Abstract
Cholesteryl ester transfer protein (CETP) transfers cholesteryl ester and triglyceride between HDL and apoB-containing lipoproteins. Anacetrapib (ANA), a reversible inhibitor of CETP, raises HDL cholesterol and lowers LDL cholesterol in dyslipidemic patients. We previously demonstrated that ANA increases macrophage-to-feces reverse cholesterol transport and fecal cholesterol excretion in hamsters, and increased preβ HDL-dependent cholesterol efflux via ABCA1 in vitro. However, the effects of ANA on in vivo preβ HDL have not been characterized. In vitro, ANA inhibited the formation of preβ, however in ANA-treated dyslipidemic hamsters, preβ HDL levels (measured by two-dimensional gel electrophoresis) were increased, in contrast to in vitro findings. Because changes in plasma preβ HDL have been proposed to potentially affect markers of cholesterol absorption with other CETP inhibitors, a dual stable isotope method was used to directly measure cholesterol absorption in hamsters. ANA treatment of hamsters (on either dyslipidemic or normal diet) had no effect on cholesterol absorption, while dalcetrapib-treated hamsters displayed an increase in cholesterol absorption. Taken together, these data support the notion that ANA promotes preβ HDL functionality in vivo, with no effects on cholesterol absorption.
Keywords: cholesteryl ester transfer protein, apolipoprotein A1, anacetrapib, dalcetrapib, high density lipoprotein
Despite the success of statins as a therapeutic intervention for coronary heart disease, a large degree of residual risk remains in the coronary heart disease population, resulting in both a continued unmet medical need as well as the pursuit of therapies which can further reduce known risk factors. Cholesteryl ester transfer protein (CETP) mediates the transfer of cholesteryl ester and triglyceride between HDL and apoB-containing lipoproteins such as LDL, and is currently a target for increasing HDL cholesterol (HDL-C) and reducing LDL cholesterol (LDL-C). Small molecule inhibitors have been developed to inhibit CETP, including torcetrapib (Pfizer), dalcetrapib (Roche), evacetrapib (Eli Lilly), and anacetrapib (ANA) (Merck). While initial clinical trials with torcetrapib established the validity of CETP inhibition as a statin-additive mechanism for reduction of LDL-C and elevation of HDL-C (1, 2), the phase III outcome trial ILLUMINATE demonstrated that torcetrapib treatment was associated with an increase in cardiovascular events, and overall mortality (3). A series of preclinical studies indicated that torcetrapib had compound-specific off-target activity that was unrelated to CETP inhibition (4–6). Dalcetrapib was evaluated in a large phase III clinical program (dal-HEART), however the phase III outcomes study (dal-OUTCOMES) was stopped early due to futility/lack of efficacy (7, 8). While a possible reason for a lack of effect on outcomes benefit with dalcetrapib might be related to its weaker inhibition of CETP (manifest as an insufficient elevation of HDL-C or lack of an effect on LDL-C), the precise answer for why dalcetrapib was ineffective remains unknown.
ANA is a potent CETP inhibitor which has not demonstrated the off-target activities of torcetrapib in both preclinical and clinical studies (9–11). In a recent 1.5 year safety study in ∼1,600 patients with cardiovascular disease (11), ANA treatment had no effect on blood pressure, electrolytes, or aldosterone, and the distribution of cardiovascular events suggested that ANA treatment would not be associated with the type of adverse effects on outcomes that were observed with torcetrapib. ANA treatment increases HDL-C by over 100% and lowers LDL-C by 30–40% as monotherapy and when coadministered with statins (9–11). In combination with the lack of any off-target, torcetrapib-like effects, the robust changes in LDL-C and HDL-C differentiate ANA from both dalcetrapib and torcetrapib, and the true effects of ANA on clinical outcomes is currently being evaluated in a large phase III outcomes study.
Despite the robust LDL-C-lowering effect of ANA, the large increase in HDL-C in response to ANA makes it important to assess the functionality of HDL particles generated in response to CETP inhibition. Previously, it was demonstrated by Yvan-Charvet et al. (12) that HDL from humans treated with ANA displays improved cholesterol efflux capacity compared with placebo. Further, we demonstrated that in dyslipidemic hamsters, ANA treatment was associated with enhanced macrophage-to-feces reverse cholesterol transport (13). ANA treatment was also associated with improvements in cholesterol efflux, including efflux through the ATP binding cassette transporter A1 (ABCA1), a process that is dependent on preβ HDL (12, 13). However, the effects of ANA on preβ HDL in vivo have not been characterized.
The purpose of the present study was to evaluate the effects of ANA on preβ HDL. This was accomplished through evaluation of preβ levels in in vitro and in vivo systems, and through comparisons between ANA and dalcetrapib. Further, we examined the effects of ANA on cholesterol absorption as another possible contributor to changes in plasma preβ HDL.
MATERIALS AND METHODS
Chemicals and compounds
ANA, dalcetrapib, and ezetimibe were synthesized by Merck Research Laboratories. 2H2O, [3,4-13C2], and [2H6]cholesterol were purchased from Sigma-Aldrich (St. Louis, MO).
In vitro generation of preβ HDL by CETP in human plasma.
