Abstract
Lysophosphatidic acid (LPA) is a bioactive lipid mediator. Concentrations of the major LPA species in mouse plasma decreased uniformly following administration of a potent selective inhibitor of the LPA-generating lysophospholipase D autotaxin, identifying an active mechanism for removal of LPA from the circulation. LPA, akylglycerol phosphate (AGP), sphingosine 1-phosphate (S1P), and a variety of structural mimetics of these lipids, including phosphatase-resistant phosphonate analogs of LPA, were rapidly eliminated (t1/2 < 30 s) from the circulation of mice following intravenous administration of a single bolus dose without significant metabolism in situ in the blood. These lipids accumulated in the liver. Elimination of intravenously administered LPA was blunted by ligation of the hepatic circulation, and ∼90% of LPA administered through the portal vein was accumulated by the isolated perfused mouse liver at first pass. At early times following intravenous administration, more LPA was associated with a nonparenchymal liver cell fraction than with hepatocytes. Primary cultures of nonparenchymal liver cells rapidly assimilated exogenously provided LPA. Our results identify hepatic uptake as an important determinant of the bioavailability of LPA and bioactive lysophospholipid mimetics and suggest a mechanism to explain changes in circulating LPA levels that have been associated with liver dysfunction in humans.
Keywords: autotaxin, lipid phosphate phosphatase, transcellular uptake, mass spectrometry
Lysophosphatidic acid (LPA) denotes a family of radyl hydrocarbon-substituted derivatives of glycerol 3-phosphate. Structural diversity in LPA species identified in biological systems encompasses species with both ester and ether linkages [(the latter of which are collectively termed alkyl glycerol phosphates (AGP)] as well as differences in hydrocarbon chain length and saturation (1). LPAs exhibit a broad range of biological activities that are initiated by LPA selective G-protein-coupled cell-surface receptors. Some LPA responses may also be mediated by the nuclear peroxisome proliferator γ receptor and the receptor for advanced glycan end products (2). LPA is present in blood plasma (3) and accumulates in human atheromas and in experimentally induced atherosclerotic lesions in mice (4). Studies using LPA receptor-deficient mice and LPA-directed small molecule therapeutics identify roles for LPA signaling in atherothrombosis and vascular injury responses (5–7). A common variant in the PPAP2B gene encoding the LPA inactivating cell surface integral membrane enzyme lipid phosphate phosphatase 3 (LPP3) may predict LPP3 mRNA expression in blood cells and is strongly associated with increased human cardiovascular disease risk. In mice, PPAP2B expression in vascular smooth muscle cells is protective against vascular injury responses (6, 8). Together, these studies point to an important role for LPA in human cardiovascular disease and focus attention on the need to understand mechanisms regulating bioavailability of this lipid in vascular cells and tissues.
LPA is present in human and rodent blood plasma at levels that are generally agreed to be excess of 100 nM where it is generated by hydrolysis of circulating lysophospholipids catalyzed by the secreted lysophospholipase D autotaxin (ATX) (9). Genetic or pharmacological manipulation of ATX expression and activity establish a close correlation between plasma LPA levels and ATX activity (10). Several highly potent and specific small molecule ATX inhibitors produce rapid and substantial decreases in plasma levels of particular LPA species in rodents (11, 12). These observations and reports of disappearance of intravenously administered LPA and the related bioactive lipid sphingosine 1-phosphate (S1P) from the circulation of mice and rats following intravenous administration (13, 14) imply that active mechanisms for either in situ degradation in blood or elimination of these lipids from the circulation exist. Definition of these mechanisms would be of obvious importance to our understanding of the role of LPA and S1P in vascular physiology and pathology and the development of bioactive lysophospholipid mimetics as pharmacological modulators of LPA and S1P metabolism and signaling. LPA and S1P are stable in plasma but can be degraded in whole blood through mechanisms that might involve LPP3 and related cell surface lipid phosphatases (13, 14). While LPA degradation in whole blood ex vivo and elimination of exogenously provided LPA from the circulation of live mice was modestly attenuated in animals with a hypomorphic mutation in another LPP enzyme, LPP1 (13), the contribution of enzymatic dephosphorylation or other potential mechanisms for degradation or elimination of LPA from the circulation is not known.
We developed methods using high-resolution MS/MS to profile and quantitate LPA species in human blood. We used these methods to investigate mechanisms responsible for elimination of LPA from the circulation of live mice. We found that the primary mechanism for elimination of exogenously supplied LPA involves rapid transcellular uptake in the liver and is largely independent of in situ degradation or accumulation in circulating blood cells.
EXPERIMENTAL PROCEDURES
Materials
Lipids were from Avanti Polar Lipids, Alabaster, AL. Evans blue dye and other reagents were from Sigma-Aldrich, St. Louis, MO. The JGW series phosphonate analogs of LPA were from Glenn Prestwich (University of Utah, Salt Lake City, UT). VPC8a202 was from Kevin Lynch (University of Virginia, Charlottesville, VA).
Animals
Mice were purchased from Jackson Laboratories, Bar Harbor, ME. Protocols and procedures conformed to current US Public Health Service policy and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Intravenous administration of lysophospholipids
Lipids were dried from organic solvents and dispersed by vortexing and sonication at a final concentration of 100 μM in 0.1%BSA/normal saline containing 5 mM Evans blue dye. Wild-type male C57/Bl6, 6–14 weeks old, and weighing 20–30 g were anesthetized using isoflurane. A vertical incision was made in the midclavicular line, and the underlying adipose tissue was retracted using forceps to expose the jugular vein. Fifty microliters (50 μl) of the lipid mixture was injected into the jugular vein using an insulin syringe, and the timer was set at the end of the injection. Blood was sampled at indicted times into K2 EDTA tubes. Fifty microliters (50 μl) of the collected anticoagulated whole blood was immediately diluted in saline, mixed gently, and centrifuged at 14,000 rpm for 0.5 min in an Eppendorf bench-top centrifuge. The cell pellets and supernatants obtained were added directly to acidified organic solvents in extraction tubes for lipid analysis or retained for measurements of Evans blue dye as detailed below.
Isolated perfused liver preparation
Single-pass rat liver perfusion was performed as previously described (15). Mice were anesthetized with urethane (1g/kg ip). The liver was perfused at a flow rate of 3 ml/min via the portal vein with Krebs-Henseleit buffer (118.5 mM NaCl, 24.9 mM NaHCO3, 1.2 mM KH2PO4, 1.19 mM MgSO4, 4.74 mM KCl, 1.27 mMCaCl2, and 5 mM glucose, pH 7.4). The perfusate was oxygenated with 95% O2–5% CO2, and the liver was maintained at 36 ± 1°C. A 50 μl bolus of C17-LPA/Evans blue dye/0.1% BSA prepared as above was injected in the inflow catheter, and the outflow was collected every 30 s for up to 5 min. Input and perfusate samples were analyzed for quantitation of C17-LPA as described below.
