overproduction in obesity and diabetes (20). We tested the hypothesis that saturated FFAs downregulate hepatic Sort1 in obesity and diabetes. As shown in Fig. 2A, both palmitate and stearic acid, the two most common saturated fatty acids in mammals, repressed Sort1 protein in primary human hepatocytes. Similarly, palmitate treatment dose-dependently inhibited Sort1 protein in HepG2 cells (Fig. 2B). In contrast, treating HepG2 cells with monosaturated fatty acid oleate did not repress Sort1 protein (Fig. 2C). Interestingly, palmitate treatment had no effect on Sort1 mRNA expression in primary human hepatocytes or in HepG2 cells (Fig. 2D, E), further implying a role of palmitate in mediating the posttranslational downregulation of Sort1 in obese mice. Palmitate is the main substrate in de novo biosynthesis of ceramides, which act as important downstream lipid mediators of elevated FFA effects on inflammation and insulin resistance in diabetes (21). Treating HepG2 cells with a ceramide analog, C6-ceramide, dose-dependently repressed Sort1 protein without affecting Sort1 mRNA expression (Fig. 2F, G), suggesting that palmitate inhibition of Sort1 protein could be at least partially mediated by the production of ceramides.
Fig. 2.
Sort1 is repressed by saturated fatty acids and C6-ceramide in HepG2 cells and human hepatocytes. (A–C) Primary human hepatocytes were treated with 0.25 mM stearic acid (SA), palmitate (PA), or vehicle for 16 h. HepG2 cells were treated with PA or oleate (OA) at indicated concentrations for 6 h or 16 h. Sort1 protein were measured by Western blot. (D, E) Primary human hepatocytes were treated with palmitate at 0.25 mM for 16 h. HepG2 cells were treated with palmitate for 16 h at indicated concentrations. (F, G) Sort1 protein and mRNA in HepG2 cells treated with C6-ceramide (C6) for 24 h at indicated concentrations. Real-time PCR assays were performed in triplicates and results are expressed as mean ± SD.
Activation of ERK signaling downregulates hepatic Sort1 in diabetic mice
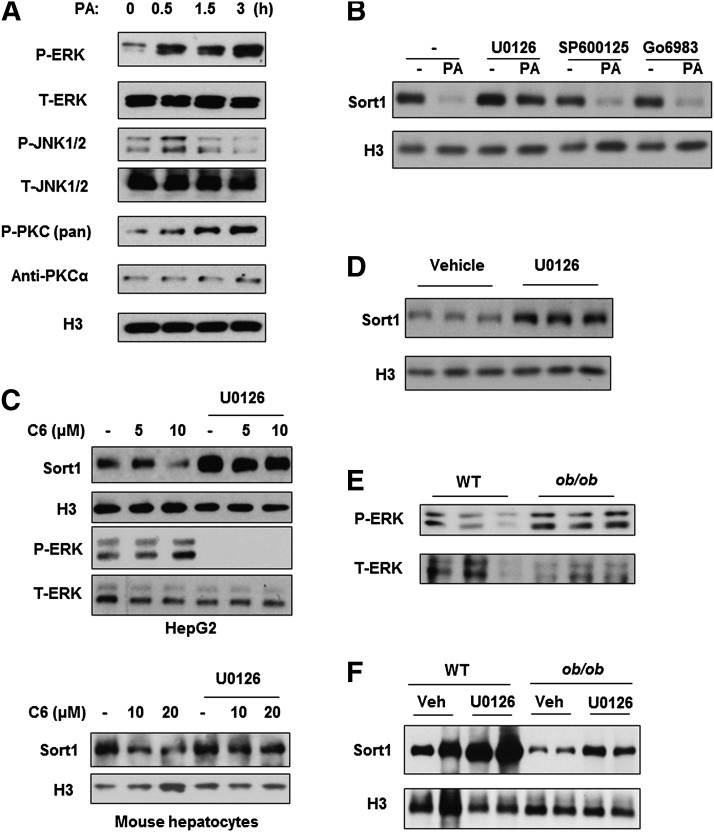