Human recombinant CETP was expressed and purified as previously described (14). Fresh EDTA plasma was isolated from human blood and stored at 4°C until use. Human plasma was treated with or without compounds in DMSO in the presence or absence of human recombinant CETP. The mixture was incubated at 37°C for 21 h. Preβ HDL concentration was measured by commercially available preβ HDL ELISA (American Diagnostics) as described by the manufacturer. To visualize the distribution of HDL fractions for comparison between in vitro incubation and freshly-isolated HDL, samples were separated by gel electrophoresis, stained, and gel images collected using the LipoprintTM system (Quantimetrix) as described by manufacturer.
Animals
All testing protocols described below were reviewed and approved by the Merck Research Laboratories Institutional Animal Care and Use Committee in Rahway, NJ. Animals were maintained in a 12 h/12 h light-dark cycle with free access to food and water in single housing condition in a temperature controlled environment (22°C). Male Syrian golden hamsters (weight ∼120 g at beginning of study) were obtained from Harlan Laboratories Inc. (Madison, WI). For in vivo studies, male Syrian golden hamsters were either maintained on regular rodent chow [7012 (5% dietary fat; 3.75 kcal/g); Teklad, Madison, WI] or placed on a high-fat cholesterol diet [D08092301 (45% kcal from lard, 0.12% cholesterol, 4.73 kcal/g); Research Diets, New Brunswick, NJ] for 3 weeks before receiving compounds.
In vivo hamster studies
Hamsters (n = 10 in each treatment group) were maintained on a regular diet [7012 (5% dietary fat; 3.75 kcal/g); Teklad, Madison, WI]. Hamsters were orally dosed with either vehicle [0.5% methyl cellulose (Sigma)], ANA (once daily, 60 mg/kg), dalcetrapib (twice daily, 200 mg/kg), or ezetimibe (admixed in feed to deliver 1.5 mg/kg/day). Another cohort of hamsters was placed on a high-fat cholesterol diet {D08092301 [45% kcal from fat (lard), 35% kcal from carbohydrate, 20% kcal from protein, and 0.12% cholesterol]; Research Diets} for 3 weeks before receiving the same treatment as above. Feces were collected for a 24 h period prior to compound treatment and again after 14 days of compound treatment. On day 14, 2 h after the final dose, hamsters received an intravenous dose of 13C-cholesterol and an oral dose of D6-cholesterol, both at 15 mg/kg. Intravenous 13C-cholesterol was injected as a filtered solution (3 mg/ml in a vehicle containing 10% ethanol and 90% Intralipid, 5 ml/kg iv dose). Hamsters also received an intraperitoneal dose of D2O at 20 ml/kg. Blood was collected from the jugular vein at 4, 24, 48, and 72 h following injection of tracer and via cardiac puncture at the final time point (96 h following tracer injection), and plasma was separated by centrifugation for determination of cholesterol absorption and de novo cholesterol synthesis.
Plasma lipid/apolipoprotein analyses, fecal cholesterol
A commercial enzymatic colorimetric kit was used for the determination of plasma total cholesterol (Wako Cholesterol E kit) according to manufacturer's instructions. For analysis of plasma lipoprotein-associated cholesterol, LipoPrint was used as described previously (13). Plasma preβ HDL levels were determined using two-dimensional (2D) gel electrophoresis followed by immunoblotting for apoA1. 2D gel electrophoresis and analysis were kindly performed by Bela Aztalos at Tufts University (Boston, MA) (15). The method used to quantify hamster plasma apoA1 was adapted from Lassman et al. (16). Briefly, 4 μl of plasma was diluted with 136 μl ammonium bicarbonate pH 8.0 and a known amount of a stable isotope-labeled peptide standard (AKPA-[2H10]LEDLR; Bachem, Torrance, CA) was spiked into plasma; 10 μl 10% sodium deoxycholate was added prior to reduction, alkylation, and tryptic digestion. These conditions allow for the complete digestion of apoA1, therefore, the apoA1 peptide concentration reflects the apoA1 protein concentration (apoA1 peptide concentration was calculated using peak area ratio between the sample and internal standard).
Fecal cholesterol was measured by extracting lipids using the Folch method (17), whereby fecal samples were homogenized in 2:1 chloroform:methanol, followed by filtration/washing with 0.9% saline, centrifugation, and drying of lower phase under nitrogen gas. The extract was reconstituted with 10% Triton X in isopropanol and analyzed using a commercial cholesterol kit (Wako Cholesterol E kit).
Ex vivo CETP activity
The ability of inhibitors to block CETP-mediated cholesteryl ester transfer in animal plasma was also measured by radioactive CETP transfer assay as previously described (14). Briefly, the assays were performed by incubating 95% plasma with [3H]cholesteryl oleate-labeled exogenous LDL up total volume of 50 ul at 37°C for 90 min. After reaction, the transfer reaction was terminated by precipitation of LDL with 20% w/v PEG 8000 (1:1 vol). The samples were centrifuged and an aliquot of the HDL-containing supernatant was counted by liquid scintillation. The inhibition of CETP activity was expressed as percentage of total radioactivity recovered in the treated animals versus controls.
