Abstract
11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) mediates glucocorticoid activation and is currently considered as therapeutic target to treat metabolic diseases; however, biomarkers to assess its activity in vivo are still lacking. Recent in vitro experiments suggested that human 11β-HSD1 metabolizes the secondary bile acid 7-oxolithocholic acid (7-oxoLCA) to chenodeoxycholic acid (CDCA) and minor amounts of ursodeoxycholic acid (UDCA). Here, we provide evidence from in vitro and in vivo studies for a major role of 11β-HSD1 in the oxidoreduction of 7-oxoLCA and compare its level and metabolism in several species. Hepatic microsomes from liver-specific 11β-HSD1-deficient mice were devoid of 7-oxoLCA oxidoreductase activity. Importantly, circulating and intrahepatic levels of 7-oxoLCA and its taurine conjugate were significantly elevated in mouse models of 11β-HSD1 deficiency. Moreover, comparative enzymology of 11β-HSD1-dependent oxidoreduction of 7-oxoLCA revealed that the guinea-pig enzyme is devoid of 7-oxoLCA oxidoreductase activity. Unlike in other species, 7-oxoLCA and its glycine conjugate are major bile acids in guinea-pigs. In conclusion, the oxidoreduction of 7-oxoLCA and its conjugated metabolites are catalyzed by 11β-HSD1, and the lack of this activity leads to the accumulation of these bile acids in guinea-pigs and 11β-HSD1-deficient mice. Thus, 7-oxoLCA and its conjugates may serve as biomarkers of impaired 11β-HSD1 activity.
Keywords: bile acid, metabolism, species, biomarker, glucocorticoid, metabolic disease
11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1) is a NADPH-dependent enzyme catalyzing the regeneration of cortisol (in humans, higher mammals) and corticosterone (in rodents) from inactive cortisone and 11-dehydrocorticosterone, respectively, thereby controlling the tissue- and cell-specific exposure to active glucocorticoids (1). Locally increased glucocorticoid levels, especially in adipose tissue, have been implicated in the pathogenesis of the metabolic syndrome, characterized by glucose intolerance, insulin resistance, dyslipidemia, and hypertension (2, 3). Evidence from experiments with transgenic mice (knockout or overexpression of 11β-HSD1) and selective inhibitors revealed beneficial effects of 11β-HSD1 inhibition on almost all major parameters of the metabolic syndrome (4–13). Data from human phase II clinical trials supported the results obtained from animal studies (14, 15). Currently, several pharmaceutical companies are developing selective 11β-HSD1 inhibitors for treatment of metabolic diseases, osteoporosis, age-associated cognitive deficiencies, and wound healing (15–17).
Suitable biomarkers to detect impaired 11β-HSD1 function in vivo and monitor inhibition of 11β-HSD1 in preclinical and clinical studies are still not available, and there is a great interest to identify such biomarkers for diagnostic purposes and to assess the efficacy of therapeutic inhibitors. Circulating concentrations of glucocorticoids and their metabolites are not suitable biomarkers because their levels in plasma and urine are a result of combined activities of 11β-HSD1 and 11β-HSD2. Furthermore, an ideal biomarker should be independent of mechanisms of negative feedback regulation, such as the control of circulating glucocorticoid levels by the hypothalamus-pituitary-adrenal (HPA) axis and stress-induced fluctuations (18, 19).
11β-HSD1 has broad substrate specificity and metabolizes, besides 11-ketoglucocorticoids, several oxidized endogenous steroids and sterols (19–25). Performing in vitro experiments, we recently identified a novel role for human 11β-HSD1 in the metabolism of the secondary bile acid 7-oxoLCA (26). We observed that recombinant human 11β-HSD1 preferentially reduced 7-oxoLCA to the 7α-hydroxylated bile acid chenodeoxycholic acid (CDCA) and, to a lesser extent, to the 7β-hydroxylated ursodeoxycholic acid (UDCA) (26). We hypothesized that the metabolism by 11β-HSD1 might be responsible for the very low circulating levels of this secondary bile acid. 7-oxoLCA is formed from CDCA and UDCA in the colon by 7α-hydroxysteroid dehydrogenases from microorganisms such as Escherichia coli, Bacteroides fragilis, and Bacteroides intestinalis (27, 28). Thereafter, 7-oxoLCA is reabsorbed in the intestine and reaches the liver through the enterohepatic cycle.
In the present study, we aimed to i) obtain further in vitro and in vivo evidence for a role of 11β-HSD1 in the oxidoreduction of 7-oxoLCA, ii) assess species-specific differences in the 11β-HSD1-dependent oxidoreduction of 7-oxoLCA, and iii) test our hypothesis that 7-oxoLCA might accumulate in transgenic 11β-HSD1-deficient mice and provide initial evidence that this bile acid might serve as a biomarker for 11β-HSD1 deficiency.
MATERIALS AND METHODS
Chemicals and reagents
CDCA, UDCA, [2,2,4,4-2H4]CDCA (CDCA-d4) (>98% isotopic purity), and [9,11,12,12-2H4]cortisol (cortisol-d4) (>98% isotopic purity) were purchased from Sigma-Aldrich (St. Louis, MO). 7-oxolithocholic acid (7-oxoLCA) was purchased from Steraloids (Newport, RI). 7-oxolithocholyltaurine (7-oxoLC-Tau) and 7-oxolithocholylglycine (7-oxoLC-Gly) were a kind gift from Dr. Alan F. Hofmann (University of California, San Diego, CA). Cell culture media were purchased from Invitrogen (Carlsbad, CA) and Sigma (Buchs, Switzerland). [1,2-3H]cortisone was obtained from American Radiolabeled Chemicals (St. Louis, MO), [1,2,6,7-3H]cortisol from Amersham Pharmacia (Piscataway, NJ), and tricyclo[3.3.1.13,7] dec-1-yl-6,7,8,9-tetrahydro-5H-1,2,4-triazolo[4,3-a]azepine (T0504) from Enamine (Kiev, Ukraine). The construction of expression plasmids has been described earlier (29, 30). Male human and guinea-pig liver microsomes were obtained from Celsis International Ltd. (Brussels, Belgium), and serum samples from 12–16 h fasted adult male mice (balb/c and C57bL/6), rats (Han Wistar and Sprague-Dawley), dog (Canis familiaris, beagle bred), guinea-pigs (Dunkin-Hartley), and hamsters (golden Syrian) were obtained from Harlan (Gannat, France).
Reduction of 7-oxoLCA by recombinant 11β-HSD1 from various species
To assess species-dependent differences in the 11β-HSD1-dependent metabolism of 7-oxoLCA, we transiently transfected HEK-293 (human embryonic kidney-293) cells [cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 units/ml penicillin, 50 μg/ml streptomycin and 2 mM glutamine] with plasmids coding for human, rat, mouse, hamster, canine, and guinea-pig 11β-HSD1 as described previously (23, 29, 30). At 48 h posttransfection (jetPRIME, Polyplus transfection, Illkirch, France) cells were detached and centrifuged, and pellets were stored at −80°C. Cell pellets were resuspended in TS2 buffer (100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2, 250 mM sucrose, and 20 mM Tris/HCl, pH 7.4), sonicated, and used immediately to measure enzyme activity.
To determine apparent Km and Vmax values, lysates were incubated for 10 min at 37°C in a total volume of 20 μl containing 500 μM NADPH and 7-oxoLCA at concentrations between 62.5 nM and 4 μM. To determine the relative content of product formed, lysates at a high total protein concentration (to achieve almost complete substrate conversion) were incubated with 1 µM of 7-oxoLCA for 10 min. Reactions were terminated by adding 80 µl of acetonitrile containing 100 nM of CDCA-d4. Concomitantly, lysates were used in parallel experiments to assess the conversion of cortisone to cortisol. Reaction products were directly measured by mass spectrometry as described previously (26, 31). The rate of all reactions was kept below 25% of substrate conversion, with the exception of the comparison of product formation from 7-oxoLCA shown in Fig. 1A.