To further delineate the mechanisms mediating saturated FFA-induced Sort1 downregulation, we first aimed to identify the downstream signaling pathway involved. Saturated FFAs have been shown to activate MAPK and PKC signaling (7, 8). Consistently, palmitate treatment induced the phosphorylation of ERK, JNK, and PKC in HepG2 cells (Fig. 3A). Interestingly, blocking ERK signaling with U0126 significantly blocked the repressive effect of palmitate on Sort1 protein (Fig. 3B). In contrast, blocking JNK or PKC signaling showed no effect on basal Sort1 protein or palmitate-induced Sort1 repression in HepG2 cells (Fig. 3B). Furthermore, treating HepG2 cells with C6-ceramide at 10 μM induced ERK phosphorylation and repressed Sort1, which was abolished by the ERK inhibitor U0126 (Fig. 3C, upper panel). Similar results were obtained in primary mouse hepatocytes (Fig. 3C, lower panel). To further test whether ERK signaling regulates Sort1 expression in vivo, we injected mice with U0126 and measured hepatic Sort1 protein after 6 h. Mice treated with U0126 showed a significant increase in hepatic Sort1 (Fig. 3D). Furthermore, obese and diabetic ob/ob mice showed higher hepatic ERK phosphorylation than WT controls (Fig. 3E), while U0126 treatment partially recovered liver Sort1 protein levels in ob/ob mice (Fig. 3F).
Fig. 3.
Activation of ERK signaling negatively regulates Sort1 in HepG2 cells and in mouse liver. (A) Phosphorylated and total ERK, JNK, and PKC were measured in HepG2 cells treated with 0.5 mM palmitate for time indicated. The anti-phospho-PKC (pan) antibody detects phosphorylated PKC isoforms α, βI/II, γ, ϵ, η, and θ. A major band is detected at ∼80 Kda in HepG2 cells. (B) HepG2 cells were pretreated with vehicle, ERK inhibitor U0126 (10 μM), JNK inhibitor SP600125 (10 μM), or PKC inhibitor Go6983 (100 nM) for 1 h followed by palmitate treatment (0.2 mM) for 16 h. Control cells were treated with vehicle. Sort1 levels were measured by Western blot. (C) HepG2 cells (upper panel) or primary mouse hepatocytes (lower panel) were pretreated with U0126 (10 μM) for 1 h followed by ceramide C6 (C6) treatment at 5, 10, or 20 μM for an additional 24 h. Sort1 protein and ERK phosphorylation were measured by Western blot. (D) Fasted WT mice were injected with vehicle or U0126 at 5 mg/kg via tail vein and liver Sort1 protein was measured 6 h later by Western blot. (E) ERK phosphorylation and total ERK protein were measured in the livers of fasted WT controls and ob/ob mice. (F) Liver Sort1 protein in WT mice or ob/ob mice intravenously injected with vehicle or U0126 for 6 h.