Analysis of plasma water labeling
The 2H-labeling of body water was determined using headspace analyses following exchange with acetone as described by Shah et al. (18). Briefly, 20 μl of sample (or standard) was reacted with 2 μl of 10 N NaOH and 4 μl of a 5% (v/v) solution of acetone in acetonitrile for 4 h at room temperature. The instrument is programmed to inject 5 ul of headspace gas from the GC vial in a splitless mode. Samples were analyzed using a 2 min isothermal run (Agilent 5973 MS coupled to a 6890 GC oven fitted with an Agilent DB-5MS column, 30 m × 250 μm × 0.15 μm, the oven was set at 170°C and helium carrier flow was set at 1.0 ml × min−1), acetone elutes at ∼1.4 min, the mass spectrometer was set to perform selected ion monitoring of m/z 58 and 59 (10 ms dwell time per ion) in the electron impact ionization mode.
Analysis of [2H] and [13C] labeling of total plasma cholesterol
The isotopic labeling of [2H]- and [13C]-labeled total plasma cholesterol was determined using GC-MS. Lipids were saponified by heating plasma (50 μl) with 1 N KOH in 80% methanol (200 μl) at 65°C for 1 h. Samples were acidified with 25 μl 6 N HCl and then extracted in 125 μl chloroform followed by vigorous vortexing for 20 s The samples were centrifuged at 3,000 rpm for 5 min, 100 μl of chloroform (lower layer) was collected and evaporated to dryness under N2. Samples were derivatized by reacting with 100 μl of pyridine:acetic anhydride (1:2, v:v) at 65°C for 1 h. Excess reagent was evaporated to dryness under N2 and the acetylated derivative was reconstituted in 50 μl ethyl acetate for analysis by GC-MS. All analyses were performed using an Agilent 5973 MS coupled to a 6890 GC oven fitted with an Agilent DB-5MS column (30 m × 250 μm × 0.15 μm). The instrument was programmed to inject 1 ul of sample using a 10:1 split (helium carrier flow was set at 1.0 ml × min−1). The starting oven temperature of 150°C was then raised at 20°C × min−1 intervals to 310°C and held for 6 min (cholesterol elutes at ∼9). The mass spectrometer was set to perform selected ion monitoring of m/z 368, 369, 370, and 373 (10 ms dwell time per ion) in the electron impact ionization mode.
Calculations and statistical analysis
Unless otherwise stated, data are presented as mean ± S.E.M. Statistical analysis was performed using 1-way ANOVA followed by Dunnett's multiple comparison test for comparison of mean values between multiple groups. Significance level was set at P < 0.05.
To quantify the contribution of cholesterol synthesis to cholesterol levels, one fits the data using a precursor:product labeling ratio to the general equation:
where n is the number of exchangeable hydrogens (assumed to equal 26 for cholesterol) (19).The change in the ratio of m/z 369:368 (i.e., M+1/M0) was used to model the product labeling. The precursor labeling was assumed to equal plasma water and the concentration of total circulating cholesterol was determined via enzymatic assay. These calculations assumed that the kinetics follow a single exponential term.
To quantify the fractional rate of cholesterol absorption the ratio of m/z 373:370, the orally administered tracer expressed relative to the intravenously administered tracer (20, 21) was compared. Although [2H6]cholesterol was administered orally, the fragment ion that is used in the analyses results in the loss of one 2H. Therefore we measured the abundance of M+5.
RESULTS
Effects of ANA in preβ HDL in vitro
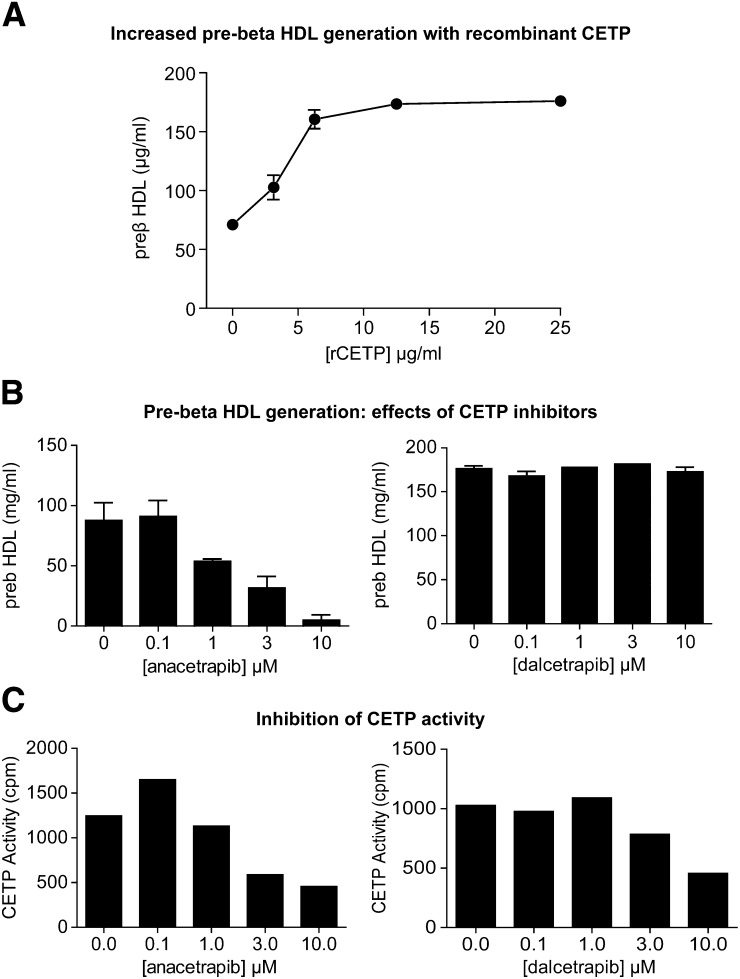
Under in vitro conditions (incubation of a human plasma sample for 21 h at 37°C), preβ HDL levels increased over time (data not shown) and increased with the addition of increasing concentrations of human recombinant CETP (Fig. 1A). In the presence of 25 ug/ml human recombinant CETP added to plasma for 21 h, ANA inhibited the generation of preβ HDL in a concentration-dependent manner (Fig. 1B), at concentrations that inhibited CETP transfer activity (Fig. 1C). By comparison, dalcetrapib had no effect on the in vitro generation of preβ HDL (Fig. 1B), despite inhibiting CETP transfer activity, albeit less potently than ANA (Fig. 1C).