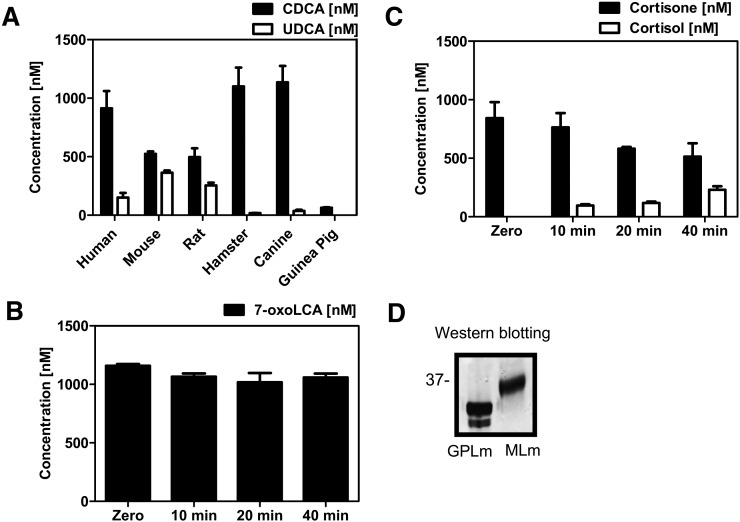
Fig. 1.
Enzymatic activity of recombinant 11β-HSD1 from various species was determined in lysates of transfected HEK-293 cells as described in Materials and Methods. (A) Stereoselective oxidoreduction of 7-oxoLCA (1 µM) to CDCA (black bars) and UDCA (white bars) by 11β-HSD1 from various species after incubation for 10 min at 37°C. (B) Guinea-pig liver microsomes were incubated for 40 min with 7-oxoLCA (1 μM) and glucose-6-phosphate (1 mM). Bile acids were extracted and quantitated by LC-MS/MS. Unconverted 7-oxoLCA is represented by black bars. Data (n = 3) represent mean ± SD. (C) Oxidoreduction of cortisone by guinea-pig liver microsomes. (D) Western blotting against 11β-HSD1 in guinea-pig (GPLm) and mouse liver microsomes (MLm).
The expression of recombinant C-terminally FLAG-tagged 11β-HSD1 in different transfection experiments was determined semiquantitatively by immunoblotting. Protein concentrations were determined by the bicinchoninic acid (BCA) method. An amount of 25 µg of total protein was resolved on 12% Bis-Tris gels (NuPage®, Invitrogen) using 1X MES as buffer (Invitrogen, NuPAGE® MES SDS Running Buffer) and then transferred to nitrocellulose membranes (iBlot®, Invitrogen). Thereafter, membranes were blocked with Odyssey® blocking buffer (LI-COR, Biosciences, Lincoln, NE) overnight at 4°C. Immunoreactions were carried out with primary anti-FLAG antibody M2 (Invitrogen) to detect the recombinant enzyme and with secondary goat anti-mouse Alexa Fluor® 790 antibody, respectively (Invitrogen). After immunoreaction of FLAG-tagged enzymes, the membranes were stripped and incubated with an antibody against β-actin (Sigma). All detection and quantification reactions were performed using a LI-COR Odyssey infrared imaging system (LI-COR, Biosciences, Lincoln, NE).
Impact of bile acids on the interconversion of glucocorticoids by 11β-HSD1 of six species
The inhibitory impact of bile acids on the interconversion of glucocorticoids by 11β-HSD1 of six species was performed as described previously (26). Briefly, lysates were incubated for 10 min at 37°C in a total volume of 22 μl containing 200 nM and 10 nCi of [1,2-3H]cortisone or [1,2,6,7-3H]cortisol and 500 μM cofactor NADPH or NADP+, respectively, and vehicle or various concentrations of bile acids. Following the interconversion of radiolabeled glucocorticoids and termination of reactions by adding methanol containing 2 mM of unlabeled cortisone and cortisol, 15 μl were spotted on Polygram SIL G-25 UV254 silica plates (Macherey-Nagel, Oensingen, Switzerland). Plates were dried, and cortisone and cortisol were resolved using a solvent system of 9:1 (v/v) chloroform/methanol. The separated steroids were analyzed by scintillation counting.
Calculation of enzyme kinetic parameters
Enzyme kinetics was analyzed by nonlinear regression using four-parameter logistic curve fitting. For statistical comparisons, the ratio t-test in GraphPad Prism 5 software was used. Results (mean ± SD) were obtained from at least three independent experiments. For calculation of Vmax, the expression level of the FLAG-tagged enzyme was normalized to the expression signal of the internal control β-actin.
Microsomal preparations and activity assays with hepatic microsomes
Microsomes from livers of wild-type and liver-specific 11β-HSD1-knockout mice were prepared as described earlier (32). The quality of microsomal preparations was validated by using a kit to measure cytochrome c reductase activity (Sigma, Saint Louis, MO). 11β-HSD1 reductase activity was determined by incubation of microsomes (0.05 mg/ml) for 60 min at 37°C in a total volume of 25 μl containing TS2 buffer, 500 μM NADPH or 1 mM glucose-6-phosphate (G6P), 1 μM 7-oxoLCA or 1 µM cortisone, and vehicle or 5 μM of the 11β-HSD1 inhibitor T0504 as indicated. Substrates and inhibitors were diluted from 10 mM stock solutions in DMSO or methanol. The final solvent concentration in all reactions was kept below 0.2%. Reactions were started by adding microsomes into freshly prepared reaction mixture and stopped by adding 500 µl of acetonitrile containing CDCA-d4 and cortisol-d4 at a concentration of 100 nM as internal standards for LC-MS/MS analysis. Thereafter, the organic phase was evaporated to dryness, and samples were reconstituted in 50% methanol/water solution, followed by injection into the LC-MS/MS instrument.
Animal experimentation
Animal studies were conducted under Home Office license and following approval of the Joint Ethics and Research Governance Committee of the University of Birmingham (Birmingham, United Kingdom) in accordance with the UK Animals (Scientific Procedures) Act, 1986. Mice were kept in a climate-controlled facility, housed under standard conditions on a 12 h light/dark cycle, and fed ad libitum with standard chow and free access to drinking water.
To assess the impact of 11β-HSD1 on the circulating and intrahepatic levels of 7-oxoLCA and its conjugated metabolites, 15-week-old wild-type mice (n = 16), 11β-HSD1 global KO (n = 8), and 11β-HSD1 liver-specific KO (n = 16), previously described in Ref. 33, were fasted overnight, and blood samples were collected by intracardiac puncture. Plasma was prepared immediately by centrifugation, and samples were stored at −80°C until further processing.
Quantification of 7-oxoLCA and its taurine and glycine conjugates by LC-MS/MS
Extraction and quantification of bile acids in plasma samples was performed essentially as described previously (31). The extraction of bile acids from liver tissue was performed by homogenizing 100 mg of tissue in 200 µl 50% methanol. Samples were spiked with 300 µl of deuterium labeled bile acids as internal standards (CDCA-d4) at a final concentration of 1 µM in acetonitrile, followed by protein precipitation by adding 1.5 ml of alkaline (5% NH4OH) ice-cold acetonitrile. Thereafter, samples were mixed continuously for 1 h and centrifuged at 11,000 g for 10 min. The supernatant was transferred to a new tube, the solvent was evaporated, and the residue was reconstituted in 100 µl of 50% methanol, followed by an additional centrifugation step to remove insoluble particles. The method was qualified on the basis of extraction efficiency, intraday accuracy, and precision for 7-oxoLCA, 7-oxoLC-Tau, and 7-oxoLC-Gly (data not shown). The method presented acceptable extraction efficiency, accuracy, and precision for the bile acids studied.