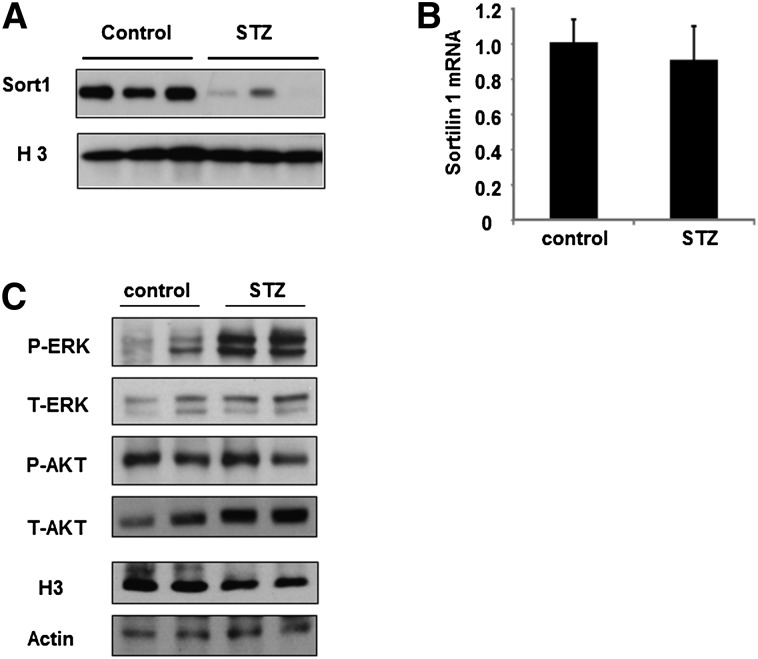
To further test whether increased FFAs are associated with reduced hepatic Sort1 in vivo, we took advantage of the STZ-treated type I diabetic mice, which have increased circulating FFAs (Table 2). STZ-treated mice also showed elevated plasma cholesterol and triglyceride levels (Table 2). Interestingly, the hepatic Sort1 protein, but not mRNA, was markedly lower in STZ-treated mice over controls (Fig. 4A, B), and such decrease in Sort1 protein was associated with higher liver ERK phosphorylation (Fig. 4C). Hepatic phosphorylation of AKT was not significantly different between the two groups after overnight fasting (Fig. 4C). Analysis of hepatic lipid contents showed that STZ-treated mice showed lower hepatic triglycerides and FFAs and unchanged cholesterol levels, consistent with perturbation of hepatic lipid metabolism under insulin-deficient conditions (Table 2).
TABLE 2.
Hepatic and serum lipid parameters in STZ-treated mice and controls
| Serum | Control | STZ |
| Triglyceride (mg/dl) | 106.4 ± 1.27 | 143.8 ± 12.6* |
| Cholesterol (mg/dl) | 108.9 ± 6.2 | 192.3 ± 13.3* |
| Free fatty acids (mM) | 0.2 ± 0.01 | 0.38 ± 0.04* |
| Liver | ||
| Triglyceride (μmole/g) | 12.6 ± 0.32 | 3.84 ± 0.06* |
| Cholesterol (μg/g) | 1.88 ± 0.14 | 2.96 ± 0.5 |
| Free fatty acids (nmole/g) | 0.26 ± 0.03 | 0.15 ± 0.01* |
n = 4. *P < 0.05 versus control mice.
Fig. 4.
STZ-treated mice showed elevated serum FFA, hepatic ERK activation, and reduced hepatic Sort1 protein. (A, B) Hepatic Sort1 protein and mRNA expression in control and STZ-treated mice. (C) Hepatic phospho-ERK, total ERK, phospho-AKT, and total AKT in control and STZ-treated mice. H3 and actin were measured as loading controls. Results are plotted as mean ± SE; n = 4. *P ≤ 0.05 versus control.