Fig. 1.
Effects of CETP inhibitors on preβ HDL generation in vitro. Human plasma was incubated at 37°C for 21 h and preβ HDL was measured as described in Materials and Methods. A: CETP-dependence of in vitro preβ HDL generation. B: Concentration-dependent inhibition of in vitro preβ HDL generation by ANA (left) but not dalcetrapib (right). C: Inhibition of CETP activity by both ANA (left) and dalcetrapib (right).
Effects of CETP inhibitors on preβ HDL levels in vivo
Preβ HDLs from hamster plasma were measured using 2D gel electrophoresis. Dyslipidemic hamsters were treated with ANA or dalcetrapib for 2 weeks and plasma collected, snap-frozen, and subjected to 2D gel electrophoresis for analysis. In this experiment, animals treated with ANA and dalcetrapib displayed reduced CETP transfer activity (55% reduction, P < 0.001 and 41% reduction, P < 0.001, respectively) and an increase in HDL-C (65 and 30% increase, respectively, P < 0.001). ANA-treated hamsters showed an increase in plasma preβ HDL levels, as a percent of total apoA1 detected in the 2D gel system (Fig. 2A). Hamsters treated with dalcetrapib showed no significant change in preβ HDL (Fig. 2A). Total plasma apoA1 was measured from hamster plasma using LC/MS. Neither ANA nor dalcetrapib showed a significant change in plasma apoA1 (Fig. 2B). The data reported in Fig. 2 are from one of three independent studies performed with ANA and dalcetrapib in dyslipidemic hamsters, all showing similar results (data not shown). From the 2D gel electrophoresis analysis, levels of α-migrating particles were also assessed (Table 1). In samples from ANA-treated animals, the greatest increase was observed in the large α particles (Table 1), with a 20% increase compared with vehicle controls. A numerical but nonsignificant increase was observed in medium and small α particles. In the case of dalcetrapib-treated animals, no significant changes were observed in preβ HDL or in large and medium α particles (Table 1), while a 23% reduction in small α particles was observed with dalcetrapib treatment (Table1).
Fig. 2.
Increased preβ HDL in dyslipidemic hamsters by ANA but not dalcetrapib. A: Preβ HDL was analyzed by 2D gel electrophoresis as described in Materials and Methods. ANA (left) increased preβ HDL as percent of total apoA-I, while dalcetrapib (right) had no effect. B: Lack of effect of treatment on total plasma apoA-I from hamsters treated with ANA (left) or dalcetrapib (right). *P < 0.05 versus vehicle (Veh). BID, twice daily.
TABLE 1.
Distribution of HDL subfractions (percent of total apoA-I)
| HDL Subfraction | Vehicle | ANA | Vehicle | Dalcetrapib |
| Preβ1 | 5.5 ± 0.8 | 8.1 ± 0.03* | 4.8 ± 0.8 | 3.1 ± 0.0 |
| Alpha (large) | 18.2 ± 1.1 | 22.0 ± 0.5** | 16.3 ± 2.6 | 17.6 ± 2.8 |
| Alpha (medium) | 42.0 ± 1.4 | 45.2 ± 1.5 | 39.8 ± 6.4 | 42.3 ± 6.7 |
| Alpha (small) | 13.2 ± 1.4 | 16.2 ± 0.9 | 17.1 ± 2.9 | 13.1 ± 2.0** |
Data presented as mean ± SEM; *P < 0.05, **P < 0.01.