Statistical analysis
Data are presented as mean ± SD. Statistical significance was assessed by Student t-test. P ≤ 0.05 was considered significant.
Docking studies
The ligands were drawn using ChemBioDraw Ultra 12.0 and the 2D structures converted into 3D structures using ChemBio3D Ultra 12.0 (1986–2010 CambridgeSoft). The docking studies were performed using GOLD (34, 35), which uses a genetic algorithm to predict binding modes for small molecules in a protein binding site. The crystal structures for human and guinea pig 11β-HSD1 were downloaded from the Protein Data Bank (PDB, www.pdb.org) (36). PDB-entry 2BEL, chain A (37), was chosen for human protein, and 3LZ6, chain A (38), for guinea-pig protein. For human 11β-HSD1, the binding site was defined as an 8 Å sphere, centered with hydroxyl-oxygen of Ser170 (x: 3.84, y: 22.49, z: 13.34). For the guinea-pig protein, the binding site was defined as an 8 Å sphere, centered by hydroxyl-oxygen of Tyr158 (x: 13.21, y: 22.34, z: 45.45). For both enzymes, GoldScore was used as a scoring function, and the program was set to define the atom types for proteins and ligands automatically. The proteins were kept rigid and ligands flexible during the docking run. To give the steroidal ligands more flexibility, the program was set to flip ring corners while searching possible binding orientations for the ligands. For each ligand, a maximum of ten binding orientations were generated, but in case the three best-ranked solutions were within RMSD of 1 Å of each other, the program was allowed to terminate the run earlier. Using these settings, the program successfully reproduced the binding orientations of the co-crystallized ligands carbenoxolone (2bel) and N-adamantan-2-yl-1-ethyl-D-prolinamide (3lz6), thus validating the docking settings.
RESULTS
Comparison of the oxidoreduction of 7-oxoLCA by 11β-HSD1 from six species
We previously showed that human 11β-HSD1 preferentially converts 7-oxoLCA to CDCA (26). Evidence from studies using rat liver microsomes indicated the formation of both CDCA and UDCA from 7-oxoLCA. In the present study, we investigated whether these observations are due to species-specific differences in the stereoselective product formation by 11β-HSD1 or whether additional enzymes might be involved in the carbonyl reduction of 7-oxoLCA in rats and mice. Recombinant 11β-HSD1 from six species were expressed in HEK-293 cells, and the carbonyl reduction of 7-oxoLCA was determined by incubation of lysates at high total protein concentration with 1 µM of 7-oxoLCA for 10 min to convert most of the substrate and to assess the relative composition of products formed. Human 11β-HSD1 preferentially formed CDCA with minor amounts of UDCA as reported earlier (26). Canine and hamster 11β-HSD1 almost completely converted the substrate and presented similar stereoselectivity to human 11β-HSD1, thus mainly producing the 7α-hydroxylated bile acid CDCA, with minor amounts of the 7β-hydroxylated UDCA. Rat and mouse 11β-HSD1 also almost completely converted the substrate and reduced 7-oxoLCA to significant amounts of both CDCA and UDCA (Fig. 1A). Surprisingly, we could detect only background activity of 7-oxoLCA oxidoreduction when using HEK-293 cell lysates expressing recombinant guinea-pig 11β-HSD1 or when using guinea-pig liver microsomes (Fig. 1A, B). Under the same conditions, cortisone was efficiently reduced by recombinant guinea-pig 11β-HSD1 (Fig. 1C, Table 1), with an activity at 4 µM cortisone that was about two times higher than that of the human enzyme, in line with an earlier report on kinetic parameters for glucocorticoids as substrates of 11β-HSD1 (29). On the other hand, 11β-HSD1 from human, mouse, rat, hamster, and dog efficiently converted 7-oxoLCA (Table 1) with Km values ranging 1.2–5.0 µM and Vmax values ranging 24–107 nmol × mg−1 × h−1. None of the 11β-HSD1 enzymes was able to catalyze the conversion of either CDCA or UDCA to 7-oxoLCA at pH 7.4 and in the presence of NADP+, suggesting that exclusively the reduction is catalyzed under physiological conditions.
TABLE 1.
Comparison of the oxidoreduction of 7-oxoLCA and cortisone by recombinant 11β-HSD1 from six species
| Species | Oxidoreduction of 7-oxoLCA |
Oxidoreduction of Cortisone | |
| Km [µM] | Vmax [nmol × h−1 × mg−1] | Activity at 4 µM [nmol × h−1 × mg−1] | |
| Canine | 2.0 ± 0.4 | 52 ± 5 | 22 ± 5 |
| Guinea-pig | — | — | 22 ± 4 |
| Hamster | 4.2 ± 0.7 | 107 ± 11 | 27 ± 7 |
| Human | 1.2 ± 0.3 | 26 ± 2 | 16 ± 1 |
| Mouse | 2.0 ± 0.3 | 24 ± 2 | 10 ± 2 |
| Rat | 5.0 ± 0.5 | 86 ± 6 | 29 ± 8 |
The results are mean ± SD (n = 3).
Inhibition of the 11β-HSD1-dependent interconversion of glucocorticoids by bile acids
Next, we investigated whether 7-oxoLCA, CDCA, or UDCA can inhibit 11β-HSD1 reductase and dehydrogenase activity, respectively, and assessed species-specific differences. A preferential inhibition of the reductase reaction was observed when using 7-oxoLCA, except for the guinea-pig enzyme, which showed weak inhibition with all three bile acids analyzed, independent of whether reduction or oxidation was measured (Table 2). The IC50 values obtained for both reductase and dehydrogenase activity did not reflect the ability of a given bile acid to serve as a substrate/product. Human and hamster 11β-HSD1 showed lowest IC50 values for 7-oxoLCA on cortisone reduction, while rat, mouse, and canine 11β-HSD1 reductase activity was equally well inhibited by 7-oxoLCA and CDCA and less efficiently by UDCA. Interestingly, although CDCA potently inhibited cortisone reduction by 11β-HSD1, there was almost no end-product inhibition of the reduction of 7-oxoLCA, and this bile acid was almost completely reduced by 11β-HSD1 (Fig. 1A). 11β-HSD1 dehydrogenase activity of all species, except guinea-pig, was most potently inhibited by CDCA, with weaker effects by 7-oxoLCA and UDCA.
TABLE 2.
Impact of bile acids on the interconversion of glucocorticoids by 11β-HSD1 from six species
| 11β-HSD1 Species | Reductase Activity IC50 (µM) | Dehydrogenase Activity IC50 (µM) | |
| Human | 7-oxoLCA | 1.1 ± 0.3 | 2.8 ± 0.8 |
| UDCA | 6.9 ± 1.2 | 2.3 ± 1.0 | |
| CDCA | 4.1 ± 0.6 | 0.3 ± 0.1 | |
| Rat | 7-oxoLCA | 2.4 ± 0.9 | 11 ± 3 |
| UDCA | 13 ± 3 | 7 ± 3 | |
| CDCA | 0.7 ± 0.1 | 0.7 ± 0.3 | |
| Mouse | 7-oxoLCA | 3.5 ± 0.6 | 12 ± 3 |
| UDCA | 32 ± 9 | 18 ± 3 | |
| CDCA | 2.3 ± 0.4 | 1.6 ± 0.1 | |
| Hamster | 7-oxoLCA | 2.3 ± 0.9 | 29 ± 7 |
| UDCA | 21 ± 8 | 13 ± 4 | |
| CDCA | 44 ± 14 | 5 ± 1 | |
| Canine | 7-oxoLCA | 0.9 ± 0.2 | 16 ± 3 |
| UDCA | 26 ± 5 | 18 ± 3 | |
| CDCA | 1 ± 0.1 | 0.9 ± 0.2 | |
| Guinea pig | 7-oxoLCA | 15 ± 4 | 11 ± 20 |
| UDCA | 55 ± 15 | 39 ± 18 | |
| CDCA | 22 ± 4 | 12 ± 2 |
The results are mean ± SD (n = 3).