Palmitate-induced ERK activation promotes Sort1 protein degradation
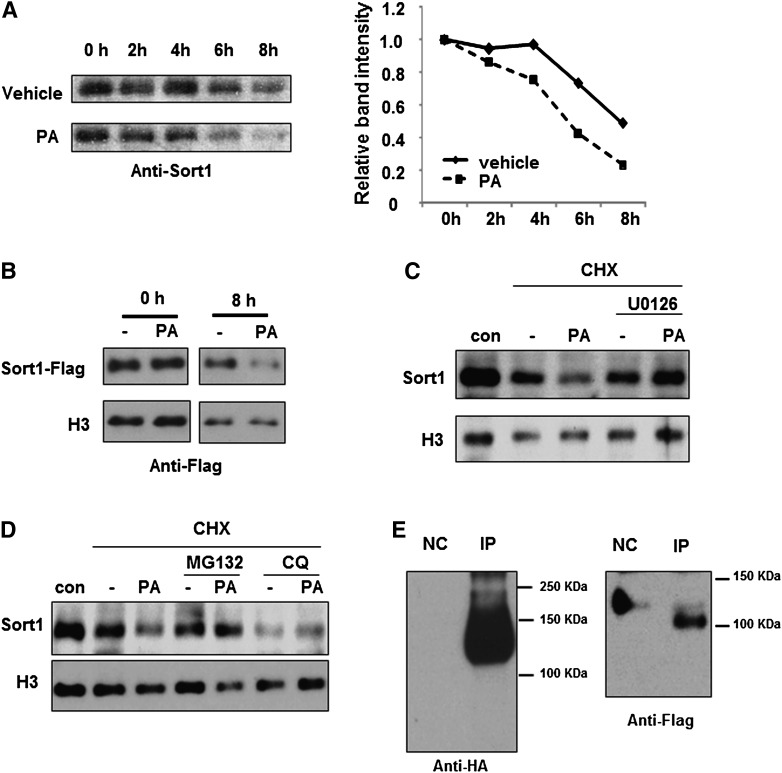
To obtain further mechanistic insights, we tested whether palmitate represses Sort1 by regulating Sort1 protein stability. By treating cells with cyclohexamide (CHX) to block protein synthesis, we estimated that Sort1 protein has a half-life of approximately ∼8 h in HepG2 cells (Fig. 5A). Treating HepG2 cells with palmitate significantly accelerated the degradation rate of endogenous Sort1 (Fig. 5A) as well as the degradation rate of ectopically expressed flag-tagged Sort1 (Fig. 5B). In addition, palmitate-induced Sort1 degradation was blocked by the ERK inhibitor U0126, supporting our hypothesis that activation of ERK results in Sort1 protein degradation (Fig. 5C). Palmitate-induced Sort1 degradation was also blocked by the proteasome inhibitor MG132 (Fig. 5D). The detection of poly-ubiquitinated Sort1 in an ubiquitination assay further suggests the possible involvement of proteasomal-dependent degradation of Sort1 (Fig. 5E). On the other hand, treating HepG2 cells with the lysosome inhibitor chloroquine alone resulted in increased Sort1 degradation, which was not further affected by palmitate (Fig. 5D).
Fig. 5.
Palmitate treatment promotes Sort1 protein degradation. (A) HepG2 cells were pretreated with 40 ng/ml CHX for 30 min followed by treatments with vehicle or 0.5 mM palmitate. In left panel, Sort1 protein was measured at indicated time points by Western blot. In right panel, band density was quantified by ImageJ and plotted with the intensity at 0 h set as 1. (B) HepG2 cells were infected with adenovirus expressing a C-terminal flag-tagged Sort1 at MOI = 1 for 24 h. Cells were then treated with 0.5 mM palmitate and 40 ng/ml CHX. Flag-tagged Sort1 protein was measured at indicated time points by Western blot with an anti-flag antibody. (C) HepG2 cells were pretreated with 10 μM U0126 and 40 ng/ml CHX for 30 min, followed by treatment with vehicle or 0.5 mM palmitate for 6 h. Sort1 protein was measured by Western blot. Control cells (con) received vehicle treatment only. (D) HepG2 cells were pretreated with 1 μM MG132, 10 μM chloroquine, and 40 ng/ml CHX for 30 min, followed by 0.5 mM palmitate treatment as shown for 6 h. Sort1 protein was measured by Western blot. (E) Detection of ubiquitinated Sort1 in HepG2 cells. The assays were performed as described in Materials and Methods. NC, negative control for immunoprecipitation.