Liver digestion and cell isolation with antibody-coated beads
Anesthetized mice were injected with a bolus of lysophospholipids/0.1% FFA BSA or saline in the jugular vein. A two-step collagenase digestion was performed as described previously (16). Briefly, the liver was first perfused with Ca2+/Mg2+-free HBSS containing 10 mM glucose, 10 mM HEPES, and 0.3 mM EDTA, and then with HBSS containing 0.05% collagenase type IV, 1.3 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, and 10 mM HEPES. Collagenase digestion was stopped with complete culture medium as described above. The cells were centrifuged twice at 50 g for 3 min at 4°C. The pellet (containing hepatocytes) was washed three times with DMEM/0.1%delipidated FBS. The nonparenchymal cells in the supernatant (endothelial, Kupffer, and stellate cells) were collected by centrifugation at 600 g for 15min at 4°C. For isolation of nonparenchymal cell subfractions, cell preparations isolated as above were resuspended in PBS and layered over a 1.037 g/ml solution of Percoll as described by others (17). Dynabeads (Invitrogen, Carlsbad, CA) were washed and incubated with monoclonal rat anti-mouse CD31 antibody (BD Biosciences, San Jose, CA). The antibody-coated beads were washed with PBS and incubated with the resuspended cells for 20 min with gentle mixing, and antibody-associated cells were recovered by centrifugation.
Accumulation of LPA by isolated liver cells
Hepatocytes, CD31+ cells (sinusoidal endothelial cells) and CD31− cells (Kupffer and stellate cells) were isolated as described above and maintained in a 37°C humidified incubator. Cells were incubated in microfuge tubes with serum-free DMEM containing C17-LPA complexed with 0.1% fatty acid free BSA at a final concentration of 10 μM for one hour with constant mixing. Cells were collected on ice, washed once with cold PBS, and processed for lipid extractions as described below. The purity of these cell preparations was estimated to be greater than 90% in line with previous reports (16).
Lipid extraction
Lipids were extracted from 50–100 μl of sample (plasma or tissue homogenate), which was added to a 5 × 100 borosilicate tube containing 2 ml methanol, 1 ml chloroform, and 0.45 ml 0.1M HCl. Tubes were vortexed for 5 min. Then 1 ml chloroform and 1.3 ml of 0.1M HCl were added, and tubes were vortexed again for 5 min at 2,500 rpm. After centrifugation, the organic phase was transferred to a 4 ml glass vial and evaporated under N2. Samples were resuspended in 100 μl methanol, vortexed, and stored in autosamplers at −20°C for HPLC/ESI/MS/MS analysis.
Evans blue dye quantitation
Evans blue dye was quantitated using absorbance spectrophotometry at 620 nm. Plasma samples were diluted with normal saline and transferred to microcuvettes.
HPLC/ESI/MS/MS analysis
Lipids were quantitated by methods reported previously using HPLC ESI selected ion-monitoring mode MS/MS assays performed on AB Sciex 4000 Q-Trap instruments. In brief, instrument settings for each analyte were optimized by direct infusion and tuning. HPLC methods were identical to or adapted from our prior reports (11, 18–21). The instrument was operated in selected ion-monitoring mode to measure the following lipid-specific precursor and product ion pairs: C17-LPA: 423.2/152.9; C17-S1P:366.1/250.2, 366.1/82.0; VPC31143R: 436.3/338.2; JGW-9: 433.3/150.8, 433.3/79.0; JGW-10: 449.3/166.8, 449.3/62.9; Br-P-LPA: 485.2/405.1, 485.2/62.9; VPC8a202: 649.3/551.1, 649.3/136.1; TF-LPA: 684.4/78.9, 684.4/644.4; 18:1-12:0 NBD PA: 796.3/280.9, 796.3/78.7; C19-LPC: 538.4/184.0; 16-O-LPA: 395.8/79.1, 395.8/96.5; C17-MAG (ammonium adduct): 345.6/327. In each case, structurally related lipids were included during the sample extraction to monitor recovery. After peak identification and integration, the lipids of interest and recovery standards were quantitated by reference to calibration curves generated by adding a range of concentrations of the lipids of interest to an appropriate matrix (plasma or tissue lipids). The lipid standards used for these calibrations were independently quantitated by phosphorous determination or accurate mass measurements. Absolute levels of the analytes in the starting sample were then determined using these calibrations with correction for the recovery standard. PF8380 was also quantitated by HPLC ESI MS/MS again using an offline calibration and measuring the following precursor product ion pairs: PF8380: 478.1/300.9; 478.1/159.0; 478.1/123.0; 478.1/89.0; 478.1/124.1. We also developed a high-resolution MS method to search for LPA and AGP species in biological samples. The chromatography method used to separate LPA and AGP species for these measurements has been reported previously (22). Data were collected using an AB Sciex 5600 Q-TOF mass spectrometer. Lipid species identified from full-scan MS data were selected for fragmentation in a second run to generate product ion spectra. Again, quantitative data were obtained from these assays by using internal recovery standards and offline calibration curves generated using independently quantitated standards.