Relative contribution of 11β-HSD1 to the oxidoreduction of 7-oxoLCA
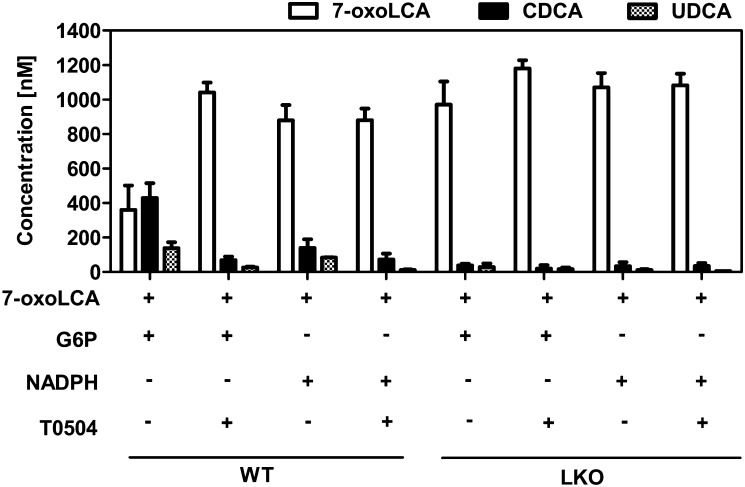
To clarify whether the oxidoreduction of 7-oxoLCA is mainly catalyzed by 11β-HSD1 or whether other enzymes are involved, we performed experiments with microsomes prepared from livers of wild-type and liver-specific 11β-HSD1-knockout mice (33). To distinguish between NADPH-dependent enzymes that are oriented to the cytosol, including cytochrome P450 enzymes, aldoketoreductases, and short-chain dehydrogenase/reductase enzymes, and enzymes that are facing the endoplasmic reticulum (ER) lumen, such as 11β-HSD1, we compared activities in the presence of NADPH (for cytoplasmic enzymes) or G6P (for luminal enzymes). In the ER lumen, NADPH is regenerated by hexose-6-phosphate dehydrogenase (H6PDH), which is primarily dependent on G6P under physiological conditions (39). Thus, intact liver microsomes, where the luminal compartment is protected by the ER membrane, contain an endogenous NADPH-regenerating system (40). After 60 min of incubation of liver microsomes from wild-type mice with G6P, approximately 60% of initially supplied 7-oxoLCA was converted to about two times more CDCA than UDCA (Fig. 2). The reaction was almost completely inhibited by the selective 11β-HSD1 inhibitor T0504 (also known as Merck-544) (41). When NADPH was added to the reaction mixture, we detected only traces of CDCA and UDCA, which were abolished by T0504, suggesting that this activity was due to a small fraction of microsomes with inverted orientation. Importantly, in experiments using liver microsomes from liver-specific 11β-HSD1-knockout mice, no conversion of 7-oxoLCA could be detected, regardless of whether G6P or NADPH was supplied to the reaction mixture. As control, we measured cytochrome c reductase activity in microsomes from livers of wild-type and liver-specific 11β-HSD1-deficient mice and found no differences between the two preparations, indicating that the preparations were qualitatively comparable (data not shown).
Fig. 2.
Microsomes from livers of wild-type or liver-specific 11β-HSD1 knockout (LKO) mice were incubated for 60 min with 7-oxoLCA (1 µM), hexose-6-phosphate (1 mM G6P) or NAPDH (1 mM), and 11β-HSD1 inhibitor T0504 (5 µM) as indicated. 7-oxoLCA is presented by white bars; CDCA and UDCA are presented by black and hatched bars, respectively. Data (n = 3) represent mean ± SD. Reactions were terminated with acetonitrile, and then extracted and measured by LC-MS/MS.
Comparison of circulating concentrations of 7-oxoLCA and its conjugates in serum from various species
Because 7-oxoLCA oxidoreductase activity was absent in guinea-pig liver microsomes and in lysates of HEK-293 cells expressing recombinant guinea-pig 11β-HSD1, but it was present in all other species tested, we hypothesized that 7-oxoLCA and its conjugated metabolites accumulate in the serum of guinea-pigs but not in the other species. Therefore, we determined the concentrations of 7-oxoLCA, 7-oxoLC-Tau, and 7-oxoLC-Gly in the serum of various species, including guinea-pig (Dunkin-Hartley), human, mouse (balb/c and C57bL/6), rat (Han Wistar and Sprague-Dawley), canine (Canis familiaris, beagle bred), and hamster (golden Syrian). We observed very high levels of 7-oxoLCA and its glycine conjugate in guinea-pig serum (Table 3). In contrast, in all other species that were investigated and that express 7-oxoLCA oxidoreductase activity, the circulating levels of 7-oxoLCA and its conjugates were very low. Taurine conjugation plays a minor role in guinea-pigs, and 7-oxoLC-Tau levels were negligible in this species.
TABLE 3.
Concentrations of 7-oxoLCA and its conjugates in the serum from several species
| Dog | Guinea-pig | Hamster | Human | Mouse (Balb/c) | Mouse (C57BL/6) | Rat (Han Wistar) | Rat (Sprague-Dawley) | |
| 7-oxoLCA | 41 ± 83 | 13593 ± 5482 | 11 ± 9 | 3 ± 1 | 20 ± 12 | 6 ± 4 | 4 ± 5 | 22 ± 28 |
| 7-oxoLC-Tau | 9 ± 11 | 3 ± 2 | 3 ± 1 | 1 ± 0.1 | 8 ± 2 | 32 ± 14 | 2 ± 0.2 | 4 ± 1 |
| 7-oxoLC-Gly | 1 ± 0.2 | 5481 ± 3557 | 1 ± 1 | 1 ± 0.1 | 5 ± 3 | ND | ND | 1 ± 2 |
The results are expressed in nM as mean ± SD (n = 8). Underlined values represent below lower limit of quantification. ND, not detected.
Impact of 11β-HSD1 disruption on circulating levels of 7-oxoLCA and its conjugates in mice
We hypothesized that 11β-HSD1 deficiency and, therefore, the inability to reduce 7-oxoLCA, leads to elevated circulating levels of 7-oxoLCA and its conjugated metabolites. To test this hypothesis, we measured the concentrations of 7-oxoLCA and its taurine and glycine conjugates in serum and in liver tissue of liver-specific and global 11β-HSD1-deficient mice (Table 4). The glycine conjugation plays a minor role in mice, in contrast to guinea-pigs, and concentrations of 7-oxoLC-Gly were very low in serum and liver tissue of liver-specific 11β-HSD1-knockout and wild-type mice. In liver tissue of liver-specific knockout and global knockout mice, 7-oxoLCA levels were approximately two times higher, although the differences did not reach statistical significance. In contrast, intrahepatic 7-oxoLC-Tau was 7-fold and 4-fold higher in liver-specific 11β-HSD1-deficient mice and in global 11β-HSD1-deficient mice compared with wild-type controls. As expected, 7-oxoLC-Tau levels were generally higher than 7-oxoLCA levels in liver tissue. Circulating levels of 7-oxoLC-Tau were somewhat higher than free 7-oxoLCA levels. Importantly, 7-oxoLCA concentrations significantly increased (6-fold) in serum of liver-specific 11β-HSD1-deficient mice and further increased (24-fold) in global-knockout mice. Circulating 7-oxoLC-Tau levels were 40-fold higher in liver-specific 11β-HSD1-knockout mice compared with wild-type mice, and they were even somewhat lower in serum of global-knockout mice (20-fold higher than wild-type mice).