DISCUSSION
Results from this study suggests that FFA-mediated posttranslational downregulation of hepatic Sort1 may promote the development and progression of diabetic dyslipidemia, the underlying cause of increased CVD risk among obese and diabetic patients. Hepatic apoB production is an important determining factor for hepatic VLDL secretion and plasma LDL-C and triglyceride levels (22). It is well known that hepatic apoB production is mainly regulated by posttranslational presecretory degradation via both proteasomal-dependent and lysosomal-dependent pathways (22). Because insulin signaling was shown to promote proteasomal-dependent apoB degradation, hepatic apoB overproduction under diabetic conditions has mainly been attributed to impaired hepatic insulin resistance (22). As recent studies demonstrated a significant impact of Sort1-mediated lysosomal-dependent apoB degradation on plasma cholesterol and triglyceride levels (12, 14), impaired hepatic Sort1 function may also contribute significantly to higher apoB production in obesity and diabetes. It should be noted, however, that two independent studies found that whole-body knockout of sort1 in mice resulted in decreased hepatic apoB secretion and lower plasma cholesterol levels (13, 14). These results are opposite to that from mice with liver-specific Sort1 overexpression or siRNA-mediated hepatic Sort1 knockdown, which showed an inverse relationship between hepatic Sort1 and plasma lipid levels (12). Although further studies are necessary to address such discrepancies from mechanistic perspectives, it is possible that complete and whole-body absence of Sort1 may result in different metabolic outcomes than those caused by variations in hepatic Sort1 levels. Besides results obtained from mouse models, only one study has so far demonstrated that the genetic variation that results in higher Sort1 mRNA expression in human livers is associated with lower plasma LDL-C (12). Here we show that Sort1 protein, but not mRNA, is significantly lower in individuals with obesity and hepatic steatosis, which are often associated with higher hepatic apoB/VLDL production. We further showed in both normal mice and obese and diabetic mice that increasing Sort1 expression in the liver significantly reduced plasma cholesterol and triglyceride levels. Our study, therefore, provides new evidence supporting an inverse relationship between hepatic Sort1 and plasma lipid levels, and it further suggests a potential role of Sort1 in the pathogenesis and/or progression of diabetic dyslipidemia.
Increased circulating FFAs play a critical role in the pathogenesis of hepatic steatosis, insulin resistance, and inflammation (23), all of which contribute to hepatic VLDL overproduction and hyperlipidemia. Our study suggests a novel molecular link between FFAs, ERK activation, and hepatic VLDL overproduction via hepatic Sort1 downregulation. On one hand, abnormal activation of MAPKs and PKC isoforms is associated with insulin receptor and insulin receptor substrate phosphorylation and inactivation, leading to insulin resistance (7, 8); on the other hand, ERK activation downregulates hepatic Sort1. These changes presumably result in decreased apoB degradation in both proteasomes and lysosomes and, therefore, apoB overproduction and dyslipidemia. Consistent with these findings, a large body of evidence has suggested that abnormal ERK activation, not only in the liver but also adipose and muscle, is critically involved in the development of obesity, insulin resistance, and altered lipid and glucose metabolism (24–26), whereas decreased ERK activation or genetic ERK1 inactivation is linked to improved metabolic homeostasis (27, 28). Palmitate is an essential substrate for de novo synthesis of ceramides, and it is well documented that oversupply of saturated FFAs, mainly palmitate, results in elevated ceramides in the circulation and insulin-resistant tissues in diabetic humans and rodent models (21). Elevated ceramides are not only implicated in inflammation and insulin resistance but also in the development of coronary artery disease (29). In an attempt to identify the downstream mediators of palmitate, we found that C6-ceramide treatment repressed Sort1 protein, but not mRNA, in HepG2 cells and mouse hepatocytes. These results indicate that it is possible that the palmitate inhibition of Sort1 in diabetic mice may be partially mediated by elevated circulating and tissue ceramides. Interestingly, in STZ-treated mice, lower hepatic Sort1 protein was associated with higher circulating FFAs but lower hepatic triglycerides and FFAs (Table 2). These results suggest that downregulation of hepatic Sort1 in these mice was not a direct result of hepatic fat accumulation per se. It is reported that STZ-treated rats showed a marked increase in C16:0 esterified ceramides in the circulation (30), and uptake of circulating LDL-ceramides is involved in inflammation and insulin resistance (31). It is possible that increased hepatic uptake of circulating ceramides plays a role in hepatic Sort1 downregulation in STZ-treated mice. Elevated circulating ceramides in obesity and type II diabetes may also play a role in hepatic Sort1 downregulation.