Effects of CETP inhibitors on HDL-C and bulk fecal cholesterol concentration in dyslipidemic Syrian golden hamsters
To examine the effects of CETP inhibitors on cholesterol absorption, a separate cohort of Syrian golden hamsters on either high-fat/dyslipidemic diet or normal diet were treated with ANA or dalcetrapib for 2 weeks. A group of hamsters was also treated with ezetimibe, a known inhibitor of cholesterol absorption (22, 23). As shown in Fig. 3A, in dyslipidemic hamsters, ANA treatment resulted in inhibition of ex vivo CETP transfer activity of 54% compared with vehicle, while dalcetrapib treatment inhibited CETP activity by 45%. Ezetimibe-treated hamsters showed no change in CETP activity. Inhibition of CETP by ANA resulted in a 52% increase in HDL-C and a 32% reduction in LDL-C (Fig. 3B). Dalcetrapib treatment was associated with a 13% increase in HDL-C, with no change in LDL-C (Fig. 3B). Ezetimibe reduced LDL-C by 47% with 33% reduction in HDL-C (Fig. 3B). During the 2 week dosing period, feces were collected from each treatment group and fecal cholesterol concentration was measured. Both ANA and dalcetrapib increased fecal cholesterol concentration by 244 and 188%, respectively (Fig. 3C). Ezetimibe increased fecal cholesterol by 616% (Fig. 3C).
Fig. 3.
Effect of CETP inhibition on plasma lipoproteins and fecal cholesterol in dyslipidemic hamsters. A: ANA and dalcetrapib (Dal) reduce CETP activity, with no effect of ezetimibe (Eze) (cholesterol absorption control). B: Increase in plasma HDL-C by ANA and dalcetrapib (left), reduction in LDL-C by ANA and ezetimibe (right). C: ANA and dalcetrapib increase fecal cholesterol content equivalently in dyslipidemic hamsters, but to a lesser extent than ezetimibe. **P < 0.01, ***P < 0.001 versus vehicle (Veh). CPM: counts per minute of 3H cholesteryl ester detected in the acceptor particle following LDL precipitation, as described in Materials and Methods.
In normolipidemic hamsters, ANA and dalcetrapib inhibited ex vivo CETP transfer activity by 38 and 35%, respectively (Fig. 4A). Treatment of hamsters with ANA was associated with a 60% increase in HDL-C, while dalcetrapib treatment was associated with a 24% increase in HDL-C (Fig. 4B). Neither CETP inhibitor affected LDL-C in normolipidemic hamsters (Fig. 4B). Ezetimibe had no effect on lipoprotein-associated cholesterol in hamsters on normal diet (Fig. 4B). Fecal cholesterol was unchanged in normolipidemic hamsters treated with either ANA or dalcetrapib (Fig. 4C). Ezetimibe, however, increased fecal cholesterol levels by 600% (Fig. 4C).
Fig. 4.
Effect of CETP inhibition on plasma lipoproteins and fecal cholesterol in normolipidemic hamsters. A: ANA and dalcetrapib reduce CETP activity, with no effect of ezetimibe (cholesterol absorption control). B: Increase in plasma HDL-C by ANA and dalcetrapib (left), lack of effect of any treatment on LDL-C (right). C: Only ezetimibe increases fecal cholesterol content in normolipidemic hamsters. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle.
Effects of CETP inhibitors on cholesterol absorption
A dual tracer approach was taken to directly measure cholesterol absorption in either dyslipidemic or normolipidemic hamsters treated with ANA or dalcetrapib, with ezetimibe-treated hamsters included as a positive control for inhibition of absorption. Figure 5A depicts the changes in the ratio of orally administered D6-cholesterol to iv administered 13C-cholesterol in treated dyslipidemic hamsters, as an area under the curve (AUC). As shown in Fig. 5A, ezetimibe-treated hamsters showed a marked reduction in cholesterol absorption, evidenced by the suppression of the oral:iv tracer relationship. Dalcetrapib-treated hamsters displayed an increase in the absorption of cholesterol, while ANA-treated hamsters had no change in cholesterol absorption. To determine whether these changes were accompanied by alterations in de novo cholesterol synthesis, the incorporation of deuterium into newly synthesized cholesterol was used as a measure of cholesterol synthesis. Ezetimibe-treated hamsters displayed a marked increase in cholesterol synthesis, while ANA- and dalcetrapib-treated hamsters showed no significant changes in cholesterol synthesis (Fig. 5B).
Fig. 5.
Dalcetrapib (Dal), but not ANA, increases cholesterol absorption in dyslipidemic hamsters. A: Area under the curve (AUC) of the ratio of oral:iv-labeled cholesterol (measure of cholesterol absorption). B: Only ezetimibe (Eze) increases incorporation of 2H into cholesterol, a measure of de novo cholesterol synthesis. ***P < 0.001, **P < 0.01 versus vehicle (Veh).
In normolipidemic hamsters (Fig. 6), ezetimibe treatment inhibited cholesterol absorption to a similar extent as in dyslipidemic hamsters (Fig. 6A). Dalcetrapib treatment was also associated with an increase in cholesterol absorption (Fig. 6A), albeit a smaller change from vehicle-treated animals as compared with dyslipidemic animals. ANA had no effect on cholesterol absorption in normolipidemic hamsters. Neither ANA nor dalcetrapib had an effect on cholesterol synthesis in normolipidemic hamsters, while ezetimibe-treated animals displayed a marked increase in cholesterol synthesis (Fig. 6B).