TABLE 4.
Circulating and intrahepatic concentrations of 7-oxoLCA and its conjugates in mouse models of 11β-HSD1 deficiency
| Circulation (nM) |
Intrahepatic (fmol/mg) |
|||||
| WT | LKO | KO | WT | LKO | KO | |
| 7-oxoLCA | 15 ± 11 | 91 ± 152** | 360 ± 529*** | 15 ± 10 | 33 ± 34 | 27 ± 21 |
| 7-oxoLC-Tau | 22 ± 19 | 875 ± 1210* | 407 ± 392*** | 200 ± 188 | 1458 ± 1705*** | 874 ± 945*** |
| 7-oxoLC-Gly | 14 ± 14 | 4 ± 6 | 18 ± 16 | 8 ± 8 | 10 ± 9 | 2 ± 1 |
The results are expressed in nM as mean ± SD. Wild-type (WT) (n = 24), liver-specific 11β-HSD1-deficient mice (LKO) (n = 16) and global 11β-HSD1-deficient mice (KO) (n = 8). Data represent mean ± SD. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
DISCUSSION
Recently, we reported that 7-oxoLCA and its taurine and glycine conjugates are substrates of human 11β-HSD1 and that they are efficiently converted to the 7α-hydroxylated CDCA and, to a lesser extent, to the 7β-hydroxylated UDCA (26). At physiological pH, 11β-HSD1 exclusively catalyzed the reduction of 7-oxoLCA, and neither CDCA nor UDCA served as substrate. In the present study, we extended these findings by comparing 11β-HSD1 from six species and observed remarkable species-specific differences. The catalytic activities of hamster and canine 11β-HSD1 resembled those of the human enzyme. These two species preferentially reduced 7-oxoLCA to CDCA, suggesting that they are suitable animal models to study potential physiological and toxicological consequences of 7-oxoLCA accumulation due to 11β-HSD1 deficiency. In contrast, rat and mouse 11β-HSD1 were not stereoselective and produced substantial amounts of both 7α- and 7β-hydroxylated forms. Interestingly, for the substrate 7-oxocholesterol, the human, rat, and mouse enzymes generated 7β-hydroxycholesterol exclusively, whereas the hamster enzyme was not stereoselective and formed both 7α- and 7β-hydroxycholesterol (23).
Importantly, recombinant guinea-pig 11β-HSD1 and guinea-pig liver microsomes were unable to reduce 7-oxoLCA, providing an explanation for the very high levels of 7-oxoLCA and 7-oxoLC-Gly in the circulation of guinea-pigs compared with the other species investigated (Table 3). Our findings are in line with an earlier study suggesting that 7-oxoLCA is a primary bile acid in this species, accounting for 30% of the total bile acids contained in bile (42). Moreover, the high IC50 values of 7-oxoLCA, CDCA, and UDCA for 11β-HSD1-dependent cortisone reduction (Table 2) suggest that the binding of these bile acids is not optimally stabilized in the substrate pocket of the guinea-pig enzyme.
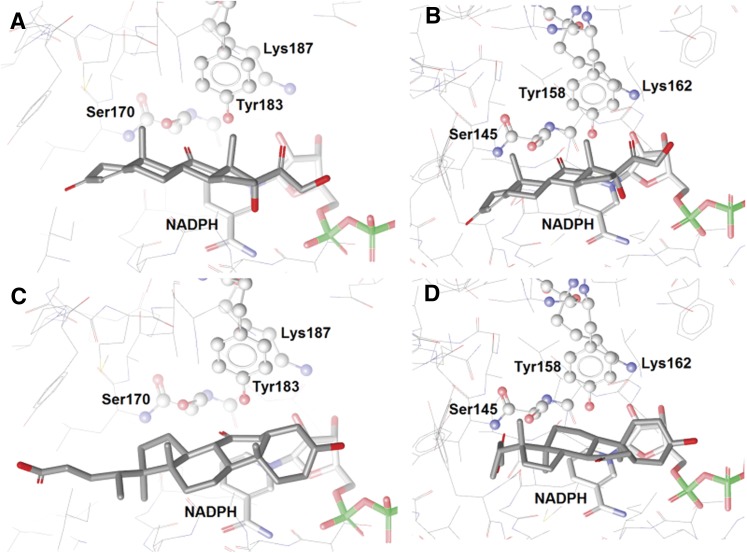
The comparison of the superimposed 3D structures of human and guinea-pig 11β-HSD1 alone did not explain the species differences in 7-oxoLCA metabolism (see supplementary Fig. I for alignment data). Therefore, 7-oxoLCA and cortisone were docked to human and guinea-pig 11β-HSD1. In the reduction reaction, the hydrogen atoms are transferred to the ligand from the catalytic amino acid Tyr and the cofactor NADPH (43, 44). Therefore, the ligand needs to be oriented such that the carbonyl group is located in the vicinity of the catalytic Tyr and the cofactor. This is the case for cortisone, which binds similarly in human and guinea-pig 11β-HSD1 (Fig. 3A, B). However, 7-oxoLCA has different binding orientations in human and guinea-pig 11β-HSD1. In the human enzyme, as reported earlier (26), 7-oxoLCA binds in an inverted orientation with its A-ring aligning with the D-ring of cortisone and with the carbonyl group in position 7 in close proximity to the catalytic center, like the carbonyl group in position 11 of cortisone (Fig. 3C). In contrast, a flipped binding mode of 7-oxoLCA is predicted for the guinea-pig enzyme, where the carbonyl group points away from the catalytic amino acids (Fig. 3D). This flipped binding orientation may be explained by steric reasons: 7-oxoLCA fits to the binding pocket, but because of the bulky side-chain in position 17 of the steroid backbone, the binding mode is unfavorable for the reduction reaction. However, when binding to the enzyme, 7-oxoLCA prevents other ligands from binding, explaining its action as a weak competitive inhibitor. These docking results support our biological findings that cortisone is metabolized by both human and guinea-pig 11β-HSD1 but that 7-oxoLCA is not reduced by the guinea-pig enzyme.
Fig. 3.
The predicted binding orientations of cortisone in (A) human and (B) guinea-pig and 7-oxoLCA in (C) human and (D) guinea-pig in the 11β-HSD binding sites. The catalytic triad Ser-Tyr-Lys is highlighted in ball-and-stick style, and the cofactor NADPH, in bold sticks. The ligands are represented in gray.
Our data clearly show that guinea-pig 11β-HSD1 is able to reduce cortisone. Thus, glucocorticoid activation is similarly catalyzed in guinea-pigs and the other species investigated. 11β-HSD1 is a highly efficient enzyme, and upon pharmacological application, cortisone and prednisone are almost completely converted to cortisol and prednisolone in the liver. Thus, the lack of the reduction of 7-oxoLCA (produced by intestinal microorganisms) by 11β-HSD1 in the liver may be responsible, at least in part, for the high circulating levels in this species. Because 11β-HSD1 seems to be the only enzyme responsible for the reduction of 7-oxoLCA, we hypothesized that this bile acid and its conjugates would accumulate in 11β-HSD1-deficient mice.
Analysis of the 7-oxoLCA levels in liver tissue indicated a trend to increase (approximately 2-fold) in liver-specific and global 11β-HSD1-knockout mice. Serum levels, however, were significantly increased 6-fold and 24-fold. Thus, serum 7-oxoLCA is a better biomarker for 11β-HSD1 deficiency than is intrahepatic 7-oxoLCA. Importantly, a significant increase in both hepatic and circulating levels of 7-oxoLC-Tau was obtained with a more pronounced increase in serum (40-fold and 20-fold) compared with liver tissue (7-fold and 4-fold). The reason why 7-oxoLC-Tau is higher in liver-specific compared with global knockout mice remains unclear but may be due to compensatory mechanisms or differences in gut microbiota. In liver-specific and global 11β-HSD1-knockout mice, unlike in guinea-pigs, taurine conjugation of 7-oxoLCA is the main route of its elimination in mice and glycine conjugation can be neglected. Based on these findings, we propose that 7-oxoLCA and 7-oxoLC-Tau, as well as the sum of these two bile acids, may represent suitable biomarkers of 11β-HSD1 deficiency.