In this study, we provide evidence demonstrating that hepatic Sort1 levels cannot only be changed by genetic variations that affect sort1 gene transcription but also by posttranslational mechanisms that affect Sort1 protein stability. We show that ERK-mediated Sort1 protein degradation may be a mechanism underlying the dissociation of hepatic Sort1 protein and mRNA in obese mice. Although it is still not fully clear how ERK activation results in Sort1 degradation, our results suggest the potential involvement of Sort1 ubiquitination and proteasome-dependent degradation. Interestingly, as we prepared this article, one study reported that cellular Sort1 turnover is regulated by Sort1 mono-ubiquitination and lysosomal-dependent degradation in HeLa cells (32). In contrast, our results from HepG2 cells (Fig. 5E) showed a typical pattern of poly-ubiquitination of Sort1, which is more frequently associated with proteasomal-dependent protein degradation. In addition, our results in Fig. 5D showed that blocking lysosome activity by chloroquine itself resulted in Sort1 degradation. After trafficking to the lysosome, Sort1 is retrograde transported back to Golgi apparatus via a retromer complex (11). It is possible that disruption of this process via lysosomal inhibition somehow leads to Sort1 protein degradation. However, our results do not rule out the possible lysosomal-dependent Sort1 turnover under normal conditions. Further study is underway to investigate the posttranslational regulation of Sort1 protein.
Our results showing decreased Sort1 protein in steatotic liver of obese individuals are important evidence of impaired hepatic Sort1 function in obese humans. However, we did not observe increased ERK activation in steatotic human livers as measured by ERK phosphorylation due to large variations (supplementary Fig. II). It should be noted that reduction of hepatic Sort1 likely resulted from chronic metabolic alterations; however, numerous known and unknown factors could acutely alter ERK signaling in human livers. This is very different from experimental conditions in which baseline levels are well controlled.
Increasing evidence suggests that saturated fatty acid accumulation plays a critical role in causing ER stress, which underlies many lipid disorders associated with diabetes and fatty liver diseases (33). Palmitate is a strong inducer of ER stress in hepatocytes (34). One recent study reports that sort1 gene transcription is repressed in obese mice by mechanisms involving activation of ER stress, mTORC1, and an ER stress-induced transcriptional factor ATF3 (35). Our results showed decreased hepatic Sort1 mRNA in Western diet-fed mice but not in ob/ob mice or in human steatotic livers. It is yet to be determined whether activation of ER stress may also account for posttranslational Sort1 downregulation in obesity and diabetes.
Despite the newly emerged role of liver Sort1 in regulating plasma lipid homeostasis in humans, experimental data on how Sort1 can be regulated is still very limited. Further insights on Sort1 regulation are important in improving our understanding of not only the physiological and pathological roles of Sort1 in lipid metabolism but also the potentials of targeting Sort1 to improve lipid homeostasis in obesity, diabetes, and fatty liver diseases.
Supplementary Material
Acknowledgments
The authors thank Ken Dorko and Huimin Yan (Bio-specimen Core, KUMC) for providing primary hepatocytes. The authors thank Dr. Steven Weinman for critical reading of this manuscript.