RESULTS
Metabolic stability of LPA species in human plasma
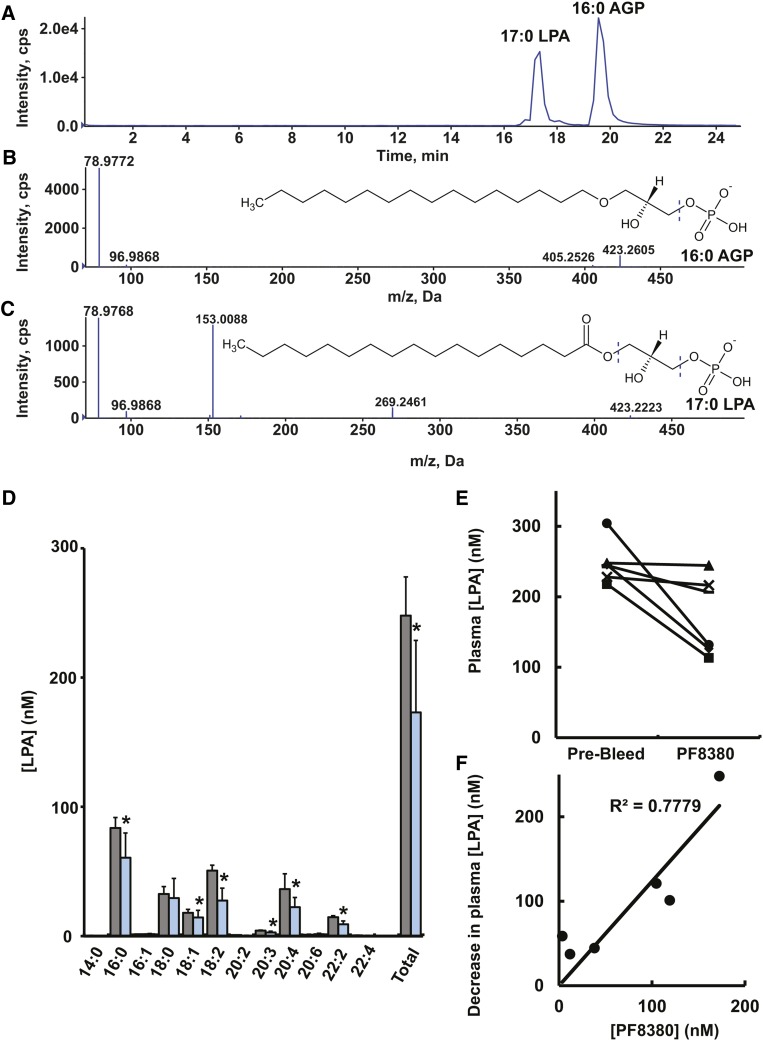
There is a general consensus that LPAs with ester-linked 18:2, 18:1, 18:0, 16:0, and 20:4 fatty acids account for ∼80% of the total LPA content in mouse and human plasma (3). LPA species with ether-linked radyl chains (AGPs) are higher affinity agonists for the LPA5 receptor, which may account for their greater potency as platelet activators. AGPs have been detected in brain (23) and chicken eggs (24), but their levels in human or mouse plasma have not been reported. To attempt to measure AGPs in mouse plasma and to investigate their potential sensitivity to ATX inhibition, we developed a method using HPLC to separate LPA species with ether and ester chains, which were identified by high-resolution MS using a quadrupole time-of-flight mass spectrometry. LPA molecular species were identified from the monoisotopic mass of their molecular anion, and alkyl versus ester LPAs were further discriminated using their distinct product ion spectra. LPAs generate the glycerol phosphate-derived product ion m/z 153, which is not observed in the product ion spectra of AGPs, which form only phosphate-derived ions of m/z 79 and 97 (Fig. 1A–C). Secondary confirmation and quantitation of lipid species were accomplished as described in Experimental Procedures. We validated our approach using a sample of human ovarian cancer ascites fluid, which contained 11 distinct AGP species (LPA16:2e, LPA18:2e, LPA16:0e, LPA20:1e, LPA18:0e, LPA20:2e, LPA24:2e, LPA12:3e, LPA18:3e, LPA16:1e, LPA10:4e) at individual concentrations in the range of 0.1–10 μM). However, as reported by many others (1–3, 11–13), LPA species were abundant in plasma from C57Bl/6 mice on a normal chow diet, but we were unable to detect any AGP species at levels above the detection limit of our assay (∼10 nM). Mice were given a single intravenous dose of 30 mg/kg PF8380, which is an nM-potency ATX inhibitor previously shown to reduce LPA levels in rat plasma following oral administration (12). PF8380 induced a statistically significant decrease in most of the major LPA species 10 min after administration, indicating that plasma levels of these lipids decline at comparable rates when their synthesis by ATX is blocked (Fig. 1D, E). The concentration of PF8380 in plasma samples from PF8380-treated animals was strongly correlated (R2 = 0.78 by linear regression) with the decrease in total plasma LPA, suggesting that variability in PF8380 dosing rather than interindividual variability in response to PF8380 accounted for the variance in decreases in plasma LPA levels observed after PF8380 administration. Inclusion of PF8380 concentrations in the range observed in plasma after dosing to freshly collected mouse plasma before lipid extraction using our standard protocol did not produce significant decreases in plasma LPA levels, indicating that ATX inhibition ex vivo or effects of PF8380 on LPA recovery from these samples did not account for our observations of decreases in plasma LPA following PF8380 dosing in vivo (data not shown).
Fig. 1.
Effect of ATX inhibition on plasma LPA levels in mice. (A–C) Extracted ion chromatograms and product ion spectra of 17:0 LPA and 16:0 AGP. (D) LPA molecular species profile in plasma collected from mice immediately before (gray bars) and 10 min after (blue bars) administration of a single 30 mg/kg dose of PF8380. Data shown are from six animals with statistical comparisons (means ± SD) made using a paired t-test. (E) Total plasma LPA levels in paired samples from individual mice determined in samples collected immediately before (prebleed) and after (PF8380) administration of a single 30 mg/kg dose of PF8380. (F) Correlation between the decrease in total plasma LPA levels and plasma level of PF8380 at 10 min post-dosing determined by linear regression analysis.
LPA is rapidly eliminated from the circulation of live mice without significant enzymatic degradation in situ
Prior reports of the elimination of radiolabeled LPA and S1P from the plasma of live mice following intravenous infusion of a single bolus dose estimated the half-lives of these lipids to be 2–3 min and ∼15 min, respectively (13, 14). Precise determination of these rates is confounded by difficulties inherent in dosing and rapidly sampling accurate volumes. To address this limitation, we infused mice with a single bolus dose of the unnatural LPA species C17-LPA in combination with Evans blue dye, a plasma volume marker that binds with high affinity to serum albumin and can be readily quantitated in plasma samples using spectrophotometry (25) allowing for simple normalization of plasma LPA measurements for variations in sampling volume or post-collection dilution. Following intravenous administration, as expected, Evans blue dye was persistent in plasma (data not shown), whereas C17 LPA measured by HPLC MS/MS was detectable at the earliest time point but subsequently disappeared extremely rapidly (Fig. 2A) Using the ratio of C17-LPA to Evans blue dye in the administered sample to calculate the quantity of LPA administered intravenously to each animal, we found that the fraction of administered LPA remaining in plasma declined extremely rapidly, to the extent that only 10–20% of the administered LPA was remaining at the earliest time point measured. This rapid decrease in plasma LPA levels occurred without significant accumulation of C17-LPA in blood cells (data not shown). C17-LPA could be degraded by phosphatases to form C17-monoacylglycerol (MAG) or A-type phospholipases (which would release a C17 fatty acid). We were unable to detect either of these metabolites in mouse plasma or whole blood following intravenous administration of C17-LPA (data not shown). These results were surprising because LPA has been reported to be degraded in whole blood through mechanisms that primarily involve enzymatic dephosphorylation; indeed, we have reported that human platelets and leukocytes both express LPPs and display phosphatase activity against exogenous LPA (6, 26). We therefore measured the rate of degradation of LPA in whole mouse blood or plasma ex vivo. C17-LPA levels declined in these incubations with significant accumulation of C17-MAG, suggesting that a major mechanism involved enzymatic dephosphorylation (Fig. 2B). By contrast, C17-LPA was much more stable in plasma (Fig. 2C). Although the rapidity of the process precluded a more detailed examination, elimination of an intravenously administered 5 nmol bolus of LPA from the circulation and degradation of a starting concentration of 1 μM of LPA in whole blood ex vivo exhibited pseudo first-order kinetics, so we estimated first-order rate constants to compare the rate of elimination of LPA from the plasma in live mice to the rates of LPA degradation observed in whole blood ex vivo. The first-order rate of elimination of intravenously administered LPA was ∼2.5-fold faster than the rate of degradation of LPA in whole blood ex vivo (Fig. 2C). Taken together, these results show that while (in keeping with previous observations) LPA can be degraded in whole blood through mechanisms that primarily involve dephosphorylation, consistent with our inability to detect C17-LPA dephosphorylation products in blood in vivo following intravenous administration of C17-LPA, the rate of degradation of LPA in blood is too slow to account for the rate of elimination of intravenously administered LPA from the plasma.