Biomarkers for impaired 11β-HSD1 activity are of great interest for clinical researchers as well as for the pharmaceutical industry to assess the in vivo efficacy of therapeutic inhibitors. Despite the fact that their efficiency can also be assessed through traditional and indirect clinical markers, such as fasting plasma glucose and lipid profiles, biomarkers for direct assessment of 11β-HSD1 inhibition in preclinical and clinical studies are still unavailable. It has been reported that 11β-HSD1-deficient mice have slightly elevated plasma levels of corticosterone and adrenocorticotropic hormone (ACTH); however, these changes seem to be strain-dependent (45, 46). Interestingly, circulating corticosterone levels were not or were only mildly elevated in global 11β-HSD1-knockout, liver-specific 11β-HSD1-knockout, H6PDH-knockout, and 11β-HSD1/H6PDH double-knockout mice when compared with wild-type mice (33, 47–49). Therefore, it is reasonable to assume that glucocorticoid levels are not substantially altered after a therapeutic intervention with 11β-HSD1 inhibitors, thus excluding the applicability of glucocorticoid levels as a biomarker of 11β-HSD1 deficiency or enzyme inhibition. Likewise, urinary levels of 11-dehydrocorticosterone (11-DHC), corticosterone, and their tetrahydro metabolites are also of limited value as a measure of hepatic inhibition because they were not significantly changed in liver-specific 11β-HSD1 knockouts. Similarly, deletion of one allele in heterozygous 11β-HSD1-knockout mice, a scenario similar to 50% enzyme inhibition, is insufficient to elicit a significant change in urinary corticosteroid metabolites compared with wild-type controls (33, 47). H6PDH-knockout, 11β-HSD1-knockout, and 11β-HSD1/H6PDH double-knockout mice do have altered urinary biomarkers, but this reflects total body loss or 11β-HSD1 reductase activity, and the value of these biomarkers to determine tissue-specific effects remains uncertain. It will be interesting to investigate in a follow-up study whether 7-oxoLCA and 7-oxoLC-Tau levels are elevated in heterozygous 11β-HSD1-deficient mice and in mice treated with selective 11β-HSD1 inhibitors.
Importantly, although 7-oxoLCA and 7-oxoLC-Tau are substrates of 11β-HSD1, they are independent of the HPA-axis feedback regulation, and they are not involved in adaptive metabolic changes. Further, they are devoid of overt toxicity, yet they have been shown to reduce the biliary lithogenic index (50). Moreover, 7-oxoLCA and 7-oxoLC-Tau are not ligands for the vitamin D receptor (VDR) (51), and they do not activate farnesoid X receptor (FXR) to the same extent as do CDCA and UDCA (data not shown). Thus, it is unlikely that these bile acids influence the activity of these nuclear receptors. Due to the fact that, in humans, circulating glycine-conjugated bile acids are more abundant than taurine-conjugated metabolites (52), we propose that 7-oxoLCA, 7-oxoLC-Gly, and the sum of these two bile acids may be used as biomarkers for impaired 11β-HSD1 function. In guinea-pigs, as in humans, circulating glycine-conjugated bile acids are more abundant than taurine-conjugated metabolites. Only traces of 7-oxoLC-Tau were detected in guinea-pig serum, whereas the concentration of 7-oxoLC-Gly was approximately 5.5 µM (Table 2). These findings are in line with observations by other investigators (53). Similar to humans and guinea-pigs, glycine conjugation seems to prevail in hamsters (54), whereas in rodents and canines, taurine conjugation prevails and only minor amounts of glycine conjugated bile acids were detected, in line with observations by other investigators (52, 55). Nevertheless, the applicability of 7-oxoLCA and its conjugates as potential biomarkers of 11β-HSD1 deficiency and inhibition requires further investigation in vivo using specific 11β-HSD1 inhibitors or in clinical situations, such as patients suffering from cortisone reductase deficiency.
CONCLUSIONS
Our results provide further evidence for an exclusive role of 11β-HSD1 in the reduction of 7-oxoLCA, which is generated by intestinal microorganisms, reaches the liver after entering the enterohepatic cycle, and is rapidly metabolized to CDCA and UDCA. Unlike glucocorticoids, the substrate 7-oxoLCA and particularly its taurine conjugate were markedly elevated in the serum of liver-specific 11β-HSD1-deficient mice. Because 7-oxo and 7-oxoLC-Tau are not under the control of the HPA axis and they do not act through classical bile acid receptors, their concentrations do not seem to be regulated by feedback mechanisms, and they may be used as biomarkers to detect impaired 11β-HSD1 activity in clinically relevant situations. Future studies are needed to validate the use of these bile acids as biomarkers for reduced 11β-HSD1 activity, for example, in animal models and patients treated with 11β-HSD1 inhibitors and in patients with cortisone reductase deficiency.
Supplementary Material
Acknowledgments
The authors thank Dr. Alan F. Hofmann (University of California, San Diego) for providing 7-oxoLC-Tau and 7-oxoLC-Gly, and Drs. François Pognan, Armin Wolf, and Heiko Schadt, Novartis AG, Basel, for critical comments.