Fig. 6.
Dalcetrapib (Dal), but not ANA, increases cholesterol absorption in normolipidemic hamsters. A: Area under the curve (AUC) of the ratio of oral:iv-labeled cholesterol (measure of cholesterol absorption). B: Only ezetimibe (Eze) increases incorporation of 2H into cholesterol, a measure of de novo cholesterol synthesis. ***P < 0.001, **P < 0.01 versus vehicle (Veh).
DISCUSSION
“Preβ HDL” refers to a subset of HDL particles that are deficient/devoid of lipid, and are critical in collecting cholesterol from peripheral tissues as the first step in reverse cholesterol transport (24, 25). While we have demonstrated that ANA treatment improves ABCA1-dependent cholesterol efflux (13), a function attributable to preβ HDL, the effects of ANA on preβ HDL in vivo have not been evaluated. The purpose of the current study was to examine the effects of ANA on preβ HDL in vivo.
ANA-treated dyslipidemic hamsters displayed an increase in the amount of large α-migrating HDL particles. This is similar to what we previously reported in Castro-Perez et al. (13), where the largest increase in cholesteryl ester content was in the large HDL2 population. While this is somewhat similar to what was reported for torcetrapib in humans (26), where α1 HDL particles were increased, there are also some key differences. Preβ HDL was not increased in the human setting, and the total apoA1 pool size was increased. In the current study, we observed an increase in preβ HDL levels with ANA, reported as a percent of total apoA-I, which did not change. Taken with the observation of no change in total apoA-I, this supports the notion that ANA treatment results in remodeling of the HDL particle fraction.
The effects of ANA on preβ HDL in vivo are in stark contrast to the in vitro system, where ANA inhibited formation of preβ HDL. In fact an increase was observed, suggesting that lipid-poor or lipid-free apoA-I is being regenerated in vivo with ANA. In a previous study, we reported an increase in ABCA1-dependent cholesterol efflux (along with an increase in ABCG1 and SRB1-mediated efflux) in hamsters treated with ANA (13), a finding similar to what was shown in humans (12), where total efflux to the HDL fraction of human subjects treated with ANA was increased compared with placebo. Taken with the increase in preβ HDL concentration, these studies support the notion that ANA is maintaining, if not increasing, preβ HDL functionality.
Importantly, one must be cautious in interpreting changes in static concentrations of preβ HDL in vivo. For example, an increase in preβ HDL could be due to inhibition of particle maturation, which would be explained by a reduction in LCAT activity. It has been demonstrated that HDL-associated LCAT activity is increased in CETP-deficient patients (27), and we reported an increase in HDL2-associated cholesteryl ester in hamsters treated with ANA (13) which contradicts the notion that particle maturation is being inhibited. Phospholipid transfer protein has also been shown to have the capacity to generate preβ HDL (28, 29). While ANA has high selectivity for CETP, an indirect or compensatory role for phospholipid transfer protein in the generation of preβ HDL cannot be ruled out.
In vitro, in isolated human plasma, formation of preβ HDL in plasma is thought to be dependent upon remodeling of large α-migrating HDL particles by CETP, which results in delipidation of the HDL particle and generates lipid-poor or lipid-free apoA1 (25, 30). Given that CETP activity is involved in this process in vitro, it follows that inhibition of CETP by small molecule inhibitors would reduce the formation of preβ HDL in an in vitro system, and this phenomenon was described recently by Neisor et al. (31). Given that a system using isolated plasma lacks other critical elements that contribute to HDL particle remodeling, such as target tissues/vascular endothelium, sources of de novo apoA-I (liver, intestine), and mechanisms of clearance of free apoA-I, it is difficult to draw firm conclusions about the effects of CETP inhibition on preβ HDL formation using an isolated in vitro system.
The fact that dalcetrapib had no effect on preβ HDL formation in the in vitro system has already been attributed to a different mode of action of dalcetrapib against CETP compared with other CETP inhibitors (31); however, the in vivo effects of dalcetrapib on hamster preβ HDL were unknown until now. In vivo, the lack of effect of dalcetrapib on preβ HDL could be attributable to the weaker inhibitory effect of dalcetrapib on CETP (e.g., requiring a greater inhibition of CETP/increase in total HDL-C). Interestingly, in patients, despite inhibition of plasma CETP activity of 50% and a 20–25% increase in HDL-C (32), no significant reduction in LDL-C has been reported with dalcetrapib. To understand this discrepancy would require additional investigation beyond the scope of the current study.
Niesor et al. (33) reported an increase in noncholesterol markers of cholesterol absorption in hamsters and humans treated with dalcetrapib. This effect was hypothesized to be another marker of preβ HDL functionality due to increased efflux of cholesterol from the basolateral side of the intestinal wall through ABCA1 to preβ HDL. Because of this, we examined cholesterol absorption directly using a dual isotope oral versus iv administration method (20, 21). The observation that ANA had no effect on cholesterol absorption is similar to what was reported earlier using more crude methods (13), and supports the notion that CETP inhibition by ANA facilitates the excretion of cholesterol into the feces, rather than affecting its absorption. Under high-fat diet conditions, ANA treatment was associated with increased cholesterol excretion, which also supports this finding. Importantly, fecal bile acids were not measured in this study, and represent a sizable proportion of sterol excretion in addition to cholesterol. Previously we reported that ANA increased fecal cholesterol and fecal bile acids in Castro-Perez et al. (13) under high-fat conditions, so the data in the current study could underestimate the total sterol loss in response to CETP inhibition. In a human study where HDL remodeling, apoA1 kinetics, and fecal sterol excretion were measured in response to CETP inhibition, torcetrapib treatment was not associated with increased sterol excretion during the monitoring period (26). Whether the results of hamster studies where ANA stimulates fecal cholesterol excretion translate to the human condition remains to be tested.