Footnotes
Abbreviations:
- CHX
- cyclohexamide
- CVD
- cardiovascular disease
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- GWAS
- genome-wide association study
- HDL-C
- HDL-cholesterol
- JNK
- c-jun N-terminal kinase
- LDL-C
- LDL-cholesterol
- MAPK
- mitogen-activated protein kinase
- MTTP
- microsome triglyceride transfer protein
- PKC
- protein kinase C
- SNP
- single nucleotide polymorphism
- Sort1
- sortilin 1
- STZ
- streptozocin
- WT
- wild-type
This work was supported by an American Diabetes Association Junior Faculty Award (T.L.), start-up funding from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health (T.L.), National Institutes of Health Grants R37-DK-58379 and R01-DK-044442 (J.Y.L.C.), and National Institutes of Health Grants AA020518 (W.-X.D.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Reaven G. M. 2002. Multiple CHD risk factors in type 2 diabetes: beyond hyperglycaemia. Diabetes Obes. Metab. 4(Suppl. 1): S13–S18 [DOI] [PubMed] [Google Scholar]
- 2.Adiels M., Boren J., Caslake M. J., Stewart P., Soro A., Westerbacka J., Wennberg B., Olofsson S. O., Packard C., Taskinen M. R. 2005. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 25: 1697–1703 [DOI] [PubMed] [Google Scholar]
- 3.Boden G., Shulman G. I. 2002. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 32(Suppl. 3): 14–23 [DOI] [PubMed] [Google Scholar]
- 4.Steinberg H. O., Baron A. D. 2002. Vascular function, insulin resistance and fatty acids. Diabetologia. 45: 623–634 [DOI] [PubMed] [Google Scholar]
- 5.Tripathy D., Aljada A., Dandona P. 2003. Free fatty acids (FFA) and endothelial dysfunction; role of increased oxidative stress and inflammation. [Comment on Steinberg, et al. 2002. Vascular function, insulin resistance and fatty acids. Diabetologia. 45: 623–634.]. Diabetologia. 46: 300–301 [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Li Y., Guan C., Li K., Wang C., Feng R., Sun C. 2010. Free fatty acid metabolic profile and biomarkers of isolated post-challenge diabetes and type 2 diabetes mellitus based on GC-MS and multivariate statistical analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 2817–2825 [DOI] [PubMed] [Google Scholar]
- 7.Cusi K. 2012. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 142: 711–725e716. [DOI] [PubMed] [Google Scholar]
- 8.Griffin M. E., Marcucci M. J., Cline G. W., Bell K., Barucci N., Lee D., Goodyear L. J., Kraegen E. W., White M. F., Shulman G. I. 1999. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 48: 1270–1274 [DOI] [PubMed] [Google Scholar]
- 9.Lewis G. F., Steiner G. 1996. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care. 19: 390–393 [DOI] [PubMed] [Google Scholar]
- 10.Taghibiglou C., Rashid-Kolvear F., Van Iderstine S. C., Le-Tien H., Fantus I. G., Lewis G. F., Adeli K. 2002. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J. Biol. Chem. 277: 793–803 [DOI] [PubMed] [Google Scholar]
- 11.Hermey G. 2009. The Vps10p-domain receptor family. Cell. Mol. Life Sci. 66: 2677–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musunuru K., Strong A., Frank-Kamenetsky M., Lee N. E., Ahfeldt T., Sachs K. V., Li X., Li H., Kuperwasser N., Ruda V. M., et al. 2010. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 466: 714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kjolby M., Andersen O. M., Breiderhoff T., Fjorback A. W., Pedersen K. M., Madsen P., Jansen P., Heeren J., Willnow T. E., Nykjaer A. 2010. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 12: 213–223 [DOI] [PubMed] [Google Scholar]
- 14.Strong A., Ding Q., Edmondson A. C., Millar J. S., Sachs K. V., Li X., Kumaravel A., Wang M. Y., Ai D., Guo L., et al. 2012. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J. Clin. Invest. 122: 2807–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linsel-Nitschke P., Heeren J., Aherrahrou Z., Bruse P., Gieger C., Illig T., Prokisch H., Heim K., Doering A., Peters A., et al. 2010. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis. 208: 183–189 [DOI] [PubMed] [Google Scholar]