Footnotes
Abbreviations:
- 11-DHC
- 11-dehydrocorticosterone
- 11β-HSD
- 11β-hydroxysteroid dehydrogenase
- 7-oxoLCA
- 7-oxolithocholic acid
- 7-oxoLC-Gly
- 7-oxolithocholylglycine
- 7-oxoLC-Tau
- 7-oxolithocholyltaurine
- BCA
- bicinchoninic acid
- CDCA
- chenodeoxycholic acid
- CDCA-d4
- [2,2,4,4-2H4]-CDCA
- cortisol-d4
- [9,11,12,12-2H4]-cortisol
- G6P
- glucose-6-phosphate
- H6PDH
- hexose-6-phosphate dehydrogenase
- HPA
- hypothalamus-pituitary-adrenal
- NNK
- nicotine-derived nitrosamine ketone
- T0504
- tricyclo[3.3.1.13,7]dec-1-yl-6,7,8,9-tetrahydro-5H-1,2,4-triazolo[4,3-a]azepine
- UDCA
- ursodeoxycholic acid
This work was supported by Swiss National Science Foundation Grant 31003A_140961 (A.O.); David Philips Fellowship BB/G023468/1, Biotechnology and Biological Sciences Research Council (BBSRC) (G.L.); Novartis Research Foundation (A.O.); Austrian Academy of Sciences (ÖAW) Grant (A.V.); Young Talents Grants (Nachwuchsförderung), University of Innsbruck (A.V.); and Erika Cremer Habilitation Program, University of Innsbruck (D.S.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.Odermatt A., Atanasov A. G., Balazs Z., Schweizer R. A. S., Nashev L. G., Schuster D., Langer T. 2006. Why is 11β-hydroxysteroid dehydrogenase type 1 facing the endoplasmic reticulum lumen? Physiological relevance of the membrane topology of 11β-HSD1. Mol. Cell. Endocrinol. 248: 15–23 [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson J. W., Stewart P. M. 2007. Modulation of glucocorticoid action and the treatment of type-2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 21: 607–619 [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson J. W., Walker E. A., Bujalska I. J., Draper N., Lavery G. G., Cooper M. S., Hewison M., Stewart P. M. 2004. 11β-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev. 25: 831–866 [DOI] [PubMed] [Google Scholar]
- 4.Kotelevtsev Y., Holmes M. C., Burchell A., Houston P. M., Schmoll D., Jamieson P., Best R., Brown R., Edwards C. R. W., Seckl J. R., et al. 1997. 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl. Acad. Sci. USA. 94: 14924–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morton N. M., Holmes M. C., Fiévet C., Staels B., Tailleux A., Mullins J. J., Seckl J. R. 2001. Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11β-hydroxysteroid dehydrogenase type 1 null mice. J. Biol. Chem. 276: 41293–41300 [DOI] [PubMed] [Google Scholar]
- 6.Morton N. M., Paterson J. M., Masuzaki H., Holmes M. C., Staels B., Fievet C., Walker B. R., Flier J. S., Mullins J. J., Seckl J. R. 2004. Novel adipose tissue–mediated resistance to diet-induced visceral obesity in 11β-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 53: 931–938 [DOI] [PubMed] [Google Scholar]
- 7.Wamil M., Battle J. H., Turban S., Kipari T., Seguret D., de Sousa Peixoto R., Nelson Y. B., Nowakowska D., Ferenbach D., Ramage L., et al. 2011. Novel fat depot-specific mechanisms underlie resistance to visceral obesity and inflammation in 11β-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 60: 1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuzaki H., Paterson J., Shinyama H., Morton N. M., Mullins J. J., Seckl J. R., Flier J. S. 2001. A transgenic model of visceral obesity and the metabolic syndrome. Science. 294: 2166–2170 [DOI] [PubMed] [Google Scholar]
- 9.Masuzaki H., Yamamoto H., Kenyon C. J., Elmquist J. K., Morton N. M., Paterson J. M., Shinyama H., Sharp M. G. F., Fleming S., Mullins J. J., et al. 2003. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J. Clin. Invest. 112: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson J. M., Morton N. M., Fievet C., Kenyon C. J., Holmes M. C., Staels B., Seckl J. R., Mullins J. J. 2004. Metabolic syndrome without obesity: hepatic overexpression of 11β-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc. Natl. Acad. Sci. USA. 101: 7088–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle C. D., Kowalski T. J. 2009. 11β-hydroxysteroid dehydrogenase type 1 inhibitors: a review of recent patents. Expert Opin. Ther. Pat. 19: 801–825 [DOI] [PubMed] [Google Scholar]
- 12.Hughes K. A., Webster S. P., Walker B. R. 2008. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors in type 2 diabetes mellitus and obesity. Expert Opin. Investig. Drugs. 17: 481–496 [DOI] [PubMed] [Google Scholar]
- 13.Hadoke P. W. F., Iqbal J., Walker B. R. 2009. Therapeutic manipulation of glucocorticoid metabolism in cardiovascular disease. Br. J. Pharmacol. 156: 689–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstock J., Banarer S., Fonseca V. A., Inzucchi S. E., Sun W., Yao W., Hollis G., Flores R., Levy R., Williams W. V., et al. 2010. The 11β-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately controlled by metformin monotherapy. Diabetes Care. 33: 1516–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas M. P., Potter B. V. L. 2011. Crystal structures of 11β-hydroxysteroid dehydrogenase type 1 and their use in drug discovery. Future Med. Chem. 3: 367–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiganescu A., Tahrani A. A., Morgan S. A., Otranto M., Desmouliere A., Abrahams L., Hassan-Smith Z., Walker E. A., Rabbitt E. H., Cooper M. S., et al. 2013. 11β-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects. J. Clin. Invest. 123: 3051–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gathercole L. L., Lavery G. G., Morgan S. A., Cooper M. S., Sinclair A. J., Tomlinson J. W., Stewart P. M. 2013. 11β-Hydroxysteroid dehydrogenase 1: translational and therapeutic aspects. Endocr. Rev. 34: 525–555 [DOI] [PubMed] [Google Scholar]
- 18.Harno E., White A. 2010. Will treating diabetes with 11β-HSD1 inhibitors affect the HPA axis? Trends Endocrinol. Metab. 21: 619–627 [DOI] [PubMed] [Google Scholar]
- 19.Odermatt A., Nashev L. G. 2010. The glucocorticoid-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 has broad substrate specificity: Physiological and toxicological considerations. J. Steroid Biochem. Mol. Biol. 119: 1–13 [DOI] [PubMed] [Google Scholar]
- 20.Muller C., Pompon D., Urban P., Morfin R. 2006. Inter-conversion of 7α- and 7β-hydroxy-dehydroepiandrosterone by the human 11β-hydroxysteroid dehydrogenase type 1. J. Steroid Biochem. Mol. Biol. 99: 215–222 [DOI] [PubMed] [Google Scholar]
- 21.Nashev L. G., Chandsawangbhuwana C., Balazs Z., Atanasov A. G., Dick B., Frey F. J., Baker M. E., Odermatt A. 2007. Hexose-6-phosphate dehydrogenase modulates 11β-hydroxysteroid dehydrogenase type 1-dependent metabolism of 7-keto- and 7β-hydroxy-neurosteroids. PLoS ONE. 2: e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hult M., Elleby B., Shafqat N., Svensson S., Rane A., Jörnvall H., Abrahmsen L., Oppermann U. 2004. Human and rodent type 1 11β-hydroxysteroid dehydrogenases are 7β-hydroxycholesterol dehydrogenases involved in oxysterol metabolism. Cell. Mol. Life Sci. 61: 992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweizer R. A. S., Zürcher M., Balazs Z., Dick B., Odermatt A. 2004. Rapid hepatic metabolism of 7-ketocholesterol by 11β-hydroxysteroid dehydrogenase type 1. J. Biol. Chem. 279: 18415–18424 [DOI] [PubMed] [Google Scholar]
- 24.Hennebert O., Pernelle C., Ferroud C., Morfin R. 2007. 7α- and 7β-hydroxy-epiandrosterone as substrates and inhibitors for the human 11β-hydroxysteroid dehydrogenase type 1. J. Steroid Biochem. Mol. Biol. 105: 159–165 [DOI] [PubMed] [Google Scholar]
- 25.Hennebert O., Le Mée S., Pernelle C., Morfin R. 2007. 5α-Androstane-3β,7α,17β-triol and 5α-androstane-3β,7β,17β-triol as substrates for the human 11β-hydroxysteroid dehydrogenase type 1. Steroids. 72: 855–864 [DOI] [PubMed] [Google Scholar]
- 26.Odermatt A., Da Cunha T., Penno C. A., Chandsawangbhuwana C., Reichert C., Wolf A., Dong M., Baker M. E. 2011. Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11beta-hydroxysteroid dehydrogenase 1. Biochem. J. 436: 621–629 [DOI] [PubMed] [Google Scholar]
- 27.Fukiya S., Arata M., Kawashima H., Yoshida D., Kaneko M., Minamida K., Watanabe J., Ogura Y., Uchida K., Itoh K., et al. 2009. Conversion of cholic acid and chenodeoxycholic acid into their 7-oxo derivatives by Bacteroides intestinalis AM-1 isolated from human feces. FEMS Microbiol. Lett. 293: 263–270 [DOI] [PubMed] [Google Scholar]
- 28.Macdonald I. A., Williams C. N., Mahony D. E., Christie W. M. 1975. NAD- and NADP-dependent 7alpha-hydroxysteroid dehydrogenases from bacteroides fragilis. Biochim. Biophys. Acta. 384: 12–24 [DOI] [PubMed] [Google Scholar]
- 29.Arampatzis S., Kadereit B., Schuster D., Balazs Z., Schweizer R. A. S., Frey F. J., Langer T., Odermatt A. 2005. Comparative enzymology of 11β-hydroxysteroid dehydrogenase type 1 from six species. J. Mol. Endocrinol. 35: 89–101 [DOI] [PubMed] [Google Scholar]
- 30.Odermatt A., Arnold P., Stauffer A., Frey B. M., Frey F. J. 1999. The N-terminal anchor sequences of 11β-hydroxysteroid dehydrogenases determine their orientation in the endoplasmic reticulum membrane. J. Biol. Chem. 274: 28762–28770 [DOI] [PubMed] [Google Scholar]
- 31.Penno C. A., Arsenijevic D., Da Cunha T., Kullak-Ublick G. A., Montani J-P., Odermatt A. 2013. Quantification of multiple bile acids in uninephrectomized rats using ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Methods. 5: 1155–1164 [Google Scholar]
- 32.Senesi S., Legeza B., Balázs Z., Csala M., Marcolongo P., Kereszturi É., Szelényi P., Egger C., Fulceri R., Mandl J., et al. 2010. Contribution of fructose-6-phosphate to glucocorticoid activation in the endoplasmic reticulum: possible implication in the metabolic syndrome. Endocrinology. 151: 4830–4839 [DOI] [PubMed] [Google Scholar]
- 33.Lavery G. G., Zielinska A. E., Gathercole L. L., Hughes B., Semjonous N., Guest P., Saqib K., Sherlock M., Reynolds G., Morgan S. A., et al. 2012. Lack of significant metabolic abnormalities in mice with liver-specific disruption of 11β-hydroxysteroid dehydrogenase type 1. Endocrinology. 153: 3236–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdonk M. L., Cole J. C., Hartshorn M. J., Murray C. W., Taylor R. D. 2003. Improved protein-ligand docking using GOLD. Proteins. 52: 609–623 [DOI] [PubMed] [Google Scholar]
- 35.Jones G., Willett P., Glen R. C., Leach A. R., Taylor R. 1997. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 267: 727–748 [DOI] [PubMed] [Google Scholar]
- 36.Berman H. M., Westbrook J., Feng Z., Gililand G., Bhat T. N., Weissig H., Shindyalov I. N., Bourne P. E. 2000. The Protein Data Bank. Nucleic Acids Res. 28: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X., Kavanagh K., Svensson S., Elleby B., Hult M., Von Delft F., Marsden B., Jornvall H., Abrahmsen L., Oppermann U.. The high resolution structures of human, murine and guinea pig 11β-hydroxysteroid dehydrogenase type 1 reveal critical differences in active site architecture. [no journal] Epub ahead of print. 2004;10.2210/pdb2bel/pdb.