Fig. 2.
Elimination of intravenously administered LPA from the circulation of live mice and LPA metabolism in mouse blood and plasma ex vivo. (A) Plasma levels of C17-LPA were determined in three individual mice at the indicated times following intravenous administration of a single bolus dose. C17-LPA remaining in the plasma at the indicated times is expressed as a fraction of the total C17-LPA administered, which was calculated at zero time using measurements of Evans blue dye in plasma as described in the text. (B) C17-LPA was incubated with whole mouse blood for the indicated times, and levels of C17-LPA and C17-MAG were determined. (C) C17-LPA was incubated with mouse plasma for the indicated times, and levels of C17-LPA and C17-MAG were determined. (D) Semilogarithmic plot of the mean data from the experiments shown in (A), (B), and (C). Data shown in (B) and (C) are means ± SD of triplicate determinations. Error bars are omitted in (D) for clarity.
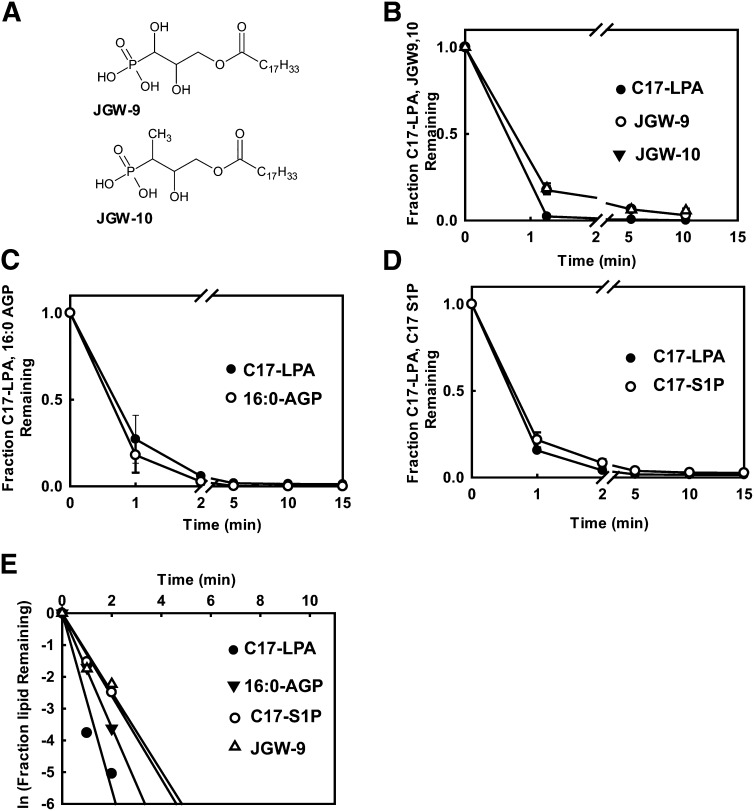
To further investigate the possible role of enzymatic degradation in the elimination of intravenously administered LPA from the plasma, we compared the rate of elimination of intravenously administered C17-LPA to that of two phosphonate-containing LPA analogs, JGW-9 and JGW-10, that are resistant to enzymatic dephosphorylation (27, 28) (Fig. 3A). The rate of elimination of these phosphatase-resistant LPA analogs was comparable to that of C17-LPA. We also found that intravenously administered 16:0 AGP (which would not be a substrate for A-type phospholipases that could putatively be involved in LPA degradation) was rapidly eliminated from the circulation. To further extend our observations, we observed that the rate of elimination of C17-S1P following intravenous administration was very similar to that of C17-LPA. Similar observations were made for several other lysophospholipids, pharmacologically active or fluorescently labeled analogs of LPA and related lipids, including VPC31143 (29), a bromophosphonate analog of LPA (Br-p-LPA), VPC8a202 (30), TopFluor®, and NBD derivatives of LPA (Table 1). Taken together, our findings indicate that the rate of elimination of intravenously administered LPA and S1P and related lysophospholipids from the circulation of live mice is at least 10-fold faster than previously reported and involves mechanisms that are independent of in situ enzymatic degradation.
Fig. 3.
Elimination of metabolically stabilized LPA analogs and related lipids from the circulation of live mice. (A) Structures of phosphonate analogs of LPA. (B–D) Plasma levels of the indicated lipids remaining in the plasma at the indicated times following single bolus dose intravenous administration are expressed as a fraction of the total amount of each lipid administered, which was calculated at zero time using measurements of Evans blue dye in plasma as described in the text. (E) Semilogarithmic plot of the mean data from the experiments shown in (B), (C), and (D). Data shown in (B), (C), and (D) are means ± SD of triplicate determinations. Error bars are omitted in (E) for clarity.
TABLE 1.
First-order rate constants and calculated half-lives for elimination of the indicated lipid species following intravenous administration of a single bolus dose to anesthetized mice
| Substrate | Rate Constant (min−1) | Half-Life (min) |
| C17-LPA (0.5 nmol) | 1.4 | 0.51 |
| C17-LPA (5 nmol) | 1.2 | 0.61 |
| C17-LPA (hepatic ligation) | 0.66 | 1.1 |
| C17-S1P | 0.79 | 0.88 |
| VPC31143R | 1.3 | 0.55 |
| JGW-9 | 0.61 | 1.1 |
| JGW-10 | 0.61 | 1.1 |
| Br-P-LPA | 0.85 | 0.82 |
| VPC8a202 | 0.36 | 2.0 |
| TF-LPA | 0.86 | 0.81 |
| NBD-PA | 0.26 | 2.7 |
| C19-LPC | 0.13 | 5.5 |
| 16-O-LPA | 1.7 | 0.4 |
| C17-MAG | 2.3 | 0.3 |
Data are means of from two to six determinations.