The observation that dalcetrapib increased cholesterol absorption confirms what had been reported using noncholesterol surrogate markers of cholesterol absorption (33). While additional studies would be necessary to determine the mechanism for this increase in cholesterol absorption, it does not seem attributable to an increase in preβ HDL, because no changes in plasma preβ HDL were observed in vivo.
In summary, ANA promotes the formation of preβ HDL in vivo in hamsters. The possibility that ANA might reduce preβ HDL, based upon in vitro methods, was ruled out using definitive 2D gel electrophoresis analysis of preβ HDL from in vivo samples. Furthermore, any contribution of changes in intestinal cholesterol absorption with ANA was also ruled out using direct measurement of absorption of isotopically labeled cholesterol. Therefore, we conclude that ANA promotes preβ HDL levels and functionality, which may have beneficial effects on coronary artery disease, via removal of cholesterol from the artery wall. However, the hypothesis of whether CETP inhibition will improve clinical outcomes in patients with coronary heart disease is currently being tested in the phase III clinical trial REVEAL.
Acknowledgments
The authors would like to thank Dr. Philip Barter (The Heart Institute, University of Sydney, Sydney, Australia) for critical review and feedback during the preparation of this manuscript.
Footnotes
Abbreviations:
- ANA
- anacetrapib
- CETP
- cholesteryl ester transfer protein
- 2D
- two-dimensional
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
REFERENCES
- 1.Davidson M. H., McKenney J. M., Shear C. L., Revkin J. H. 2006. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels. J. Am. Coll. Cardiol. 48: 1774–1781 [DOI] [PubMed] [Google Scholar]
- 2.McKenney J. M., Davidson M. H., Shear C. L., Revkin J. H. 2006. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels on a background of atorvastatin. J. Am. Coll. Cardiol. 48: 1782–1790 [DOI] [PubMed] [Google Scholar]
- 3.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J. P., Komajda M., Lopez-Sendon J., Mosca L., Tardif J., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122 [DOI] [PubMed] [Google Scholar]
- 4.Forrest M. J., Bloomfield D., Briscoe R. J., Brown R. N., Cumiskey A. M., Ehrhart J., Hershey J. C., Keller W. J., Ma X., McPherson H. E., et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154: 1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DePasquale M., Cadelina G., Knight D., Loging W., Winter S., Blasi E., Perry D., Keiser J. 2009. Mechanistic studies of blood pressure in rats treated with a series of cholesteryl ester transfer protein inhibitors. Drug Dev. Res. 70: 35–48 [Google Scholar]
- 6.Hu X., Dietz J. D., Xia C. S., Knight D. R., Loging W. T., Smith A. H., Yuan H. D., Perry D. A., Keiser J. 2009. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 150: 2211–2219 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099 [DOI] [PubMed] [Google Scholar]
- 8.Landmesser U., von Eckardstein A., Kastelein J. J., Deanfield J., Lüscher T. G. 2012. Increasing high-density lipoprotein cholesterol by cholesteryl ester transfer protein-inhibition: a rocky road and lessons learned? The early demise of the dal-HEART programme. Eur. Heart J. 33: 1712–1715 [DOI] [PubMed] [Google Scholar]
- 9.Krishna R., Bergman A. J., Jin B., Fallon M., Cote J., Van Hoydonck P., Laethem T., Gendrano I. N., 3rd, Van Dyck K., Hilliard D., et al. 2008. Multiple-dose pharmacodynamics and pharmacokinetics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Clin. Pharmacol. Ther. 84: 679–683 [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield D., Carlson G. L., Sapre A., Tribble D., McKenney J. M., Littlejohn W. T., 3rd, Sisk C. M., Mitchel Y., Pasternak R. C. 2009. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 157: 352–360 [DOI] [PubMed] [Google Scholar]
- 11.Cannon C. P., Shah S., Dansky H. M., Davidson M., Brinton E. A., Gotto A. M., Stepanavage M., Liu S. X., Gibbons P., Ashraf T. B., et al. 2010. Safety of anacetrapib in patients with or at high risk for coronary orally administered D6-cholesterol heart disease. N. Engl. J. Med. 363: 2406–2415 [DOI] [PubMed] [Google Scholar]
- 12.Yvan-Charvet L., Kling J., Pagler T., Li H., Hubbard B., Fisher T., Sparrow C. P., Taggart A. K., Tall A. R. 2010. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 30: 1430–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro-Perez J., Briand F., Gagen K., Wang S. P., Chen Y., McLaren D. G., Shah V., Vreeken R. U., Hankemeier T., Sulpice T., et al. 2011. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. J. Lipid Res. 52: 1965–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranalletta M., Bierilo K. K., Chen Y., Milot D., Chen Q., Tung E., Houde C., Elowe N. H., Garcia-Calvo M., Porter G., et al. 2010. Biochemical characterization of cholesteryl ester transfer protein inhibitors. J. Lipid Res. 51: 2739–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asztalos B. F., Sloop C. H., Wong L., Roheim P. S. 1993. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apoA-I-containing subpopulations. Biochim. Biophys. Acta. 1169: 291–300 [DOI] [PubMed] [Google Scholar]