- 38.Cheng H., Hoffman J., Le P., Nair S. K., Cripps S., Matthews J., Smith C., Yang M., Kupchinsky S., Dress K., et al. 2010. The development and SAR of pyrrolidine carboxamide 11β-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 20: 2897–2902 [DOI] [PubMed] [Google Scholar]
- 39.Clarke J. L., Mason P. J. 2003. Murine hexose-6-phosphate dehydrogenase: a bifunctional enzyme with broad substrate specificity and 6-phosphogluconolactonase activity. Arch. Biochem. Biophys. 415: 229–234 [DOI] [PubMed] [Google Scholar]
- 40.Meyer A., Vuorinen A., Zielinska A. E., Da Cunha T., Strajhar P., Lavery G. G., Schuster D., Odermatt A. 2013. Carbonyl reduction of triadimefon by human and rodent 11β-hydroxysteroid dehydrogenase 1. Biochem. Pharmacol. 85: 1370–1378 [DOI] [PubMed] [Google Scholar]
- 41.Hermanowski-Vosatka A., Balkovec J. M., Cheng K., Chen H. Y., Hernandez M., Koo G. C., Le Grand C. B., Li Z., Metzger J. M., Mundt S. S., et al. 2005. 11β-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J. Exp. Med. 202: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tint G. S., Xu G. R., Batta A. K., Shefer S., Niemann W., Salen G. 1990. Ursodeoxycholic acid, chenodeoxycholic acid, and 7-ketolithocholic acid are primary bile acids of the guinea pig. J. Lipid Res. 31: 1301–1306 [PubMed] [Google Scholar]
- 43.Oppermann U. C., Filling C., Berndt K. D., Persson B., Benach J., Ladenstein R., Jornvall H. 1997. Active site directed mutagenesis of 3β/17β-hydroxysteroid dehydrogenase establishes differential effects on short-chain dehydrogenase/reductase reactions. Biochemistry. 36: 34–40 [DOI] [PubMed] [Google Scholar]
- 44.Kavanagh K. L., Jornvall H., Persson B., Oppermann U. 2008. Medium- and short-chain dehydrogenase/reductase gene and protein families: the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 65: 3895–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris H. J., Kotelevtsev Y., Mullins J. J., Seckl J. R., Holmes M. C. 2001. Intracellular regeneration of glucocorticoids by 11β-hydroxysteroid dehydrogenase (11β-HSD)-1 plays a key role in regulation of the hypothalamic-pituitary-adrenal axis: analysis of 11β-HSD-1-deficient mice. Endocrinology. 142: 114–120 [DOI] [PubMed] [Google Scholar]
- 46.Carter R. N., Paterson J. M., Tworowska U., Stenvers D. J., Mullins J. J., Seckl J. R., Holmes M. C. 2009. Hypothalamic-pituitary-adrenal axis abnormalities in response to deletion of 11β-HSD1 is strain-dependent. J. Neuroendocrinol. 21: 879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abrahams L., Semjonous N. M., Guest P., Zielinska A., Hughes B., Lavery G. G., Stewart P. M. 2012. Biomarkers of hypothalamic-pituitary-adrenal axis activity in mice lacking 11β-HSD1 and H6PDH. J. Endocrinol. 214: 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semjonous N. M., Sherlock M., Jeyasuria P., Parker K. L., Walker E. A., Stewart P. M., Lavery G. G. 2011. Hexose-6-phosphate dehydrogenase contributes to skeletal muscle homeostasis independent of 11β-hydroxysteroid dehydrogenase type 1. Endocrinology. 152: 93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogoff D., Ryder J. W., Black K., Yan Z., Burgess S. C., McMillan D. R., White P. C. 2007. Abnormalities of glucose homeostasis and the hypothalamic-pituitary-adrenal axis in mice lacking hexose-6-phosphate dehydrogenase. Endocrinology. 148: 5072–5080 [DOI] [PubMed] [Google Scholar]
- 50.Salen G., Verga D., Batta A., Tint G., Shefer S. 1982. Effect of 7-ketolithocholic acid on bile acid metabolism in humans. Gastroenterology. 83: 341–347 [PubMed] [Google Scholar]
- 51.Makishima M., Lu T. T., Xie W., Whitfield G. K., Domoto H., Evans R. M., Haussler M. R., Mangelsdorf D. J. 2002. Vitamin D receptor as an intestinal bile acid sensor. Science. 296: 1313–1316 [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Canaveras J. C., Donato M. T., Castell J. V., Lahoz A. 2012. Targeted profiling of circulating and hepatic bile acids in human, mouse and rat using an UPLC-MRM-MS-validated method. J. Lipid Res. 53: 2231–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guertin F., Loranger A., Lepage G., Roy C. C., Yousef I. M., Domingo N., Chanussot F., Lafont H., Tuchweber B. 1995. Bile formation and hepatic plasma membrane composition in guinea-pigs and rats. Comp. Biochem. Physiol. B. 111: 523–531 [DOI] [PubMed] [Google Scholar]
- 54.Cowles R. L., Lee J-Y., Gallaher D. D., Stuefer-Powell C. L., Carr T. P. 2002. Dietary stearic acid alters gallbladder bile acid composition in hamsters fed cereal-based diets. J. Nutr. 132: 3119–3122 [DOI] [PubMed] [Google Scholar]
- 55.Washizu T., Tomoda I., Kaneko J. J. 1991. Serum bile acid composition of the dog, cow, horse and human. J. Vet. Med. Sci. 53: 81–86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.