Intravenously administered LPA is rapidly accumulated by the liver
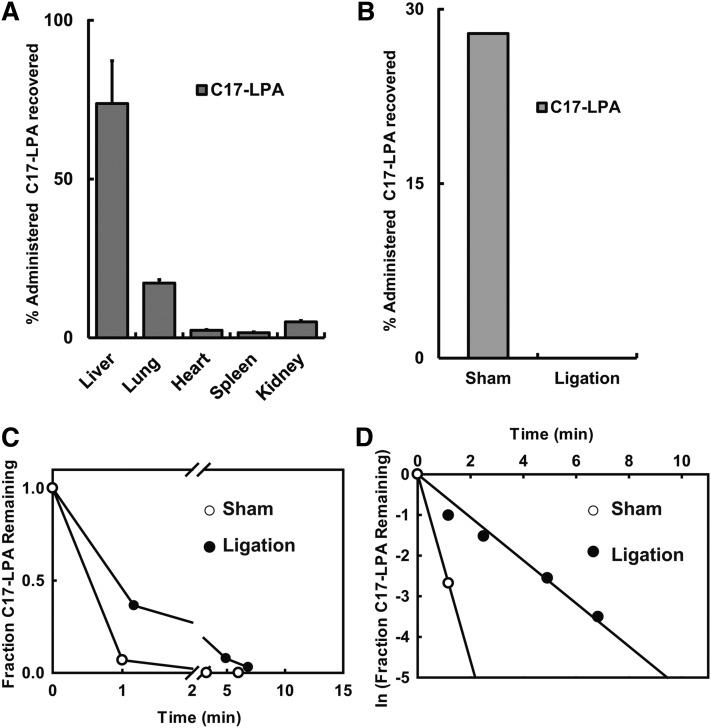
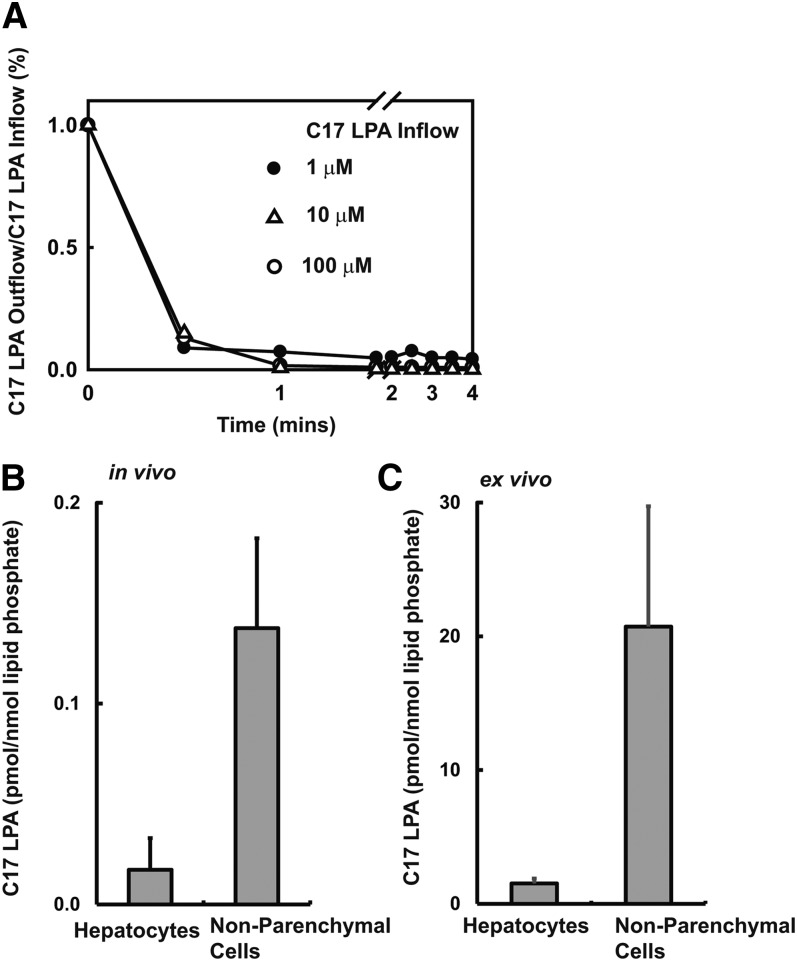
To identify the tissue responsible for accumulation of intravenously administered lysophospholipids, we determined the tissue distribution of C17-LPA in mice 15 min following a single bolus dose intravenous administration. We found that more than 80% of tissue-associated C17-LPA accumulated in the liver (Fig. 4A). We made similar observations for a number of other LPA analogs (Table 2). Consistent with this finding, we observed that ligation of the dual hepatic blood supply significantly attenuated the rate of removal of intravenously administered LPA from the circulation. Accumulation of LPA in the liver was detected in the sham but not the ligated animals, indicating that the effect of ligation on the rate of elimination of intravenously administered C17-LPA was likely a direct result of impaired hepatic accumulation of the lipid. We were concerned that our observations might be secondary to the circulatory collapse induced by this intervention and the vascular congestion proximal to the ligation site. To address these issues, we examined hepatic extraction of C17-LPA using a single-pass perfusion liver perfusion assay. In these experiments, the liver extracted 85–90% of a single bolus dose of LPA at first pass, and this rapid rate of elimination was maintained over a 100-fold LPA concentration range (Fig. 5A). In these experiments, particularly at higher C17-LPA inflow concentrations, we observed time-dependent increases in liver-associated C17-MAG and incorporation of the C17 fatty acid into other classes of phospholipids, indicating that liver-associated C17-LPA can be dephosphorylated and further metabolized (data not shown). Although we found that mouse bile contains an LPA species profile comparable to that of mouse plasma, less than 1% of the input C17-LPA was recovered in bile in this liver perfusion assay (data not shown).
Fig. 4.
Intravenously administered LPA accumulates in the liver. (A) Accumulation of intravenously administered C17-LPA in the indicated tissues expressed as a percentage of total C17-LPA accumulated in all tissues (mean ± SD of triplicate determinations). (B) Effect of surgical ligation of the hepatic circulation on liver accumulation of intravenously administered LPA in mice (mean ± SD of triplicate determinations). (C) Plasma levels of C17-LPA following intravenous administration in sham-operated (control) mice or mice with surgical ligation of the hepatic circulation. (D) Semilogarithmic plot of the data shown in (C).
TABLE 2.