- 16.Lim K. L., Chew K. C., Tan J. M., Wang C., Chung K. K., Zhang Y., Tanaka Y., Smith W., Engelender S., Ross C. A., et al. 2005. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J. Neurosci. 25: 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Twisk J., Gillian-Daniel D. L., Tebon A., Wang L., Barrett P. H., Attie A. D. 2000. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 105: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T., Owsley E., Matozel M., Hsu P., Novak C. M., Chiang J. Y. 2010. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 52: 678–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamagate A., Qu S., Perdomo G., Su D., Kim D. H., Slusher S., Meseck M., Dong H. H. 2008. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118: 2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roden M. 2006. Mechanisms of disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2: 335–348 [DOI] [PubMed] [Google Scholar]
- 21.Chavez J. A., Summers S. A. 2012. A ceramide-centric view of insulin resistance. Cell Metab. 15: 585–594 [DOI] [PubMed] [Google Scholar]
- 22.Fisher E. A. 2012. The degradation of apolipoprotein B100: multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochim. Biophys. Acta. 1821: 778–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. C., Horton J. D., Hobbs H. H. 2011. Human fatty liver disease: old questions and new insights. Science. 332: 1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujishiro M., Gotoh Y., Katagiri H., Sakoda H., Ogihara T., Anai M., Onishi Y., Ono H., Abe M., Shojima N., et al. 2003. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol. Endocrinol. 17: 487–497 [DOI] [PubMed] [Google Scholar]
- 25.Tan Y., Ichikawa T., Li J., Si Q., Yang H., Chen X., Goldblatt C. S., Meyer C. J., Li X., Cai L., Cui T. 2011. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 60: 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izawa Y., Yoshizumi M., Fujita Y., Ali N., Kanematsu Y., Ishizawa K., Tsuchiya K., Obata T., Ebina Y., Tomita S., Tamaki T. 2005. ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells. Exp. Cell Res. 308: 291–299 [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y., Zhang W., Pendleton E., Leng S., Wu J., Chen R., Sun X. J. 2009. Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats. J. Endocrinol. 203: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S. J., Pfluger P. T., Kim J. Y., Nogueiras R., Duran A., Pages G., Pouyssegur J., Tschop M. H., Diaz-Meco M. T., Moscat J. 2010. A functional role for the p62-ERK1 axis in the control of energy homeostasis and adipogenesis. EMBO Rep. 11: 226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X. C., Goldberg I. J., Park T. S. 2011. Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv. Exp. Med. Biol. 721: 19–39 [DOI] [PubMed] [Google Scholar]
- 30.Fox T. E., Bewley M. C., Unrath K. A., Pedersen M. M., Anderson R. E., Jung D. Y., Jefferson L. S., Kim J. K., Bronson S. K., Flanagan J. M., Kester M. 2011. Circulating sphingolipid biomarkers in models of type 1 diabetes. J. Lipid Res. 52: 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boon J., Hoy A. J., Stark R., Brown R. D., Meex R. C., Henstridge D. C., Schenk S., Meikle P. J., Horowitz J. F., Kingwell B. A., et al. 2013. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 62: 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumaresq-Doiron K., Jules F., Lefrancois S. 2013. Sortilin turnover is mediated by ubiquitination. Biochem. Biophys. Res. Commun. 433: 90–95 [DOI] [PubMed] [Google Scholar]
- 33.Gentile C. L., Frye M. A., Pagliassotti M. J. 2011. Fatty acids and the endoplasmic reticulum in nonalcoholic fatty liver disease. Biofactors. 37: 8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y., Wang D., Topczewski F., Pagliassotti M. J. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291: E275–E281 [DOI] [PubMed] [Google Scholar]
- 35.Ai D., Baez J. M., Jiang H., Conlon D. M., Hernandez-Ono A., Frank-Kamenetsky M., Milstein S., Fitzgerald K., Murphy A. J., Woo C. W., et al. 2012. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. J. Clin. Invest. 122: 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.