- 16.Lassman M. E., McLaughlin T. M., Somers E. P., Stefanni A. C., Chen Z., Murphy B. A., Bierilo K. K., Flattery A. M., Wong K. K., Castro-Perez J. M., et al. 2012. A rapid method for cross-species quantitation of apolipoproteins A1, B48 and B100 in plasma by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 26: 101–108 [DOI] [PubMed] [Google Scholar]
- 17.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissue. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 18.Shah V., Herath K., Previs S. F., Hubbard B. K., Roddy T. P. 2010. Headspace analyses of acetone: a rapid method for measuring the 2H-labeling of water. Anal. Biochem. 404: 235–237 [DOI] [PubMed] [Google Scholar]
- 19.Previs S. F., Mahsut A., Kulick A., Dunn K., Andrews-Kelly G., Johnson C., Bhat G., Herath K., Miller P. L., Wang S. P., et al. 2011. Quantifying cholesterol synthesis in vivo using (2)H(2)O: enabling back-to-back studies in the same subject. J. Lipid Res. 52: 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turley S. D., Herndon M. W., Dietschy J. M. 1994. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J. Lipid Res. 35: 328–339 [PubMed] [Google Scholar]
- 21.Zilversmit D. B., Hughes L. B. 1974. Validation of a dual-isotope plasma ratio method for measurement of cholesterol absorption in rats. J. Lipid Res. 15: 465–473 [PubMed] [Google Scholar]
- 22.Van Heek M., France C. F., Compton D. S., McLeod R. L., Yumibe N. P., Alton K. B., Sybertz E. J., Davis H. R., Jr 1997. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J. Pharmacol. Exp. Ther. 283: 157–163 [PubMed] [Google Scholar]
- 23.van Heek M., Austin T. M., Farley C., Cook J. A., Tetzloff G. G., Davis H. R. 2001. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes. 50: 1330–1335 [DOI] [PubMed] [Google Scholar]
- 24.Barter P. J., Rye K. A. 1996. Molecular mechanisms of reverse cholesterol transport. Curr. Opin. Lipidol. 7: 82–87 [DOI] [PubMed] [Google Scholar]
- 25.Liang H. Q., Rye K. A., Barter P. J. 1994. Dissociation of lipid-free apolipoprotein A-I from high density lipoproteins. J. Lipid Res. 35: 1187–1199 [PubMed] [Google Scholar]
- 26.Brousseau M. E., Diffenderfer M. R., Millar J. S., Nartsupha C., Asztalos B. F., Welty F. K., Wolfe M. L., Rudling M., Björkhem I., Angelin B., et al. 2005. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler. Thromb. Vasc. Biol. 25: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuura F., Wang N., Chen W., Jiang X. C., Tall A. R. 2006. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J. Clin. Invest. 116: 1435–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lie J., de Crom R., Jauhiainen M., van Gent T., van Haperen R., Scheek L., Jansen H., Ehnholm C., van Tol A. 2001. Evaluation of phospholipid transfer protein and cholesteryl ester transfer protein as contributors to the generation of pre beta-high-density lipoproteins. Biochem. J. 360: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lusa S., Jauhiainen M., Metso J., Somerharju P., Ehnholm C. 1996. The mechanism of human plasma phospholipid transfer protein-induced enlargement of high-density lipoprotein particles: evidence for particle fusion. Biochem. J. 313: 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asztalos B. F., Roheim P. S. 1995. Presence and formation of ‘free apolipoprotein A-I-like’ particles in human plasma. Arterioscler. Thromb. Vasc. Biol. 15: 1419–1423 [DOI] [PubMed] [Google Scholar]
- 31.Niesor E. J., Magg C., Ogawa N., Okamoto H., von der Mark E., Matile H., Schmid G., Clerc R. G., Chaput E., Blum-Kaelin D., et al. 2010. Modulating cholesteryl ester transfer protein activity maintains efficient pre-β-HDL formation and increases reverse cholesterol transport. J. Lipid Res. 51: 3443–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein E. A., Roth E. M., Rhyne J. M., Burgess T., Kallend D., Robinson J. G. 2010. Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur. Heart J. 31: 480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niesor E. J., Chaput E., Staempfli A., Blum D., Derks M., Kallend D. 2011. Effect of dalcetrapib, a CETP modulator, on non-cholesterol sterol markers of cholesterol homeostasis in healthy subjects. Atherosclerosis. 219: 761–767 [DOI] [PubMed] [Google Scholar]