Intravenously administered lysophospholipids and their analogs accumulate predominantly in the liver
| Tissue | Liver | Lung | Heart | Spleen | Kidney |
| C17-LPA | 73.7 ± 4.4 | 4.4 ± 1.4 | 2.5 ± 0.5 | 13.8 ± 2.7 | 5.5 ± 2.3 |
| C17-S1P | 86 ± 3 | 2.5 ± 0.6 | 1.3 ± 0.4 | 6.1 ± 1.9 | 4.2 ± 0.9 |
| JGW9 | 91 ± 0.7 | 4.2 ± 0.3 | 0.8 ± 0.2 | 1.3 ± 0.1 | 2.6 ± 0.4 |
| JGW10 | 92.7 ± 0.7 | 3.6 ± 0.2 | 0.5 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.4 |
| TFLPA | 92.2 ± 1 | 4.8 ± 0.8 | 0.1 ± 0.1 | 1.5 ± 0.3 | 1.3 ± 0.2 |
| VPC8a202 | 83.3 ± 3.4 | 2.2 ± 0.2 | 7.5 ± 1.7 | 6 ± 2.6 | 1.1 ± 0.4 |
| VPC31143R | 60 ± 5.1 | 5.1 ± 0.7 | 9.1 ± 2.7 | 21.6 ± 7.1 | 4.2 ± 0.1 |
| NBD-PA | 70.5 ± 2.9 | 5.7 ± 0.4 | 8.8 ± 0.3 | 10.6 ± 3.1 | 4.4 ± 0.4 |
| FTY720P | 68.6 ± 3.8 | 30 ± 3.6 | 0 ± 0 | 0 ± 0 | 1.4 ± 0.2 |
The values shown are masses of the different lipids recovered in the indicated tissues as a percentage of the total mass of these lipids recovered in all tissues following a single 5 nmol bolus injection. Data are means ± SD of from three to six separate experiments.
Fig. 5.
LPA accumulation by perfused liver and isolated liver cells. (A) Livers were perfused, and the concentration of C17-LPA in the perfusate outflow was expressed as a fraction of the C17-LPA concentration in the inflow determined at escalating inflow C17-LPA concentrations. (B) C17-LPA association with hepatocytes and nonparenchymal liver cells after a single bolus intravenous administration. (C) Association of C17-LPA with isolated hepatocytes and nonparenchymal liver cells cultured ex vivo. Data in (B) and (C) are means ± SD of triplicate determinations.
Uptake and metabolism of LPA by isolated liver cells
To identify which liver cells are primarily responsible for rapid uptake of LPA from the plasma, we examined the distribution of intravenously administered C17-LPA and its metabolites in liver cell populations isolated after rapid collagenase digestion, separation of hepatocytes using differential centrifugation, and immunoaffinity isolation to separate populations of PECAM expressing (sinusoidal endothelial) cells from other nonparenchymal cells (Kupffer and stellate cells). We found that C17-LPA was primarily associated with the nonhepatocyte cells, most notably the PECAM-positive endothelial cell fraction (Fig. 5B and data not shown). We directly evaluated the ability of these primary liver cell preparations to accumulate exogenously added C17-LPA in culture. Again, we observed that C17-LPA was more efficiently accumulated by isolated nonparenchymal cells than by isolated primary hepatocytes (Fig. 5B). In separate experiments, we found that cultured primary human liver sinusoidal endothelial cells and primary human umbilical vein endothelial cells exhibited markedly more pronounced assimilation of exogenously provided C17-LPA than did cultured Hep G2 heptatoma cells (data not shown). These results suggest that nonparenchymal cells, most likely the endothelial cells lining sinusoidal blood vessels, mediate rapid accumulation of circulating lysophospholipids by the liver.
DISCUSSION
Our data extend observations reported by us and others (11, 12) to show that levels of all major rodent plasma LPA species decline following pharmacological inhibition of the LPA-synthesizing enzyme ATX. As part of this study, we developed a high-resolution MS method for profiling and quantitating LPA and AGP species. Because alternative pathways for AGP production have been proposed (22), we hoped to use this method to determine whether plasma AGP levels were decreased following ATX inhibition. Although this method detected previously reported abundant LPA species in plasma from mice on a normal diet, surprisingly, levels of AGP were below the detection limit of our assay, and these lipids were not detected when mouse plasma was incubated ex vivo under conditions in which levels of LPA were substantially increased (data not shown). As noted, our method could detect multiple AGP species in human ovarian cancer ascites fluid, which is consistent with prior reports (31), and we have observed measurable levels of AGPs in plasma samples from some human subjects as well as mice with genetic or diet-induced hyperlipidemia (data not shown). Further work will be needed to determine whether AGP levels are sensitive to ATX inhibition.
Plasma levels of all major LPA species decline when ATX is inhibited. To investigate the mechanisms involved in removal of LPA from the circulation, we devised surgical and MS-based methods to monitor the elimination and tissue distribution of intravenously administered LPA in mice. We found that the rate of elimination of LPA from mouse plasma was significantly more rapid than previously reported by other workers (13, 14), which we suspect relates to our ability to precisely determine the administered LPA dose by using the plasma volume marker Evans blue dye. Although LPA can be degraded in whole blood ex vivo, our results indicate that enzymatic dephosphorylation is not directly involved in these rapid decreases in plasma LPA levels following either ATX inhibition or intravenous LPA administration. This result is consistent with our observation that inactivation of major vascular cell LPP, LPP3 in vascular smooth muscle cells, or vascular endothelial cells does not increase plasma LPA levels or the rate of elimination of intravenously administered LPA (Ref. 6 and unpublished observations). The relationship between our findings and the decrease in plasma LPA levels and elimination of intravenously administered LPA reported in LPP1-hypomorphic mice (13) remains to be established.
Our results indicate that intravenously administered LPA is actively accumulated in the liver. Studies using an isolated liver perfusion system indicate that ∼90% of LPA accumulates in the liver during the first pass. The majority of liver-associated LPA, at least at early times following intravenous administration, is found in association with nonparenchymal cells of the liver, which includes sinusoidal epithelial cells, Kupffer cells, and stellate cells. In preliminary experiments, we found that the majority of C17-LPA in this fraction was associated with cells that could be further isolated by immunoaffinity absorption using CD31 (PECAM)-selective antibodies, suggesting a role for sinusoidal endothelial cells in rapid LPA uptake by the liver. These observations were substantiated by our finding that this nonparenchymal cell fraction was much more effective at assimilating C17-LPA when cultured in vitro than were isolated hepatocytes and that cultured lines of human vascular endothelial and liver sinusoidal epithelial cells can also assimilate C17-LPA when exposed to this lipid in culture (data not shown). Association with and metabolism of extracellular LPA by cells in culture has been reported previously (32) and proposed to be important in transcellular mechanisms of LPA signaling involving activation of the nuclear peroxisome proliferator γ receptor (33). A mechanism for S1P accumulation by erythrocytes exists (34), but the relationship of this to the better understood process of cellular S1P export is not known (35). In our hands, LPA accumulation by cultured cells was unaffected by manipulations of several candidate genes that might affect this process, including LPPs, SPNS2, which functions as a facilitated transporter for S1P, and members of the organic anion transporter family (data not shown). Further work will be needed to identify the mechanism(s) responsible.
Although it is possible that the rate of elimination of intravenously administered LPA does not reflect the behavior of the bulk of LPA in plasma, our observations raise the possibility that at least a fraction of plasma LPA is rapidly being accumulated and metabolized in the liver. This process could simply constitute a mechanism for scavenging albumin-bound lysophospholipids from the plasma for reentry into hepatic pathways of lipid metabolism or it could result in modulation of systemic or localized LPA signaling. Our results also suggest an explanation for observations linking LPA to pathologies associated with liver dysfunction. A role for the liver in elimination and metabolism of circulating LPA is supported by observations that plasma LPA levels are increased in patients with chronic hepatitis C and are correlated strongly with the severity of liver dysfunction and fibrosis (36, 37). LPA also plays a causative role in cholestatic pruritis characteristic of liver diseases, including biliary cirrhosis, primary sclerosing cholangitis, and intrahepatic cholestasis of pregnancy in which post-hepatic biliary elimination is impaired (38). While in one case these pathologies may be linked to increases in circulating ATX levels (38), it is plausible that impairment of the role of the liver in elimination of circulating LPA could contribute to increases in plasma LPA levels.
Finally, our study raises the possibility that elimination from the plasma leading to accumulation and metabolism in the liver needs to be considered as a mechanism that limits the bioavailability of LPA and S1P. These processes could therefore be an important determinant of the pharmacological efficacy of structural mimetics of these lipids developed as therapeutics. For example, phosphonate and thiophosphate analogs of LPA that are comparable to the JGW-9 and JGW-10 compounds studied here have potent LPA receptor-selective agonist and antagonist actions in vitro, but our studies indicate that their efficacy in vivo would likely be limited by rapid elimination from the circulation (39). Enzymatic dephosphorylation appears to be a major fate of lipids that accumulate in the liver that would terminate their receptor-directed signaling actions. On the other hand, in the case of S1P-mimetic prodrugs (e.g., FTY720) that can be interconverted between their alcohol and phosphorylated forms by sphingosine kinase-mediated phosphorylation, plasma elimination and metabolism in cells and tissues might enable their therapeutic actions by increasing conversion to the active, phosphorylated form. Targeting, circumventing, or exploiting the process described in this article to produce more effective bioactive lysophospholipid-mimetic therapeutics will require identification of the molecular mechanisms responsible for transcellular accumulation of these lipids.
Footnotes
Abbreviations:
- AGP
- alkyl glycerol phosphate
- ATX
- autotaxin
- Br-P-LPA
- 1-Bromo-3(S)-hydroxy-4-(palmitoyloxy) butylphosphonate
- C16-LPA
- 1-O-hexadecyl-2-hydroxy-sn-glycero-3-phosphate
- C17-LPA
- 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphate
- C17-LPC
- 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine
- C17-MAG
- 1-heptadecanoyl-rac-glycerol
- C17-S1P
- D-erythro-sphingosine-1-phosphate
- C17-sphingosine
- D-erythro-sphingosine (C17 base)
- C19-LPC
- 1-nonadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine
- FTY720
- 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol, hydrochloride
- FTY720P
- 2-amino-2[2-(4-octylphenyl)ethyl]-1,3-propanediol, mono dihydrogen phosphate ester
- JGW-10
- 1,3(S)-Dihydroxy-4-(oleoyloxy)butyl]phosphonate
- JGW-9
- 3(S)-Hydroxy-4-(oleoyloxy)butyl]phosphonate
- LPA
- lysophosphatidic acid
- LPP3
- lipid phosphate phosphatase 3
- MAG
- monoacylglycerol
- NBD-PA
- 1-oleoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphate
- TopFluor®-LPA
- 1-{12-[4-(dipyrrometheneboron difluoride) butanoyl]amino}dodecanoyl-2-hydroxy-sn-glycero-3-phosphate
- S1P
- sphingosine 1-phosphate
- VPC31143R
- N-{(1R)-2-hydroxy-1-[(phosphonooxy)methyl]ethyl}(9Z)octadec-9-enamide
- VPC8a202
- (R)-3-(4-hydroxyphenyl)-2-palmitamidopropanoic acid
This work was supported by grants from the National Institutes of Health and Department of Veterans Affairs (to S.S.S. and A.J.M.). A.K.S. was a fellow of the American Heart Association Great Rivers Affiliate.
REFERENCES
- 1.Tigyi G. 2013. New trends in lysophospholipid research. Biochim. Biophys. Acta. 1831: 1. [DOI] [PubMed] [Google Scholar]
- 2.Morris A. J., Smyth S. S. 2013. Lysophosphatidic acid and cardiovascular disease: seeing is believing. J. Lipid Res. 54: 1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris A. J., Selim S., Salous A., Smyth S. S. 2009. Blood relatives: dynamic regulation of bioactive lysophosphatidic acid and sphingosine-1-phosphate metabolism in the circulation. Trends Cardiovasc. Med. 19: 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haseruck N., Erl W., Pandey D., Tigyi G., Ohlmann P., Ravanat C., Gachet C., Siess W. 2004. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 103: 2585–2592 [DOI] [PubMed] [Google Scholar]
- 5.Panchatcharam M., Miriyala S., Yang F., Rojas M., End C., Vallant C., Dong A., Lynch K., Chun J., Morris A. J., et al. 2008. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ. Res. 103: 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panchatcharam M., Miriyala S., Salous A., Wheeler J., Dong A., Mueller P., Sunkara M., Escalante-Alcalde D., Morris A. J., Smyth S. S. 2013. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 33: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y., Makarova N., Tsukahara R., Guo H., Shuyu E., Farrar P., Balazs L., Zhang C., Tigyi G. 2009. Lysophosphatidic acid-induced arterial wall remodeling: requirement of PPARgamma but not LPA1 or LPA2 GPCR. Cell. Signal. 21: 1874–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta N. N. 2011. A genome-wide association study in Europeans and South Asians identifies 5 new loci for coronary artery disease. Circ Cardiovasc Genet. 4: 465–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umezu-Goto M., Kishi Y., Taira A., Hama K., Dohmae N., Takio K., Yamori T., Mills G. B., Inoue K., Aoki J., et al. 2002. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., Arai H. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281: 25822–25830 [DOI] [PubMed] [Google Scholar]
- 11.Albers H. M., Dong A., van Meeteren L. A., Egan D. A., Sunkara M., van Tilburg E. W., Schuurman K., van Tellingen O., Morris A. J., Smyth S. S., et al. 2010. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. USA. 107: 7257–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gierse J., Thorarensen A., Beltey K., Bradshaw-Pierce E., Cortes-Burgos L., Hall T., Johnston A., Murphy M., Nemirovskiy O., Ogawa S., et al. 2010. A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J. Pharmacol. Exp. Ther. 334: 310–317 [DOI] [PubMed] [Google Scholar]
- 13.Tomsig J. L., Snyder A. H., Berdyshev E. V., Skobeleva A., Mataya C., Natarajan V., Brindley D. N., Lynch K. R. 2009. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 419: 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkataraman K., Lee Y. M., Michaud J., Thangada S., Ai Y., Bonkovsky H. L., Parikh N. S., Habrukowich C., Hla T. 2008. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102: 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coy D. J., Wooton-Kee C. R., Yan B., Sabeva N., Su K., Graf G., Vore M. 2010. ABCG5/ABCG8-independent biliary cholesterol excretion in lactating rats. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G228–G235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seglen P. O. 1976. Preparation of isolated rat liver cells. Methods Cell Biol. 13: 29–83 [DOI] [PubMed] [Google Scholar]
- 17.Do H., Healey J. F., Waller E. K., Lollar P. 1999. Expression of factor VIII by murine liver sinusoidal endothelial cells. J. Biol. Chem. 274: 19587–19592 [DOI] [PubMed] [Google Scholar]
- 18.Federico L., Ren H., Mueller P. A., Wu T., Liu S., Popovic J., Blalock E. M., Sunkara M., Ovaa H., Albers H. M., et al. 2012. Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol. Endocrinol. 26: 786–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausmann J., Kamtekar S., Christodoulou E., Day J. E., Wu T., Fulkerson Z., Albers H. M., van Meeteren L. A., Houben A. J., van Zeijl L., et al. 2011. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 18: 198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulkerson Z., Wu T., Sunkara M., Kooi C. V., Morris A. J., Smyth S. S. 2011. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J. Biol. Chem. 286: 34654–34663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selim S., Sunkara M., Salous A. K., Leung S. W., Berdyshev E. V., Bailey A., Campbell C. L., Charnigo R., Morris A. J., Smyth S. S. 2011. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin. Sci. (Lond.). 121: 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gellett A. M., Kharel Y., Sunkara M., Morris A. J., Lynch K. R. 2012. Biosynthesis of alkyl lysophosphatidic acid by diacylglycerol kinases. Biochem. Biophys. Res. Commun. 422: 758–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiura T., Nakane S., Kishimoto S., Waku K., Yoshioka Y., Tokumura A., Hanahan D. J. 1999. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim. Biophys. Acta. 1440: 194–204 [DOI] [PubMed] [Google Scholar]
- 24.Nakane S., Tokumura A., Waku K., Sugiura T. 2001. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: distinct molecular species compositions. Lipids. 36: 413–419 [DOI] [PubMed] [Google Scholar]
- 25.Morris C. J. 1944. The determination of plasma volume by the Evans blue method: the analysis of haemolysed plasma. J. Physiol. 102: 441–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smyth S. S., Sciorra V. A., Sigal Y. J., Pamuklar Z., Wang Z., Xu Y., Prestwich G. D., Morris A. J. 2003. Lipid phosphate phosphatases regulate lysophosphatidic acid production and signaling in platelets: studies using chemical inhibitors of lipid phosphate phosphatase activity. J. Biol. Chem. 278: 43214–43223 [DOI] [PubMed] [Google Scholar]
- 27.Jiang G., Xu Y., Fujiwara Y., Tsukahara T., Tsukahara R., Gajewiak J., Tigyi G., Prestwich G. D. 2007. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. ChemMedChem. 2: 679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Xu X., Gajewiak J., Tsukahara R., Fujiwara Y., Liu J., Fells J. I., Perygin D., Parrill A. L., Tigyi G., et al. 2009. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 69: 5441–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch K. R., Macdonald T. L. 2002. Structure-activity relationships of lysophosphatidic acid analogs. Biochim. Biophys. Acta. 1582: 289–294 [DOI] [PubMed] [Google Scholar]
- 30.East J. E., Kennedy A. J., Tomsig J. L., De Leon A. R., Lynch K. R., Macdonald T. L. 2010. Synthesis and structure-activity relationships of tyrosine-based inhibitors of autotaxin (ATX). Bioorg. Med. Chem. Lett. 20: 7132–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Z., Wu M., Elson P., Kennedy A. W., Belinson J., Casey G., Xu Y. 2001. Fatty acid composition of lysophosphatidic acid and lysophosphatidylinositol in plasma from patients with ovarian cancer and other gynecological diseases. Gynecol. Oncol. 83: 25–30 [DOI] [PubMed] [Google Scholar]
- 32.van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. 1992. Metabolic conversion of the biologically active phospholipid, lysophosphatidic acid, in fibroblasts. Biochim. Biophys. Acta. 1125: 110–112 [DOI] [PubMed] [Google Scholar]
- 33.McIntyre T. M., Pontsler A. V., Silva A. R., St Hilaire A., Xu Y., Hinshaw J. C., Zimmerman G. A., Hama K., Aoki J., Arai H., et al. 2003. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. USA. 100: 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanel P., Andreani P., Graler M. H. 2007. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 21: 1202–1209 [DOI] [PubMed] [Google Scholar]
- 35.Kim R. H., Takabe K., Milstien S., Spiegel S. 2009. Export and functions of sphingosine-1-phosphate. Biochim. Biophys. Acta. 1791: 692–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe N., Ikeda H., Nakamura K., Ohkawa R., Kume Y., Tomiya T., Tejima K., Nishikawa T., Arai M., Yanase M., et al. 2007. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 81: 1009–1015 [DOI] [PubMed] [Google Scholar]
- 37.Watanabe N., Ikeda H., Nakamura K., Ohkawa R., Kume Y., Aoki J., Hama K., Okudaira S., Tanaka M., Tomiya T., et al. 2007. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J. Clin. Gastroenterol. 41: 616–623 [DOI] [PubMed] [Google Scholar]
- 38.Kremer A. E., Martens J. J., Kulik W., Rueff F., Kuiper E. M., van Buuren H. R., van Erpecum K. J., Kondrackiene J., Prieto J., Rust C., et al. 2010. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 139: 1008–1018, 1018.e1. [DOI] [PubMed] [Google Scholar]
- 39.Tigyi G. 2010. Aiming drug discovery at lysophosphatidic acid targets. Br. J. Pharmacol. 161: 241–270 [DOI] [PMC free article] [PubMed] [Google Scholar]